Structure Analysis and Study of Biological Activities of Condensed Tannins from Bruguiera gymnorhiza (L.) Lam and Their Effect on Fresh-Cut Lotus Roots
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolics and Condensed Tannins Contents in the Leaf of B. gymnorhiza
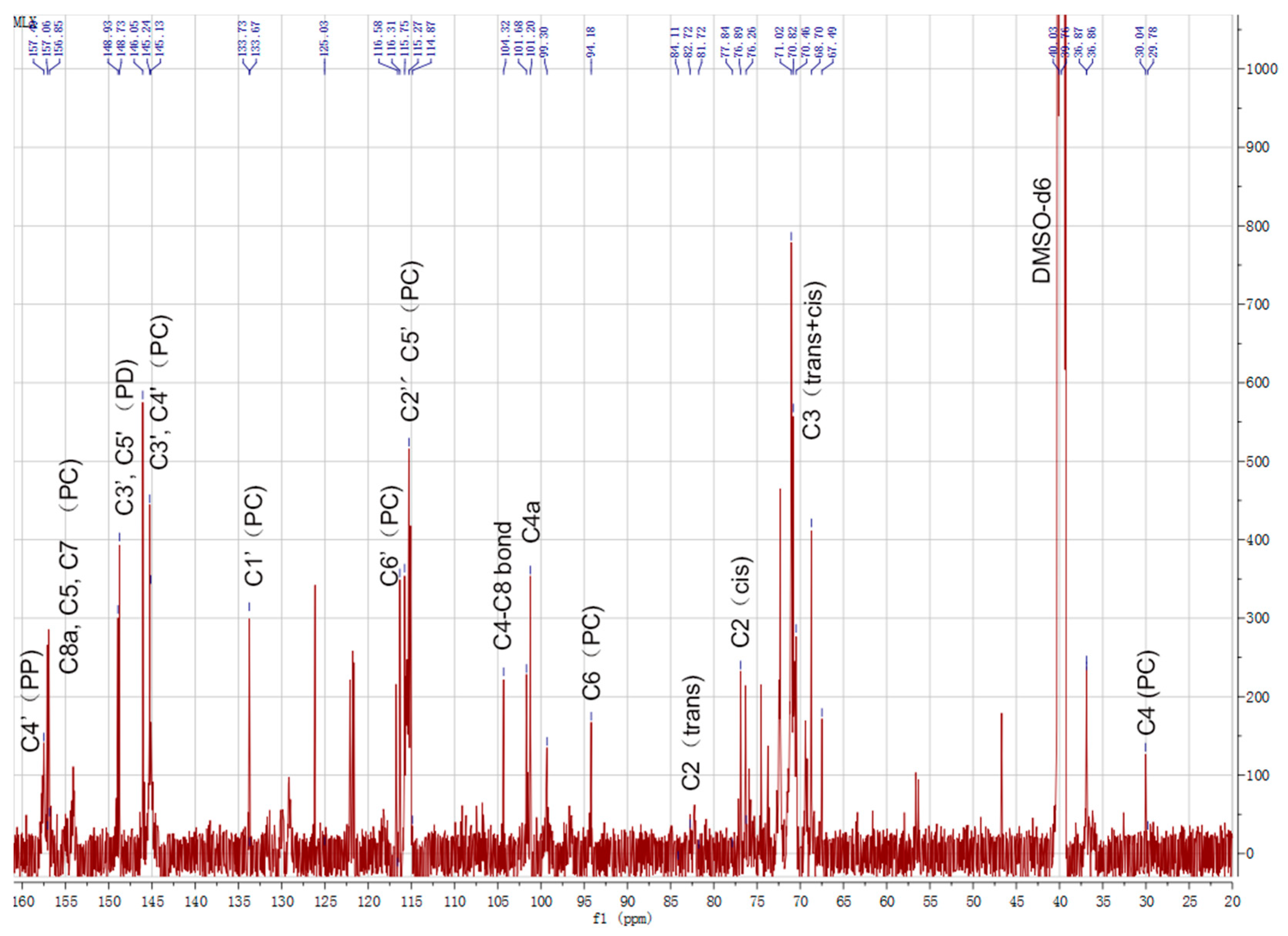
2.2. 13C-NMR Analysis of CTs
2.3. RP-HPLC Analysis
2.4. Inhibition Kinetics of Mushroom Tyrosinase
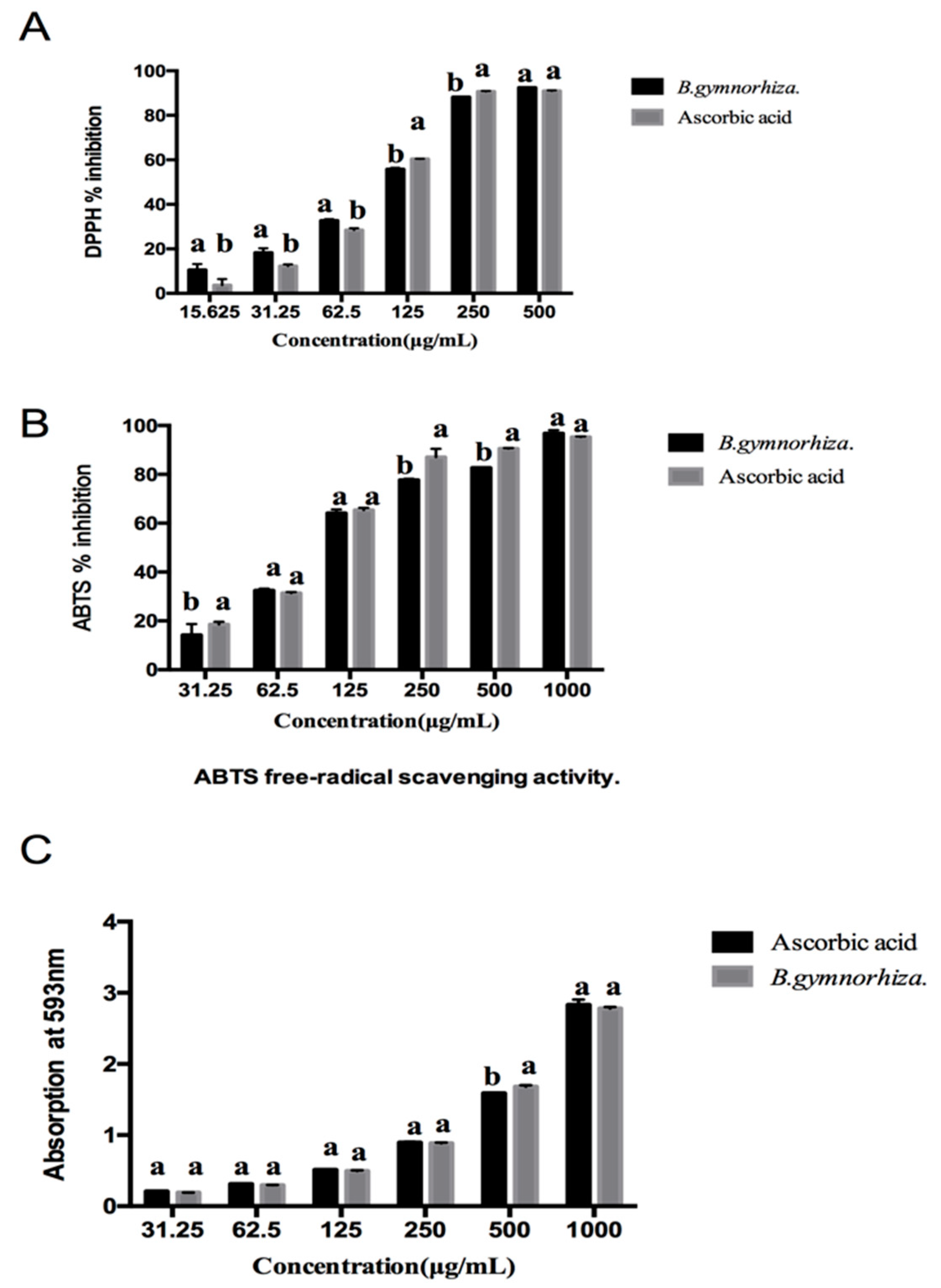
2.5. Antioxidant Activity of CTs
2.6. Evaluation of CTs Treatment on Fresh-Cut Lotus Root
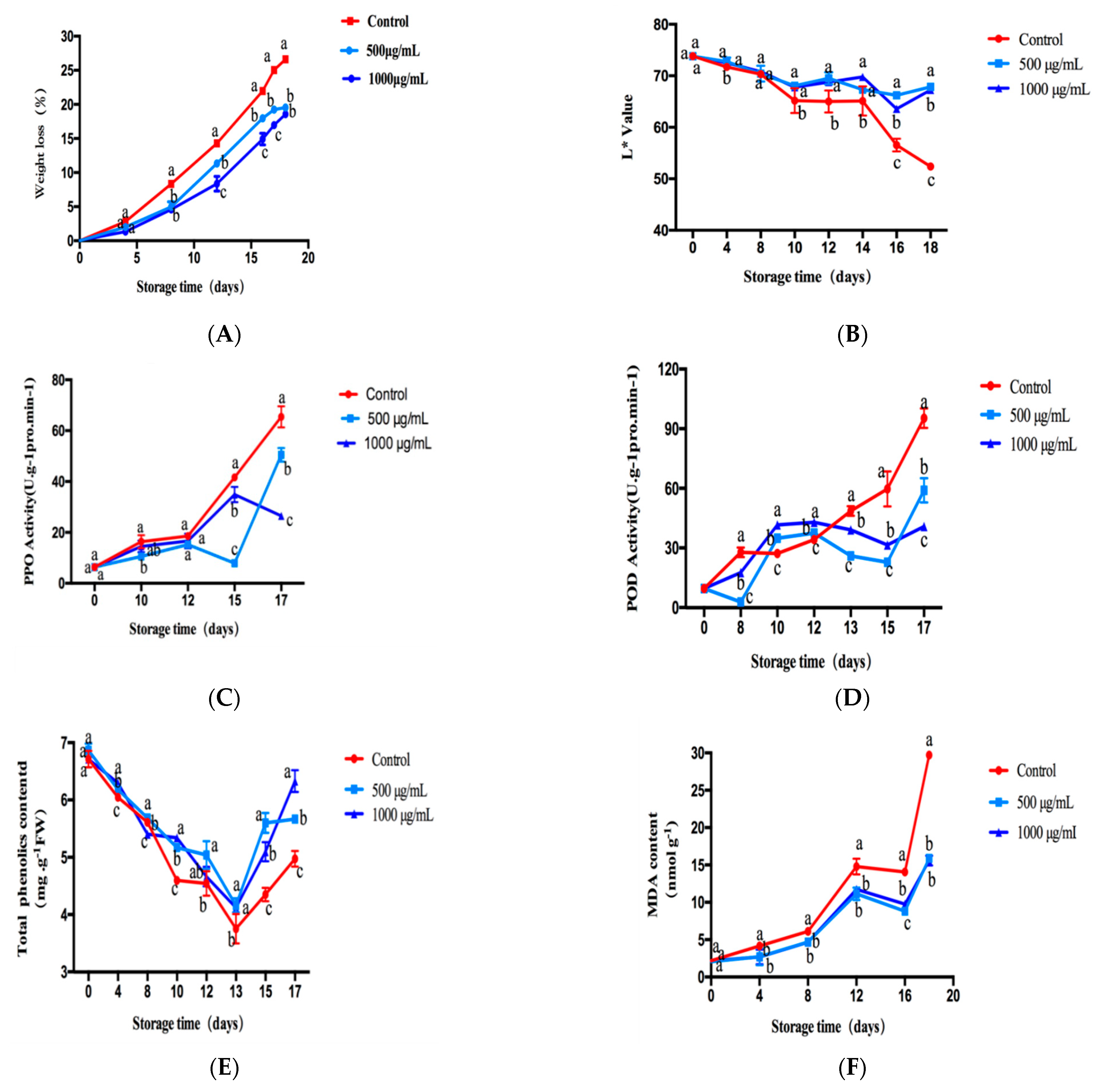
2.6.1. Effect of CTs on the Weight-Loss Ratio of Fresh-Cut Lotus Root
2.6.2. Effect of CTs on L* of Fresh-Cut Lotus Root
2.6.3. Effect of CT Treatment on Activities of PPO and POD in Fresh-Cut Lotus Root
2.6.4. Effect of CTs Treatment on TPC and MDA Content in Fresh-Cut Lotus Root
3. Materials and Methods
3.1. Chemicals and Plant Material
3.2. Extraction and Purification of the CTs
3.3. Determination of Total Phenolics and Proanthocyanidins Content
3.4. 13C-NMR Analysis
3.5. Performance Liquid Chromatography Analysis
3.6. Tyrosinase Inhibitory Activities
3.7. DPPH Assay
3.8. Scavenging Activity on ABTS Radical
3.9. FRAP Assay
3.10. Measurement of Fresh-Cut Lotus Root Storage Quality
3.11. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| CTs | condensed tannins |
| AAE | ascorbic acid equivalent |
| AF/EAF | afzelechin/epafodoxine |
| ABTS | 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid diammonium salt) |
| C/EC | catechin/epicatechin |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | ferric ion reducing antioxidant power |
| IC50 | 50% inhibiting concentration |
| UV | ultraviolet |
| 13C-NMR | 13C nuclear magnetic resonance |
| Km | Michaelis-Menten constant |
| l-DOPA | l-3,4-dihydroxyphenylalanine |
| PBS | phosphate buffer |
| PC | procyanidin |
| PD | prodelphinidins |
| PP | propelargonidin |
| TFA | trifluoroacetate acid |
| PPO | polyphenol oxidase |
| POD | peroxidase |
| MDA | malondialdehyde |
| TPC | total phenolics content |
| Vc | ascorbic acid |
| Vm | maximum velocity |
| L* | whiteness value |
| mDP | mean polymerization degree |
| OD | optical density |
| GAE | gallic acid concentration |
| DW | dry weight |
| LOX | lipoxygenase |
| APX | ascorbate peroxidase |
| PAL | phenylalnine ammonialyase |
| CAT | catalase |
References
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Duke, N.C. Mangrove floristics and biogengraphy. In Tropical Mangrove Ecosystem; Robertson, A., Ed.; American Geographical Union: Washington, DC, USA, 1992; pp. 63–100. [Google Scholar]
- Bunyapraphatsara, N. Medicinal and food plants in the mangrove area. J. Trop. Med. Plants 2000, 1, 138–142. [Google Scholar]
- Yu, L.; Jin, G.; Ye, B. Effects of environmental factors on the distribution and genetic polymorphism of mangrove plant, Bruguiera gymnorrhiza. Strait Pharm. J. 2009, 21, 68–72. [Google Scholar]
- Mukkamala Lakshmi, M.P. Ajay Parida, Molecular marker assisted intraspecific variationand species relationships in the Indian mangrovetribe Rhizophorea. Aquat. Bot. 2002, 74, 201–217. [Google Scholar]
- Lin, P.; Lin, Y.; Yang, Z.; Wang, S. Research status, civil utilization and prospect on marine mangrove drug in China: A review. Mar. Sci. 2005, 29, 76–82. [Google Scholar]
- Andaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Othman, S. Bruguiera gymnorrhiza Lamk. In Prosea, Plant Resource of South-East Asia Timber Treesl: Lesser Known Timbers Sosef MSM; Hong, L.T., Prawirohatmodjos, Eds.; Bogor Indones Press: Bogor, Indonesia, 1998; Volume 5, pp. 122–125. [Google Scholar]
- Haslam, E. Vegetable tannins-lessons of a phytochemical lifetime. Phytochemistry 2007, 68, 2713–2721. [Google Scholar] [CrossRef]
- Chai, W.-M.; Wei, Q.-M.; Deng, W.-L.; Zheng, Y.-L.; Chen, X.-Y.; Huang, Q.; Ou-Yang, C.; Peng, Y.-Y. Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and DNA damage protection activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Ou-Yang, C.; Ma, Z.; Song, S.; Huang, Q.; Wei, Q.; Peng, Y. Anti-α-glucosidase and antityrosinase activity of condensed tannins from the bark of Clausena lansium (Lour.) Skeels with antiproliferative and apoptotic properties in B16 mouse melanoma cells. Process Biochem. 2019, 86, 205–214. [Google Scholar] [CrossRef]
- Yu, R.J.; Liu, H.B.; Yu, Y.; Liang, L.; Xu, R.; Liang, C.; Tang, J.S.; Yao, X.S. Anticancer activities of proanthocyanidins from the plant Urceola huaitingii and their synergistic effects in combination with chemotherapeutics. Fitoterapia 2016, 112, 175–182. [Google Scholar] [CrossRef]
- Ree, T.V. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Zhang, H.; Yamamoto, E.; Murphy, J.; Locas, A. Microbiological safety of ready-to-eat fresh-cut fruits and vegetables sold on the Canadian retail market. Int. J. Food Microbiol. 2020, 335, 108855. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Brecht, J.K.; Abrahan, C.E.; Bornhorst, E.R.; Luo, Y.; Monge, A.L.; Vorst, K.; Brown, W. Improving temperature management and retaining quality of fresh-cut leafy greens by retrofitting open refrigerated retail display cases with doors. J. Food Eng. 2021, 292, 110271. [Google Scholar] [CrossRef]
- Cabezudo, I.; Ayelen Ramallo, I.; Alonso, V.L.; Furlan, R.L.E. Effect directed synthesis of a new tyrosinase inhibitor with anti-browning activity. Food Chem. 2020, 341, 128232. [Google Scholar] [CrossRef]
- Song, W.; Liu, L.L.; Ren, Y.J.; Wei, S.D.; Yang, H.B. Inhibitory effects and molecular mechanism on mushroom tyrosinase by condensed tannins isolation from the fruit of Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chow. Int. J. Biol. Macromol. 2020, 165, 1813–1821. [Google Scholar] [CrossRef]
- Peng, K.; Zhou, Y.; Wang, Y.; Wang, G.; Huang, Y.; Cao, J. Inclusion of condensed tannins in Lateolabrax japonicus diets: Effects on growth, nutrient digestibility, antioxidant and immune capacity and copper sulphate stress resistance. Aquac. Rep. 2020, 18, 100525. [Google Scholar] [CrossRef]
- Steinmetz, W.E. NMR assignment and characterization of proton exchange of the ellagitannin granatin B. Magn. Reson. Chem. 2010, 48, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-X.; Liang, G.; Chai, W.-M.; Feng, H.-L.; Zhou, H.-T.; Shi, Y.; Chen, Q.-X. Antioxidant and antityrosinase proanthocyanidins from Polyalthia longifolia leaves. J. Biosci. Bioeng. 2014, 118, 583–587. [Google Scholar] [CrossRef]
- Diwani, N.; Fakhfakh, J.; Athmouni, K.; Belhaj, D.; El Feki, A.; Allouche, N.; Ayadi, H.; Bouaziz-Ketata, H. Optimization, extraction, structure analysis and antioxidant properties of flavan-3-ol polymers: Proanthocyanidins isolated from Periploca angustifolia using surface response methodology. Ind. Crop. Prod. 2020, 144, 112040. [Google Scholar] [CrossRef]
- Fujimaki, T.; Mori, S.; Horikawa, M.; Fukui, Y. Isolation of proanthocyanidins from red wine, and their inhibitory effects on melanin synthesis in vitro. Food Chem. 2018, 248, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Bilia, A.R.; Gabbiani, C.; Messori, L.; Skaltsa, H. Proanthocyanidin glycosides from the leaves of Quercus ilex L. (Fagaceae). Tetrahedron Lett. 2009, 50, 1771–1776. [Google Scholar] [CrossRef]
- Sylvain Guyot, N.M. Jean-Francois Drilleau, Thiolysis-HPLC characterization of apple procyanidins covering a large range of polymerization states. Food Chem. 2001, 49, 14–20. [Google Scholar] [CrossRef]
- Lin, Y.F.; Hu, Y.H.; Lin, H.T.; Liu, X.; Chen, Y.H.; Zhang, S.; Chen, Q.X. Inhibitory effects of propyl gallate on tyrosinase and its application in controlling pericarp browning of harvested longan fruits. J. Agric. Food Chem. 2013, 61, 2889–2895. [Google Scholar] [CrossRef]
- Mark McDonald, I.M.; Scalber, A. Precipitation of Metal Ions by Plant Polyphenols: Optimal Conditions and Origin of Precipitation. Food Chem. 1996, 44. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Song, W.; Zhao, Z.; Zhao, Y. Isolation of proanthocyanidins from Pinus thunbergii needles and tyrosinase inhibition activity. Process Biochem. 2021, 100, 245–251. [Google Scholar] [CrossRef]
- Chai, W.M.; Lin, M.Z.; Wang, Y.X.; Xu, K.L.; Huang, W.Y.; Pan, D.D.; Zou, Z.R.; Peng, Y.Y. Inhibition of tyrosinase by cherimoya pericarp proanthocyanidins: Structural characterization, inhibitory activity and mechanism. Food Res. Int. 2017, 100, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Isao Kubo, I.K.-H. Flavonols from Saffron Flower: Tyrosinase Inhibitory Activity and Inhibition Mechanism. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef]
- Espin, J.C.; Wichers, H.J. Slow-Binding Inhibition of Mushroom (Agaricus bisporus) Tyrosinase Isoforms by Tropolone. Food Chem. 1999, 47, 2638–2644. [Google Scholar] [CrossRef]
- Yang, T.-T.; Guan, J.-P.; Tang, R.-C.; Chen, G. Condensed tannin from Dioscorea cirrhosa tuber as an eco-friendly and durable flame retardant for silk textile. Ind. Crop. Prod. 2018, 115, 16–25. [Google Scholar] [CrossRef]
- Song, W.; Qin, S.-T.; Fang, F.-X.; Gao, Z.-J.; Liang, D.-D.; Liu, L.-L.; Tian, H.-T.; Yang, H.-B. Isolation and Purification of Condensed Tannin from the Leaves and Branches of Prunus cerasifera and Its Structure and Bioactivities. Appl. Biochem. Biotechnol. 2017, 185, 464–475. [Google Scholar] [CrossRef]
- Petchidurai, G.; Nagoth, J.A.; John, M.S.; Sahayaraj, K.; Murugesan, N.; Pucciarelli, S. Standardization and quantification of total tannins, condensed tannin and soluble phlorotannins extracted from thirty-two drifted coastal macroalgae using high performance liquid chromatography. Bioresour. Technol. Rep. 2019, 7, 100273. [Google Scholar] [CrossRef]
- Bianchi, S.; Gloess, A.N.; Kroslakova, I.; Mayer, I.; Pichelin, F. Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and Silver fir (Abies alba [Mill.]) using MALDI-TOF mass spectrometry. Ind. Crop. Prod. 2014, 61, 430–437. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds-nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Deng, Y.-T.; Liang, G.; Shi, Y.; Li, H.-L.; Zhang, J.; Mao, X.-M.; Fu, Q.-R.; Peng, W.-X.; Chen, Q.-X.; Shen, D.-Y. Condensed tannins from Ficus altissima leaves: Structural, antioxidant, and antityrosinase properties. Process Biochem. 2016, 51, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Cappellari, L.D.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Improving Phenolic Total Content and Monoterpene in Mentha × piperita by Using Salicylic Acid or Methyl Jasmonate Combined with Rhizobacteria Inoculation. Int. J. Mol. Sci. 2019, 21, 50. [Google Scholar] [CrossRef] [Green Version]
- Zurita, J.; Diaz-Rubio, M.E.; Saura-Calixto, F. Improved procedure to determine non-extractable polymeric proanthocyanidins in plant foods. Int. J. Food Sci. Nutr. 2012, 63, 936–939. [Google Scholar] [CrossRef]
- Chai, W.M.; Shi, Y.; Feng, H.L.; Qiu, L.; Zhou, H.C.; Deng, Z.W.; Yan, C.L.; Chen, Q.X. NMR, HPLC-ESI-MS, and MALDI-TOF MS analysis of condensed tannins from Delonix regia (Bojer ex Hook.) Raf. and their bioactivities. J. Agric. Food Chem. 2012, 60, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J. Reversed-Phase HPLC following Thiolysis for Quantitative Estimation and Characterization of the Four Main Classes of Phenolic Compounds in Different Tissue Zones of a French Cider Apple Variety (Malus domestica Var. Kermerrien). Agric. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Covington, A.D. The Chemistry of Tanning Materials; Butterworth Heinemann-ElsevierOxford: Oxford, UK, 2006; pp. 22–35. [Google Scholar]
- Chai, W.M.; Shi, Y.; Feng, H.L.; Xu, L.; Xiang, Z.H.; Gao, Y.S.; Chen, Q.X. Structure characterization and anti-tyrosinase mechanism of polymeric proanthocyanidins fractionated from kiwifruit pericarp. J. Agric. Food Chem. 2014, 62, 6382–6389. [Google Scholar] [CrossRef] [PubMed]
- Vignault, A.; Gonzalez-Centeno, M.R.; Pascual, O.; Gombau, J.; Jourdes, M.; Moine, V.; Iturmendi, N.; Canals, J.M.; Zamora, F.; Teissedre, P.L. Chemical characterization, antioxidant properties and oxygen consumption rate of 36 commercial oenological tannins in a model wine solution. Food Chem. 2018, 268, 210–219. [Google Scholar] [CrossRef]
- Abu Zarin, M.; Wan, H.Y.; Isha, A.; Armania, N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness 2016, 5, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Relationships between DNA damage protection activity, total phenolic content, condensed tannin content and antioxidant potential among Indian barley cultivars. Biocatal. Agric. Biotechnol. 2017, 11, 201–206. [Google Scholar] [CrossRef]
- Shao, L.L.; Wang, X.L.; Chen, K.; Dong, X.W.; Kong, L.M.; Zhao, D.Y.; Hider, R.C.; Zhou, T. Novel hydroxypyridinone derivatives containing an oxime ether moiety: Synthesis, inhibition on mushroom tyrosinase and application in anti-browning of fresh-cut apples. Food Chem. 2018, 242, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Ji, H.; Zhang, C.; Deng, C.; Cao, W.; Mao, W.; Gao, J. Inactivation of polyphenol oxidase from Pacific white shrimp by dense phase carbon dioxide. Innov. Food Sci. Emerg. Technol. 2011, 12, 635–641. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Zeng, T.; Nie, Q.; Zhang, F.; Zhu, L. Hydrogen sulfide inhibits enzymatic browning of fresh-cut lotus root slices by regulating phenolic metabolism. Food Chem. 2015, 177, 376–381. [Google Scholar] [CrossRef] [PubMed]
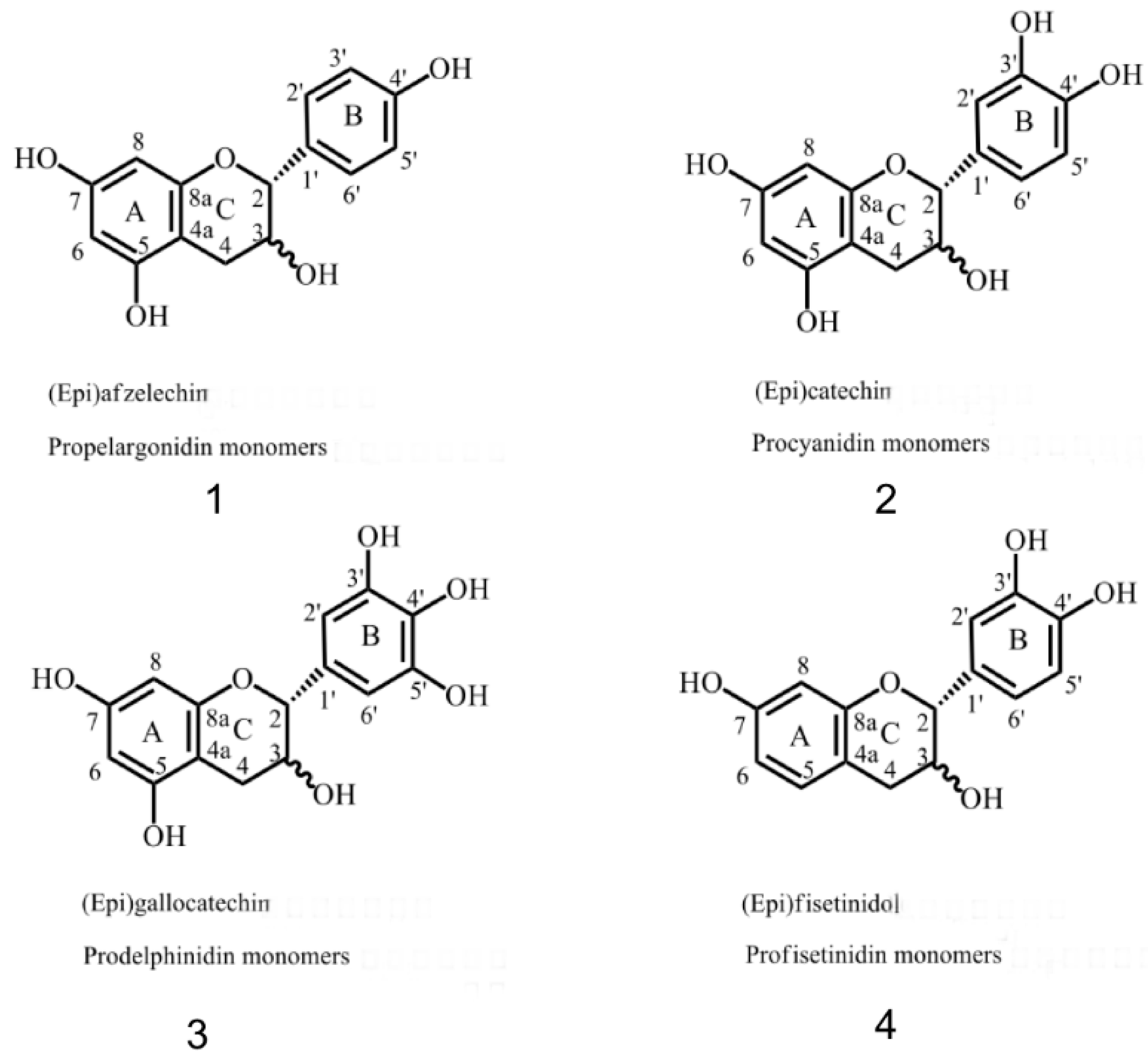
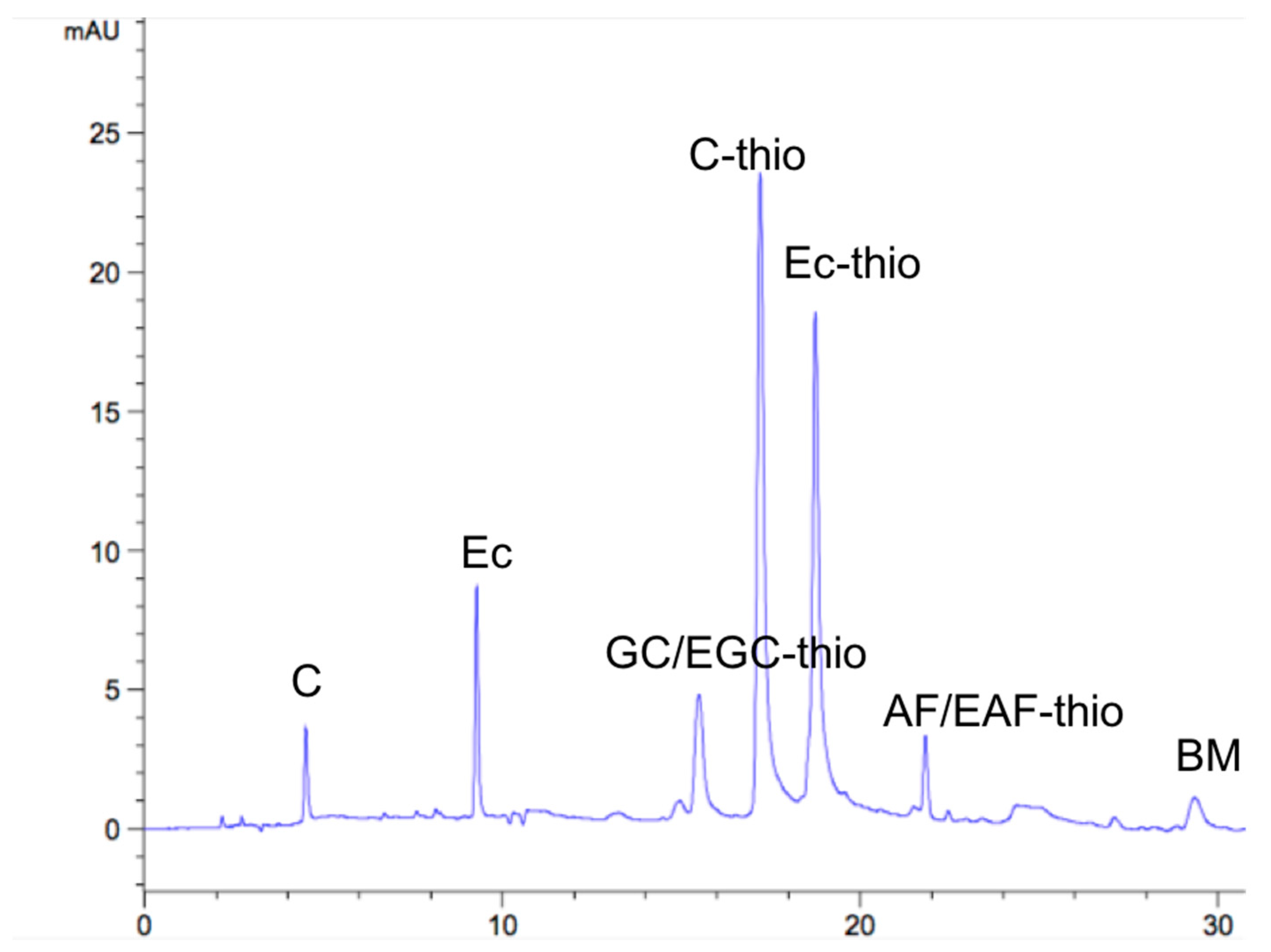

| Flavan-3-ol | The End of the Unit | Extended Unit Adduct | Solvent | ||||
|---|---|---|---|---|---|---|---|
| C | EC | GC/EGC | C | EC | AF/EAF | BM | |
| Standards | 4.4 | 9.5 | 15.4 | 17.0 | 18.4 | 21.5 | 27.5 |
| CTs | 4.55 | 9.35 | 15.53 | 17.25 | 18.64 | 21.89 | 29.63 |
| Samples | DPPH (IC50 μg/mL) | ABTS (IC50 μg/mL) | FRAP (mg AAE/g) |
|---|---|---|---|
| B. gymnorhiza | 88.81 ± 0.135 b | 105.03 ± 0.134 a | 1052.27 ± 4.170 |
| Ascorbic acid | 97.65 ± 0.153 a | 89.55 ± 0.274 b | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, T.; Wang, Q.; Liu, J.; Lu, Y.; Shi, Y. Structure Analysis and Study of Biological Activities of Condensed Tannins from Bruguiera gymnorhiza (L.) Lam and Their Effect on Fresh-Cut Lotus Roots. Molecules 2021, 26, 1369. https://doi.org/10.3390/molecules26051369
Liu X, Chen T, Wang Q, Liu J, Lu Y, Shi Y. Structure Analysis and Study of Biological Activities of Condensed Tannins from Bruguiera gymnorhiza (L.) Lam and Their Effect on Fresh-Cut Lotus Roots. Molecules. 2021; 26(5):1369. https://doi.org/10.3390/molecules26051369
Chicago/Turabian StyleLiu, Xuelian, Ting Chen, Qin Wang, Jiaai Liu, Yuhao Lu, and Yan Shi. 2021. "Structure Analysis and Study of Biological Activities of Condensed Tannins from Bruguiera gymnorhiza (L.) Lam and Their Effect on Fresh-Cut Lotus Roots" Molecules 26, no. 5: 1369. https://doi.org/10.3390/molecules26051369








