Radiofrequency Irradiation Attenuates High-Mobility Group Box 1 and Toll-like Receptor Activation in Ultraviolet B–Induced Skin Inflammation
Abstract
:1. Introduction
2. Results
2.1. Inhibitory Effect of RF on Inflammatory Cytokine Secreted by UVB-Effected Keratinocytes
2.2. Inhibition Effect on Macrophage Activation by RF-Irradiated Keratinocytes
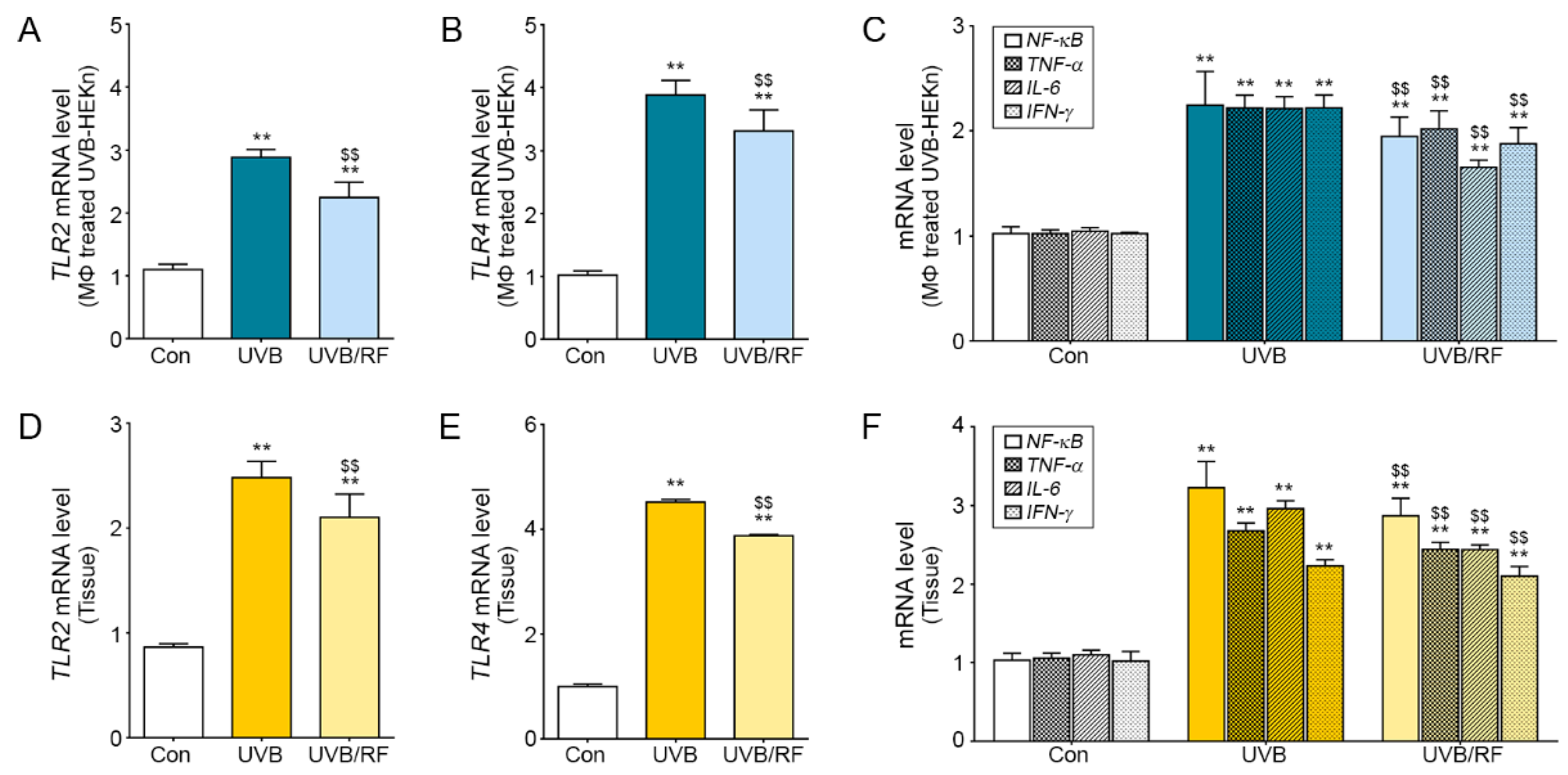
2.3. Inhibitory Effects on the TLR Pathway of Macrophages Affected by RF-Irradiated Keratinocytes
2.4. The Inhibitory Effects of RF Irradiation on Macrophages Affect Keratinocyte Proliferation and Pigment Accumulation
3. Discussion
4. Materials and Methods
4.1. UVB-Induced Skin Inflammation Models
4.1.1. Animal Model (In Vivo)
4.1.2. Cell Model (In Vitro)
4.2. RF Irradiation Conditions for Patients
4.3. Devices for RF Irradiation
4.4. Cell Proliferation Measurement
4.5. Sample Preparation
4.5.1. Paraffin-Embedded Tissue Section Processing
4.5.2. RNA Extraction and cDNA Synthesis
4.6. Quantitative Real-Time Polymerase Chain Reaction
4.7. Immunostaining Using 3,3-Diaminobenzidine
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef]
- Melnikova, V.O.; Ananthaswamy, H.N. Cellular and molecular events leading to the development of skin cancer. Mutat. Res. 2005, 571, 91–106. [Google Scholar] [CrossRef]
- Fisher, M.S.; Kripke, M.L. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science 1982, 216, 1133–1134. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Köck, A.; Schwarz, T.; Kirnbauer, R.; Urbanski, A.; Perry, P.; Ansel, J.C.; A Luger, T. Human keratinocytes are a source for tumor necrosis factor alpha: Evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J. Exp. Med. 1990, 172, 1609–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luger, T.A.; Schwarz, T. Evidence for an Epidermal Cytokine Network. J. Investig. Dermatol. 1990, 95, 100S–104S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Abaseri, T.B.; Putta, S.; Hansen, L.A. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis 2006, 27, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skobowiat, C.; Slominski, A.T. UVB Activates Hypothalamic-Pituitary-Adrenal Axis in C57BL/6 Mice. J. Investig. Dermatol. 2015, 135, 1638–1648. [Google Scholar] [CrossRef] [Green Version]
- Skobowiat, C.; Postlethwaite, A.E.; Slominski, A.T. Skin Exposure to Ultraviolet B Rapidly Activates Systemic Neuroendocrine and Immunosuppressive Responses. Photochem. Photobiol. 2017, 93, 1008–1015. [Google Scholar] [CrossRef]
- Guttridge, D.C.; Albanese, C.; Reuther, J.Y.; Pestell, R.G.; Baldwin, A.S., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 1999, 19, 5785–5799. [Google Scholar] [CrossRef] [Green Version]
- Freedberg, I.M.; Tomic-Canic, M.; Komine, M.; Blumenberg, M. Keratins and the keratinocyte activation cycle. J. Investig. Dermatol. 2001, 116, 633–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Chen, X.; Zhao, J.; Martin, B.; Zepp, J.A.; Ko, J.S.; Gu, C.; Cai, G.; Ouyang, W.; Sen, G.; et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 2015, 212, 1571–1587. [Google Scholar] [CrossRef]
- Nygaard, U.; Bogaard, E.; Niehues, H.; Hvid, M.; Deleuran, M.; Johansen, C.; Vestergaard, C. The “Alarmins” HMBG1 and IL-33 Downregulate Structural Skin Barrier Proteins and Impair Epidermal Growth. Acta Derm. Venereol. 2017, 97, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Erlandsson Harris, H.; Andersson, U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 2004, 34, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, H.; Chen, F.; Wang, Y.; Ma, J.; Wang, G. The HMGB1-RAGE/TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia. Exp. Ther. Med. 2019, 17, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Taticchi, A.; Urbani, S.; Albi, E.; Servili, M.; Codini, M.; Traina, G.; Balloni, S.; Patria, F.F.; Perioli, L.; Beccari, T.; et al. In Vitro Anti-Inflammatory Effects of Phenolic Compounds from Moraiolo Virgin Olive Oil (MVOO) in Brain Cells via Regulating the TLR4/NLRP3 Axis. Molecules 2019, 24, 4523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexis, A.F.; Sergay, A.B.; Taylor, S.C. Common dermatologic disorders in skin of color: A comparative practice survey. Cutis 2007, 80, 387–394. [Google Scholar]
- Masu, S.; Seiji, M. Pigmentary incontinence in fixed drug eruptions. Histologic and electron microscopic findings. J. Am. Acad. Dermatol. 1983, 8, 525–532. [Google Scholar] [CrossRef]
- Taylor, S.; Grimes, P.; Lim, J.; Im, S.; Lui, H. Postinflammatory hyperpigmentation. J. Cutan Med. Surg. 2009, 13, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lockey, R. Dermatology for the allergist. World Allergy Organ. J. 2010, 3, 202–215. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Lee, M.J. Therapeutic Efficacy and Safety of Invasive Pulsed-Type Bipolar Alternating Current Radiofrequency on Melasma and Rebound Hyperpigmentation. Med. Lasers 2017, 6, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.B.; Na, J.; Zheng, J.M.; Lim, J.M.; Kang, J.-S.; Lee, J.H.; Lee, S.E. In vivo skin reactions from pulsed-type, bipolar, alternating current radiofrequency treatment using invasive noninsulated electrodes. Skin Res. Technol. 2018, 24, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Lee, J.H. Adverse Events after Noninvasive Radiofrequency Treatment for Cosmetic Uses. Med Lasers 2015, 4, 16–19. [Google Scholar] [CrossRef]
- Sesto, A.; Navarro, M.; Burslem, F.; Jorcano, J.L. Analysis of the ultraviolet B response in primary human keratinocytes using oligonucleotide microarrays. Proc. Natl. Acad. Sci. USA 2002, 99, 2965–2970. [Google Scholar] [CrossRef] [Green Version]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 14051411. [Google Scholar] [CrossRef]
- Ryser, S.; Schuppli, M.; Gauthier, B.; Hernandez, D.R.; Roye, O.; Hohl, D.; German, B.; Holzwarth, J.A.; Moodycliffe, A.M. UVB-induced skin inflammation and cutaneous tissue injury is dependent on the MHC class I-like protein, CD1d. J. Investig. Dermatol. 2014, 134, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Bashir, M.M.; Sharma, M.R.; Werth, V.P. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. J. Investig. Dermatol. 2009, 129, 994–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.S. Toll-like receptors in skin. Adv. Dermatol. 2008, 24, 71–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, J.; Kim, J.H.; Kim, J.M.; Kwon, T.R.; Choi, S.Y.; Li, K.; Kim, B.J. Effects of Intradermal Radiofrequency Treatment and Intense Pulsed Light Therapy in an Acne-induced Rabbit Ear Model. Sci. Rep. 2019, 9, 5056. [Google Scholar] [CrossRef]
- Michalczyk, T.; Biedermann, T.; Bottcher-Haberzeth, S.; Klar, A.S.; Meuli, M.; Reichmann, E. UVB exposure of a humanized skin model reveals unexpected dynamic of keratinocyte proliferation and Wnt inhibitor balancing. J. Tissue Eng. Regen. Med. 2018, 12, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Kim, B.C.; Han, N.-K.; Lee, Y.-S.; Kim, T.; Yun, J.-H.; Kim, N.; Pack, J.-K.; Lee, J.-S. Effects of combined radiofrequency radiation exposure on the cell cycle and its regulatory proteins. Bioelectromagnetics 2011, 32, 169–178. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E. HMGB1, IL-1α, IL-33 and S100 proteins: Dual-function alarmins. Cell. Mol. Immunol. 2017, 14, 43–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaowattanapanit, S.; Silpa-Archa, N.; Kohli, I.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. J. Am. Acad. Dermatol. 2017, 77, 607–621. [Google Scholar] [CrossRef]
- Calderhead, R.G. Photobiological Basics of Photomedicine: A Work of Art Still in Progress. Med Lasers 2017, 6, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31. [Google Scholar] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Oh, S.; Yoon, J.H.; Kang, D.; Son, M.; Byun, K. Radiofrequency Irradiation Attenuates High-Mobility Group Box 1 and Toll-like Receptor Activation in Ultraviolet B–Induced Skin Inflammation. Molecules 2021, 26, 1297. https://doi.org/10.3390/molecules26051297
Kim HM, Oh S, Yoon JH, Kang D, Son M, Byun K. Radiofrequency Irradiation Attenuates High-Mobility Group Box 1 and Toll-like Receptor Activation in Ultraviolet B–Induced Skin Inflammation. Molecules. 2021; 26(5):1297. https://doi.org/10.3390/molecules26051297
Chicago/Turabian StyleKim, Hyoung Moon, Seyeon Oh, Jung Hyun Yoon, Donghwan Kang, Myeongjoo Son, and Kyunghee Byun. 2021. "Radiofrequency Irradiation Attenuates High-Mobility Group Box 1 and Toll-like Receptor Activation in Ultraviolet B–Induced Skin Inflammation" Molecules 26, no. 5: 1297. https://doi.org/10.3390/molecules26051297






