Effect of Syringopicroside Extracted from Syringa oblata Lindl on the Biofilm Formation of Streptococcus suis
Abstract
:1. Introduction
2. Results and Discussion
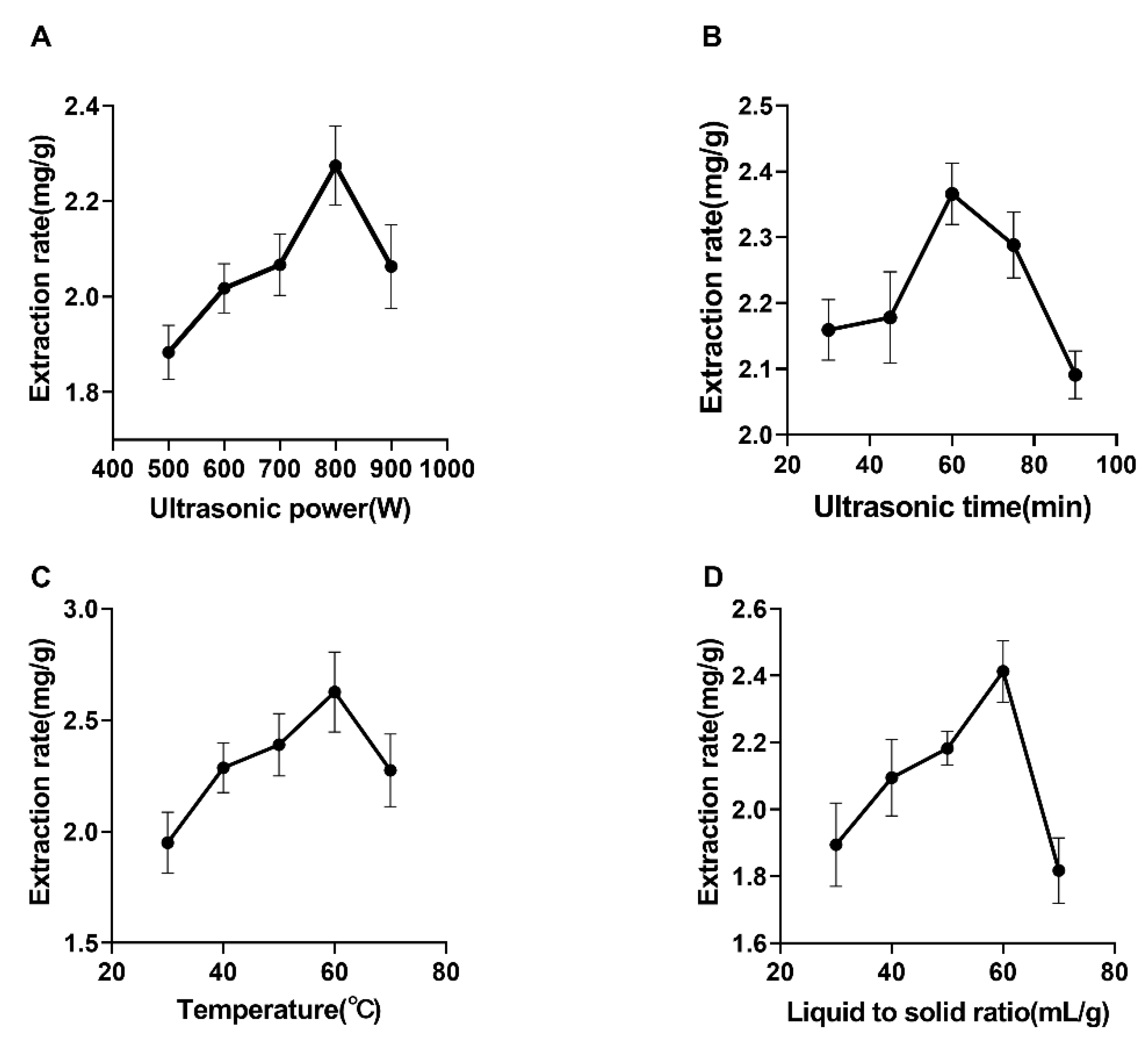
2.1. Effect of Ultrasonic Power on the Extraction of Syringopicroside
2.2. Effect of Ultrasonic Time on Extraction of Syringopicroside
2.3. Effect of Temperature on the Extraction of Syringopicroside
2.4. Effect of a Liquid-to-Solid Ratio on The Extraction of Syringopicroside
2.5. Optimization of the UAE Process with RSM
2.5.1. Statistical Analysis and Model Fitting
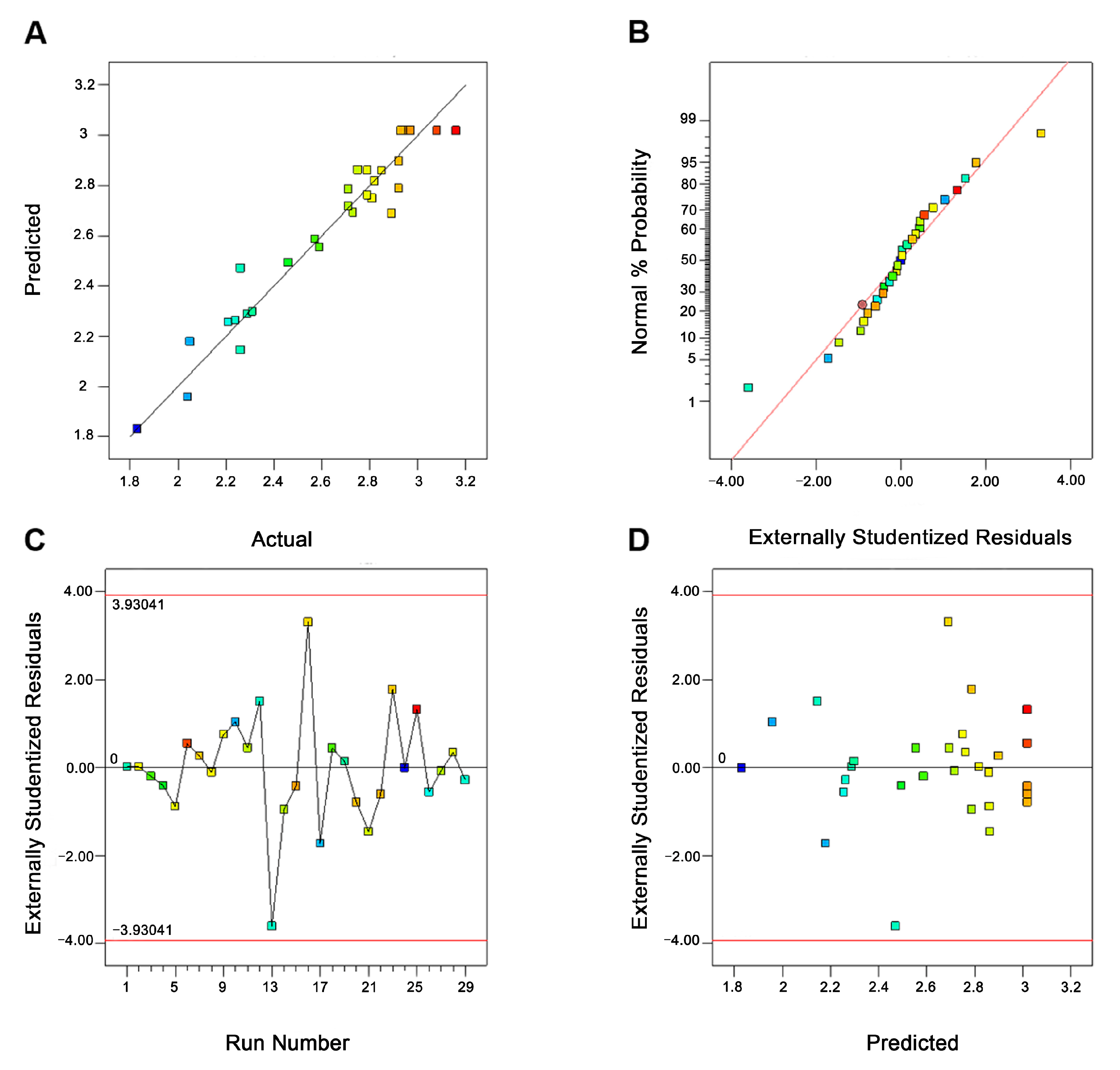
2.5.2. Model Adequacy
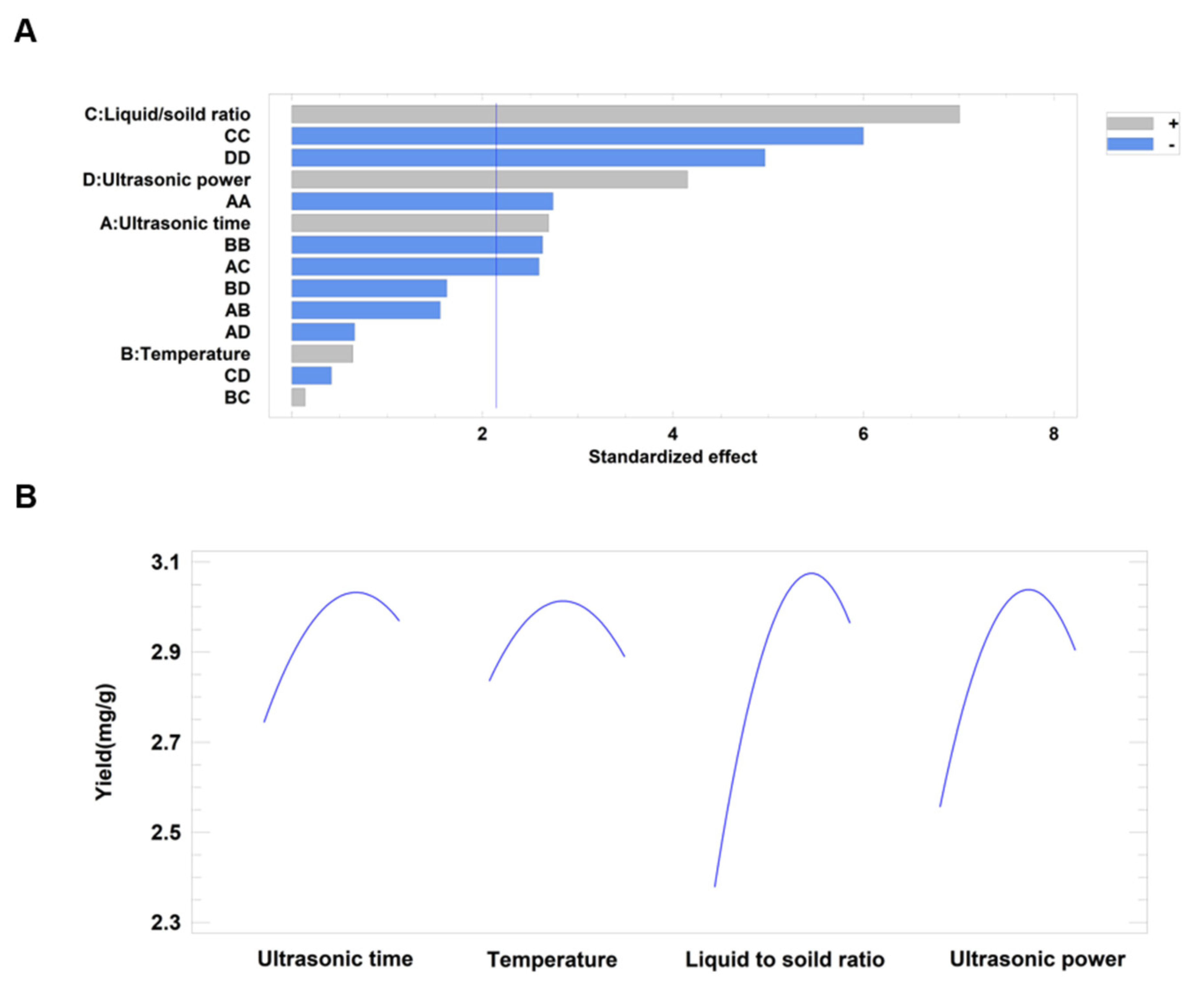
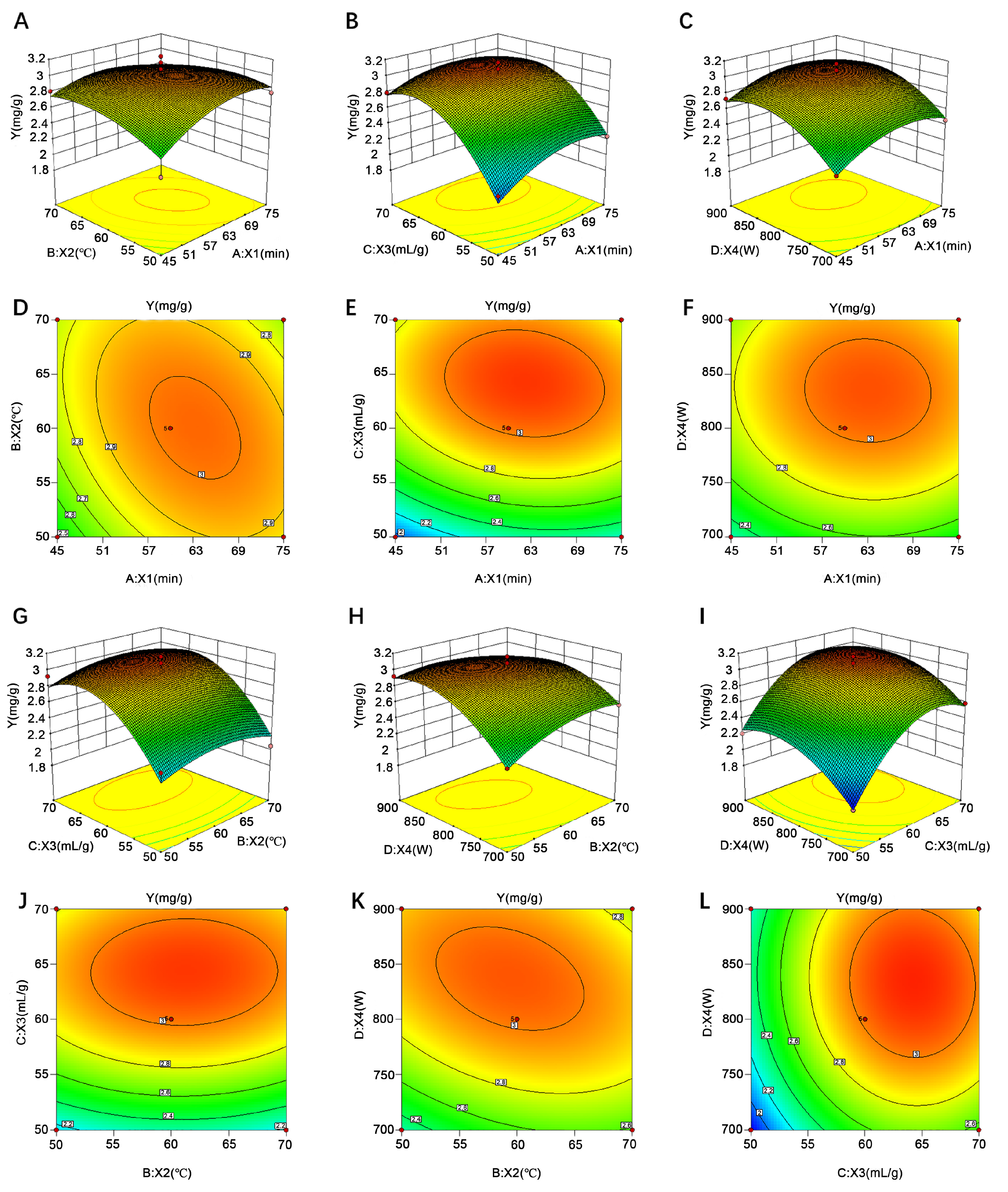
2.5.3. Influence of Variables upon Syringopicroside Yield
2.5.4. Verification of the Model
2.6. Separation and Identification of Syringopicroside
2.7. Effect of Syringopicroside on Biofilm Formation of S. suis
2.7.1. Minimum Inhibition Concentration (MIC) and Crystal Violet Staining Assay
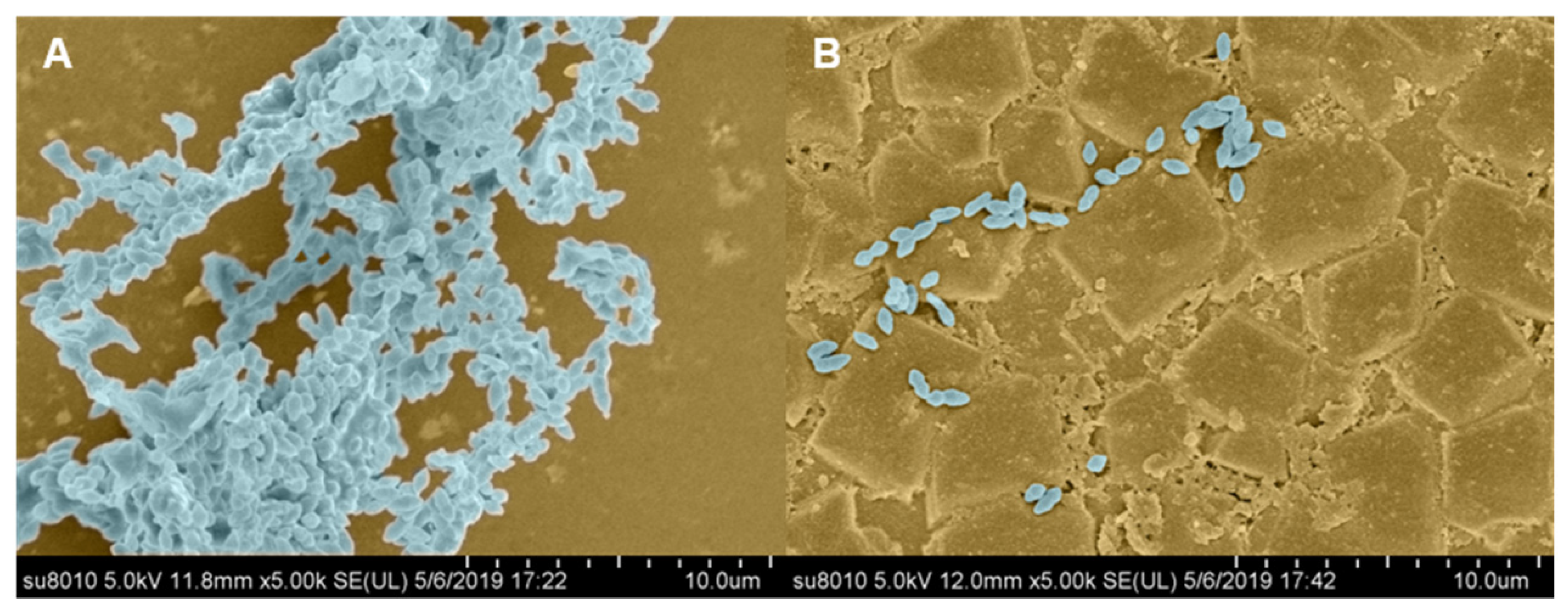
2.7.2. SEM Observation
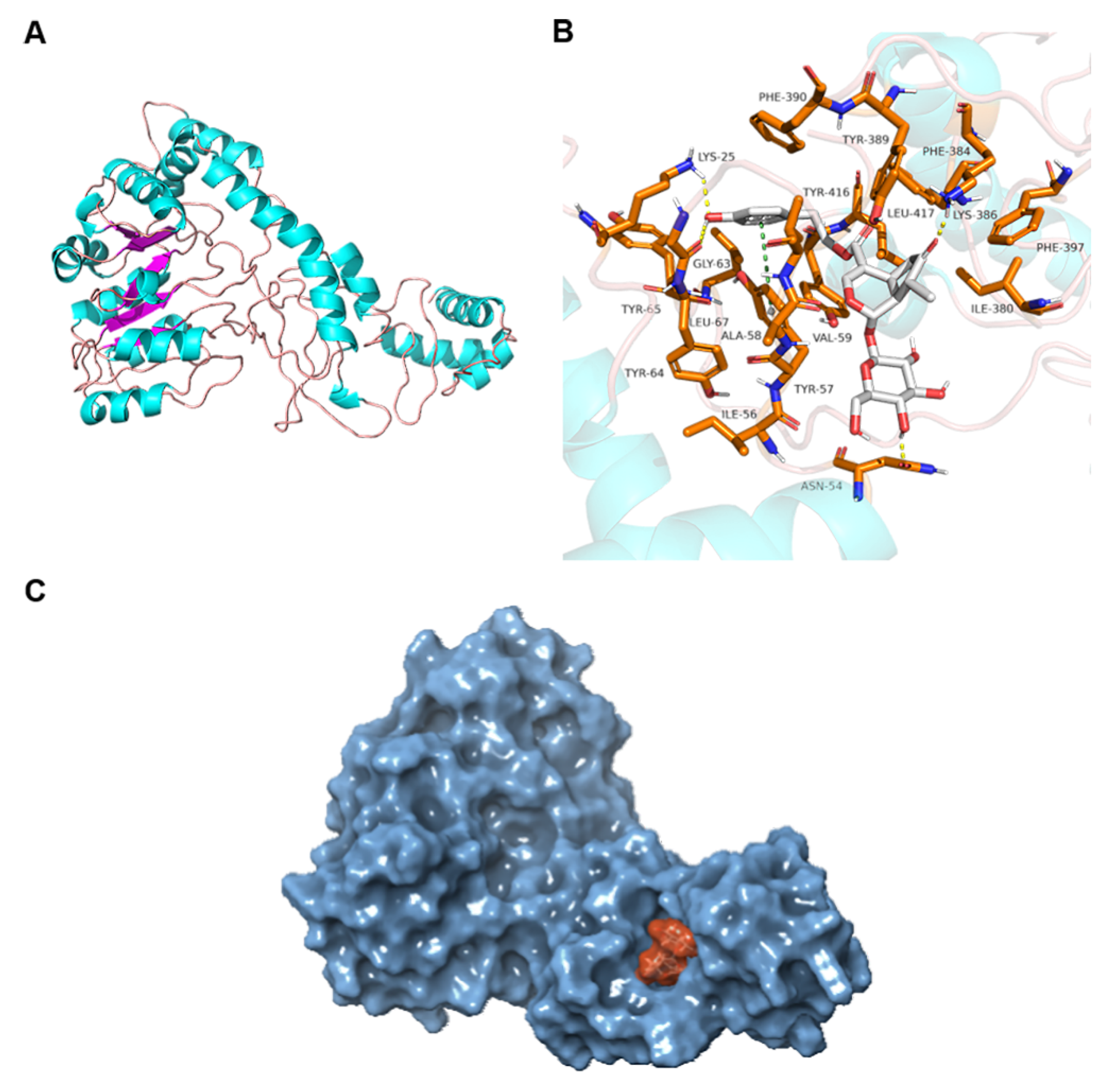
2.8. Molecular Docking
3. Materials and Methods
3.1. Bacteria Strain and Cultural Condition
3.2. Sample Preparation and Reagents
3.3. Single-Factor Experiments for Extraction of Syringopicroside
3.4. Experimental Design
3.5. Separation of Syringopicroside
3.6. Identification of Syringopicroside
3.7. Antibacterial Activity Evaluation of Syringopicroside
3.7.1. MIC Assay
3.7.2. Biofilm Assay
3.8. SEM Observation
3.9. Molecular Docking
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhao, M.; Tang, W.; Li, J.; Bai, L.; Wang, J.; Zhang, W.; Zhang, S. Two New Monoterpenoids from the Fresh Leaves of Syringa oblata. Chem. Nat. Compd. 2016, 52, 1023–1025. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; Chinese Pharmacopoeia Commission: Beijing, China, 2015.
- Zhang, X.; Lu, S.; Han, J.; Sun, S.; Wang, L.; Li, Y. Preparation, characterization and in vivo distribution of solid lipid nanoparticles loaded with syringopicroside. Pharmazie 2011, 66, 404–407. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, N.; Liu, Z.; Song, Z.; Wu, P.; Shao, J.; Zhang, J.; Yin, J. The antibacterial activity of syringopicroside, its metabolites and natural analogues from Syringae Folium. Fitoterapia 2016, 110, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, Y.Y.; Zhou, Y.H.; Xing, X.X.; Qu, Q.W.; Chen, X.Y.; Ding, W.Y.; Cheng, G.L.; Wei, A.J.; Feng, X.W. Process optimization of Syringa oblata Lindl. by response surface methodology and its effect on Staphylococcus xylosus biofilm. RSC Adv. 2019, 9, 36088–36096. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, J.; Zhou, C.; Gan, L. Preparative Separation and Enrichment of Syringopicroside from Folium syringae Leaves with Macroporous Resins. J. Biomed. Biotechnol. 2010, 2010, 572570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Yang, Y.; Wang, S.; Gao, L.F.; Chen, J.Q.; Ren, Y.Z.; Ding, W.Y.; Muhammad, I.; Li, Y.H. Syringa oblata Lindl. Aqueous Extract Is a Potential Biofilm Inhibitor in S. suis. Front. Pharmacol. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubalo, M.C.; Curko, N.; Tomasevic, M.; Ganic, K.K.; Redovnikovic, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Gottschalk, M.; Mariela, S.; Xu, J. Streptococcus suis infections in humans: The Chinese experience and the situation in North America. Anim. Health Res. Rev. 2007, 8, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Wertheim, H.F.L.; Nihia, H.D.T.; Taylor, W.; Schultsz, C. Streptococcus suis: An Emerging Human Pathogen. Clin. Infect. Dis. 2009, 48, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Bello-Onaghise, G.; Wang, G.; Han, X.; Nsabimana, E.; Cui, W.Q.; Yu, F.; Zhang, Y.F.; Wang, L.G.; Li, Z.Z.; Cai, X.H.; et al. Antiviral Strategies of Chinese Herbal Medicine Against PRRSV Infection. Front. Microbiol. 2020, 11, 1756. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Chung, P.H.; So, T.; Wong, L.; Choi, K.M.; Cheung, D.T.; Kam, K.M.; Chuang, S.K.; Tsang, T. Streptococcus suis infection in Hong Kong: An emerging infectious disease? Epidemiol. Infect. 2008, 136, 1691–1697. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zheng, H.; Gottschalk, M.; Bai, X.M.; Lan, R.T.; Ji, S.B.; Liu, H.C.; Xu, J.G. Development of Multiplex PCR Assays for the Identification of the 33 Serotypes of Streptococcus suis. PLoS ONE 2013, 8, e72070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lun, Z.R.; Wang, Q.P.; Chen, X.G.; Li, A.X.; Zhu, X.Q. Streptococcus suis: An emerging zoonotic pathogen. Lancet Infect Dis. 2007, 7, 201–209. [Google Scholar] [CrossRef]
- Takeuchi, D.; Kerdsin, A.; Akeda, Y.; Chiranairadul, P.; Loetthong, P.; Tanburawong, N.; Areeratana, P.; Puangmail, P.; Khamisara, K.; Pinyo, W. Impact of a Food Safety Campaign on Streptococcus suis Infection in Humans in Thailand. Am. J. Trop. Med. Hyg. 2017, 96, 1370–1377. [Google Scholar] [CrossRef]
- Huong, V.T.L.; Ha, N.; Huy, N.T.; Horby, P.; Nghia, H.D.T.; THiem, V.D.; Zhu, X.T.; Hoa, N.T.; Hien, T.T.; Zamora, J. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg. Infect. Dis. 2014, 20, 1105–1114. [Google Scholar] [CrossRef]
- Schultsz, C.; Jansen, E.; Keikzer, W.; Rothkamp, P.; Duim, B.; Wagenaar, J.A.; van der Ende, A. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PLoS ONE 2012, 7, e33854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seneviratne, C.J.; Wang, Y.; Jin, L.; Wong, S.S.; Herath, T.D.; Samaranayake, L.P. Unraveling the resistance of microbial biofilms: Has proteomics been helpful? Proteomics 2012, 12, 651–665. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.X.; Sun, L.Y.; Grenier, D.; Yi, L. Streptococcus suis biofilm: Regulation, drug-resistance mechanisms, and disinfection strategies. Appl. Microbiol. Biotechnol. 2018, 102, 9121–9129. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boolchandani, M.; D’Souza, A.W.; Dantas, G. Sequencing-based methods and resources to study antimicrobial resistance. Nat. Rev. Genet. 2019, 20, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.W.; Chen, X.R.; Tang, Y.; Cui, W.Q.; Li, D.L.; God’spower, B.O.; Yang, Y. Study on microwave assisted extraction of chrysophanol and its intervention in biofilm formation of Streptococcus suis. RSC Adv. 2019, 9, 28996–29004. [Google Scholar] [CrossRef] [Green Version]
- Parrino, B.; Carbone, D.; Cirrincione, G.; Diana, P.; Cascioferro, S. Inhibitors of antibiotic resistance mechanisms: Clinical applications and future perspectives. Future Med. Chem. 2020, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Bai, J.W.; Bu, X.Y.; Tang, Y.; Han, W.Q.; Li, D.L.; Wang, L.B.; Yang, Y.; Xu, Y.Q. Microwave-assisted aqueous two-phase extraction of phenolic compounds from Ribes nigrum L. and its antibacterial effect on foodborne pathogens. Food Control 2020, 119, e107449. [Google Scholar] [CrossRef]
- Okura, M.; Takamatsu, D.; Maruyama, F.; Nozawa, T.; Nakagawa, I.; Osaki, M.; Sekizaki, T.; Gottschalk, M.; Kumagai, Y.; Hamada, S. Genetic Analysis of Capsular Polysaccharide Synthesis Gene Clusters from All Serotypes of Streptococcus suis: Potential Mechanisms for Generation of Capsular Variation. Appl. Environ. Microbiol. 2013, 79, 2796–2806. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.E.; De Vries, R.; Slot, R.V.T.; Smits, M.A. The cps locus Streptococcus suis serotype 2: Genetic determinant for the synthesis of sialic acid. Microb. Pathog. 2000, 29, 127–134. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Gao, L.F.; Cai, H.; Zhou, Y.H.; Yang, Y.B.; Xu, C.G.; Ding, W.Y.; Chen, J.Q.; Muhammad, I. Rutin Inhibits Streptococcus suis Biofilm Formation by Affecting CPS Biosynthesis. Front. Pharmacol. 2017, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Al-dhabi, N.A.; Ponmurugan, K.; Maran, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of pectin from sisal waste. Carbohydr. Polym. 2015, 115, 732–738. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Capelo, J.L.; Lavilla, I.; Bendicho, C. Comparison of ultrasound-assisted extraction and microwave-assisted digestion for determination of magnesium, manganese and zinc in plant samples by flame atomic absorption spectrometry. Talanta 2000, 53, 433–441. [Google Scholar] [CrossRef]
- Chen, F.L.; Zhang, Q.; Liu, J.L.; Gu, H.Y.; Yang, L. An efficient approach for the extraction of orientin and vitexin from Trollius chinensis flowers using ultrasonic circulating technique. Ultrason. Sonochem. 2017, 37, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Lu, T.H.; Wang, M.X.; Zou, X.T.; Zhang, Y.; Yang, X.D.; Dong, Y.; Zhou, H.L. Flavonoids from Morus alba L. leaves: Optimization of extraction by response surface methodology and comprehensive evaluation of their antioxidant, antimicrobial, and inhibition of α-amylase activities through analytical hierarchy process. Molecules 2019, 24, 2398. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhang, L.C.; Li, Q.P.; Jin, W.F.; Chen, W.Y.; Han, J.; Zhang, Y.Y. Simultaneous optimization for ultrasound-assisted extraction and antioxidant activity of flavonoids from Sophora flavescens using response surface methodology. Molecules 2019, 24, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soumen, D.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Lapkin, A.A.; Plucinski, P.K.; Cutler, M. Comparative Assessment of Technologies for Extraction of Artemisinin. J. Nat. Prod. 2006, 69, 1653–1664. [Google Scholar] [CrossRef]
- Gogate, P.R.; Wilhelm, A.M.; Pandit, A.B. Some Aspects of the design of sonochemical reactors. Ultrason. Sonochem. 2003, 10, 325–330. [Google Scholar] [CrossRef]
- Hemwimol, S.; Pavasant, P.; Shotipruk, A. Ultrasound-Assisted Extraction of Anthraquinones from Roots of Morinda citrifolia. Ultrason. Sonochem. 2006, 13, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.A.; Zhang, Z.Q.; Yue, X.F.; Fan, X.H.; Li, T.; Chen, S.F. Response surface optimization of ultrasound-assisted oil extraction from autoclaved almond powder. Food Chem. 2009, 116, 513–518. [Google Scholar] [CrossRef]
- Nuerxiati, R.; Abuduwaili, A.; Mutailifu, P.; Wubulikasimu, A.; Rustamova, N.; Cui, J.X.; Aisa, H.A.; Yili, A. Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep). Int. J. Biol. Macromol. 2019, 141, 431–443. [Google Scholar] [CrossRef]
- Tian, S.Y.; Hao, C.C.; Xu, G.K.; Yang, J.J.; Sun, R.G. Optimization conditions for extracting polysaccharide from Angelica sinensis and its antioxidant activities. J. Food Drug Anal. 2017, 25, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.Q.; Liu, G.J.; Yu, Z.Y.; Song, X.M.; Li, X.G.; Wang, L.B.; Liu, L.; Dai, J. Purification, characterization and antiglycation activity of a novel polysaccharide from black currant. Food Chem. 2015, 199, 694–701. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Extraction of natural anthocyanin and colors from pulp of jamun fruit. J. Food Sci. Technol. Mysore. 2015, 52, 3617–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senrayan, J.; Venkatachalam, S. Optimization of ultrasound- assisted solvent extraction (UASE) based on oil yield, antioxidant activity and evaluation of fatty acid composition and thermal stability of Coriandrum sativum L. seed oil. Food Sci. Biotechnol. 2019, 28, 377–386. [Google Scholar] [CrossRef]
- Gupta, B.S.; Ako, J.E. Application of guar gum as a flocculant aid in food processing and potable water treatment. Eur. Food Res. Technol. 2005, 221, 746–751. [Google Scholar] [CrossRef]
- Yang, Y.B.; Wang, S.; Wang, C.; Huang, Q.Y.; Bai, J.W.; Chen, J.Q.; Chen, X.Y.; Li, Y.H. Emodin affects biofilm formation and expression of virulence factors in Streptococcus suis ATCC700794. Arch. Microbiol. 2015, 197, 1173–1180. [Google Scholar] [CrossRef]
- Nenadis, N.; Vervoort, J.; Boeren, S.; Tsimidou, M.Z. Syringa oblata Lindl var. alba as a source of oleuropein and related compounds. J. Sci. Food Agric. 2007, 87, 160–166. [Google Scholar] [CrossRef]
- Geremia, R.; Rinaudo, M. Biosynthesis, structure, and physical properties of some bacterial polysaccharides. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; Dekker: New York, NY, USA, 2005; pp. 411–430. [Google Scholar]
- Yother, J. Capsules of Streptococcus pneumoniae and other bacteria: Paradigms for polysaccharide biosynthesis and regulation. Annu. Rev. Microbiol. 2011, 65, 563–581. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Kos, V.; Whitfield, C. ABC transporters involved in export of cell surface glycoconjugates. Microbiol. Mol. Biol. Rev. 2010, 74, 341–362. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.B.; Mota, R.; Santos, C.L.; De Philipps, R.; Tamagnini, P. Assembly and Export of Extracellular Polymeric Substances (EPS) in Cyanobacteria: A Phylogenomic Approach. Adv. Bot. Res. 2013, 65, 235–279. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, X.R.; Gao, L.F.; Chem, M.; Cui, W.Q.; Ding, W.Y.; Chen, X.Q.; Bello-Onaghise, G.; Li, Y.H. Spectrum-Effect Relationships Between the Bioactive Ingredient of Syringa oblata Lindl. Leaves and Its Role in Inhibiting the Biofilm Formation of Streptococcus suis. Front. Pharmacol. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Jing, X.; Li, G. Optimization of Vacuum Microwave-Mediated Extraction of Syringoside and Oleuropein from Twigs of Syringa oblata. J. Anal. Methods Chem. 2018, 2018, 6179013. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, Z.X.; Zhao, M.M.; Wu, C.Z.; Wang, L.B.; Xu, Y.Q. Microwave-assisted extraction of an acidic polysaccharide from Ribes nigrum L.: Structural characteristics and biological activities. Ind. Crop. Prod. 2020, 147, 112249. [Google Scholar] [CrossRef]
- Wei, H.; Ruan, J.L.; Lei, Y.F.; Yang, C. Enrichment and purification of flavones from rhizomes of Abacopteris penangiana by macroporous resins. Chin. J. Nat. Med. 2012, 10, 119–124. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, X.R.; Wang, J.P.; Cui, W.Q.; Xing, X.X.; Chen, X.Y.; Ding, W.Y.; Godspower, B.O.; Eliphaz, N.; Sun, M.Q. Transcriptomic analysis reveals flavonoid biosynthesis of Syringa oblata Lindl. in response to different light intensity. BMC Plant. Biol. 2019, 19, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Q.W.; Wang, J.P.; Cui, W.Q.; Zhou, Y.H.; Xing, X.X.; Che, R.X.; Liu, X.; Chen, X.R.; Bello-Onaghise, G.; Dong, C.L.; et al. In vitro activity and In vivo efficacy of Isoliquiritigenin against Staphylococcus xylosus ATCC 700404 by IGPD target. PLoS ONE 2019, 14, e0226260. [Google Scholar] [CrossRef] [Green Version]
- Rolf, A.; Amos, B.; Wu, C.H.; Baker, W.C.; Brigitte, B.; Serenella, F.; Elisabeth, G.; Huang, H.; Rodrigo, L.; Michele, M. UniProt: The Universal Protein knowledgebase. Nucleic Acids Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef]
- Song, Y.F.; DiMaio, F.; Wang, R.Y.R.; Kim, D.; Miles, C.; Brunette, T.J.; Thompson, J.; Baker, D. High resolution comparative modeling with RosettaCM. Structure 2013, 21, 1735–1742. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chen, J.; Gindulyte, T.; He, A.J.S.; He, Q.; Li, B.A.; Shoemaker, P.A.; Thiessen, B.; Yu, L.; Zaslavsky, J.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 21, D1102–D1109. [Google Scholar] [CrossRef] [Green Version]
- Lill, M.A.; Danielson, M.L. Computer-aided drug design platform using PyMOL. J. Comput.-Aided Mol. Des. 2011, 25, 13–19. [Google Scholar] [CrossRef]


| Run | Variable Levels | Y (mg/g) | ||||
|---|---|---|---|---|---|---|
| X1 (min) | X2 (°C) | X3 (mL/g) | X4 (W) | Observed | Predicted | |
| 1 | −1 | 0 | 0 | −1 | 2.29 ± 0.13 | 2.29 |
| 2 | 1 | 0 | 0 | 1 | 2.82 ± 0.11 | 2.82 |
| 3 | 0 | 1 | 0 | −1 | 2.57 ± 0.09 | 2.59 |
| 4 | 1 | 0 | 0 | −1 | 2.46 ± 0.12 | 2.49 |
| 5 | 1 | −1 | 0 | 0 | 2.79 ± 0.03 | 2.86 |
| 6 | 0 | 0 | 0 | 0 | 3.08 ± 0.06 | 3.02 |
| 7 | 0 | −1 | 0 | 1 | 2.92 ± 0.04 | 2.90 |
| 8 | 0 | 0 | 1 | 1 | 2.85 ± 0.06 | 2.86 |
| 9 | −1 | 1 | 0 | 0 | 2.81 ± 0.05 | 2.75 |
| 10 | −1 | 0 | −1 | 0 | 2.04 ± 0.10 | 1.96 |
| 11 | −1 | 0 | 0 | 1 | 2.73 ± 0.07 | 2.69 |
| 12 | 0 | −1 | −1 | 0 | 2.26 ± 0.03 | 2.15 |
| 13 | −1 | −1 | 0 | 0 | 2.26 ± 0.07 | 2.47 |
| 14 | 1 | 0 | 1 | 0 | 2.71 ± 0.11 | 2.79 |
| 15 | 0 | 0 | 0 | 0 | 2.97 ± 0.09 | 3.02 |
| 16 | 1 | 1 | 0 | 0 | 2.89 ± 0.05 | 2.69 |
| 17 | 0 | 1 | −1 | 0 | 2.05 ± 0.06 | 2.18 |
| 18 | 0 | 0 | 1 | −1 | 2.59 ± 0.06 | 2.55 |
| 19 | 0 | −1 | 0 | −1 | 2.31 ± 0.03 | 2.30 |
| 20 | 0 | 0 | 0 | 0 | 2.93 ± 0.04 | 3.02 |
| 21 | 0 | 1 | 1 | 0 | 2.75 ± 0.05 | 2.86 |
| 22 | 0 | 0 | 0 | 0 | 2.95 ± 0.05 | 3.02 |
| 23 | 0 | −1 | 1 | 0 | 2.62 ± 0.08 | 2.58 |
| 24 | 0 | 0 | −1 | −1 | 1.83 ± 0.08 | 1.83 |
| 25 | 0 | 0 | 0 | 0 | 3.16 ± 0.11 | 3.02 |
| 26 | 0 | 0 | −1 | 1 | 2.21 ± 0.10 | 2.26 |
| 27 | 0 | 1 | 0 | 1 | 2.71 ± 0.06 | 2.72 |
| 28 | −1 | 0 | 1 | 0 | 2.51 ± 0.04 | 2.56 |
| 29 | 1 | 0 | −1 | 0 | 2.24 ± 0.07 | 2.26 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 3.12 | 14 | 0.22 | 18.73 | <0.0001 ** |
| X1 | 0.13 | 1 | 0.13 | 11.30 | 0.0047 ** |
| X2 | 0.032 | 1 | 0.032 | 2.69 | 0.1230 |
| X3 | 0.96 | 1 | 0.96 | 81.01 | <0.0001 ** |
| X4 | 0.40 | 1 | 0.40 | 33.61 | <0.0001 ** |
| X1X2 | 0.051 | 1 | 0.051 | 4.26 | 0.0581 |
| X1X3 | 0.000 | 1 | 0.000 | 0.000 | 1.0000 |
| X1X4 | 1.600 × 10−3 | 1 | 1.600 × 10−3 | 0.13 | 0.7193 |
| X2X3 | 0.029 | 1 | 0.029 | 2.43 | 0.1413 |
| X2X4 | 0.055 | 1 | 0.055 | 4.64 | 0.0491 * |
| X3X4 | 3.600 × 10−3 | 1 | 3.600 × 10−3 | 0.30 | 0.5909 |
| X12 | 0.26 | 1 | 0.26 | 21.69 | 0.0004 ** |
| X22 | 0.15 | 1 | 0.15 | 12.38 | 0.0034 ** |
| X32 | 1.23 | 1 | 1.23 | 103.53 | <0.0001 ** |
| X42 | 0.35 | 1 | 0.35 | 29.34 | <0.0001 ** |
| Residual | 0.17 | 14 | 0.012 | - | - |
| Lack-of-fit | 0.13 | 10 | 0.013 | 1.32 | 0.4237 |
| Pure error | 0.039 | 4 | 9.670 × 10−3 | - | - |
| Cor total | 3.28 | 28 | - | - | - |
| C.V% | - | 4.20 | - | - | |
| R2 | - | 0.9493 | - | - | |
| Adj-R2 | - | 0.8986 | - | - | |
| Donor Force | Receptor Force | Interaction Type |
|---|---|---|
| syringopicroside: OH | ASN54: O | Hydrogen bond |
| syringopicroside: OH | GLY63: O | Hydrogen bond |
| LYS25: OH | syringopicroside: O | Hydrogen bond |
| LYS386: OH | syringopicroside: O | Hydrogen bond |
| TYR57 | syringopicroside | π-π stacking |
| Coded Symbol | Independent Variable | Units | Levels | ||
|---|---|---|---|---|---|
| 1 | 0 | −1 | |||
| X1 | Ultrasonic time | min | 75 | 60 | 45 |
| X2 | Temperature | °C | 70 | 60 | 50 |
| X3 | Liquid-to-solid ratio | mL/g | 70:1 | 60:1 | 50:1 |
| X4 | Ultrasonic power | W | 900 | 800 | 700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Bai, J.; Yang, Y.; Bai, X.; Bello-Onaghise, G.; Xu, Y.; Li, Y. Effect of Syringopicroside Extracted from Syringa oblata Lindl on the Biofilm Formation of Streptococcus suis. Molecules 2021, 26, 1295. https://doi.org/10.3390/molecules26051295
Tang Y, Bai J, Yang Y, Bai X, Bello-Onaghise G, Xu Y, Li Y. Effect of Syringopicroside Extracted from Syringa oblata Lindl on the Biofilm Formation of Streptococcus suis. Molecules. 2021; 26(5):1295. https://doi.org/10.3390/molecules26051295
Chicago/Turabian StyleTang, Yang, Jingwen Bai, Yu Yang, Xuedong Bai, God’spower Bello-Onaghise, Yaqin Xu, and Yanhua Li. 2021. "Effect of Syringopicroside Extracted from Syringa oblata Lindl on the Biofilm Formation of Streptococcus suis" Molecules 26, no. 5: 1295. https://doi.org/10.3390/molecules26051295






