Recoverable Palladium-Catalyzed Carbon-Carbon Bond Forming Reactions under Thermomorphic Mode: Stille and Suzuki-Miyaura Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Synthesis
2.2. Thermomorphic Property Study
2.3. Recoverable Pd Complex-Catalyzed Stille Reaction of Aryl Halides
2.3.1. Aryl Iodides
2.3.2. Aryl Bromides
2.3.3. Purification of Crude Product Mixture from Pd-Catalyzed Stille Reaction
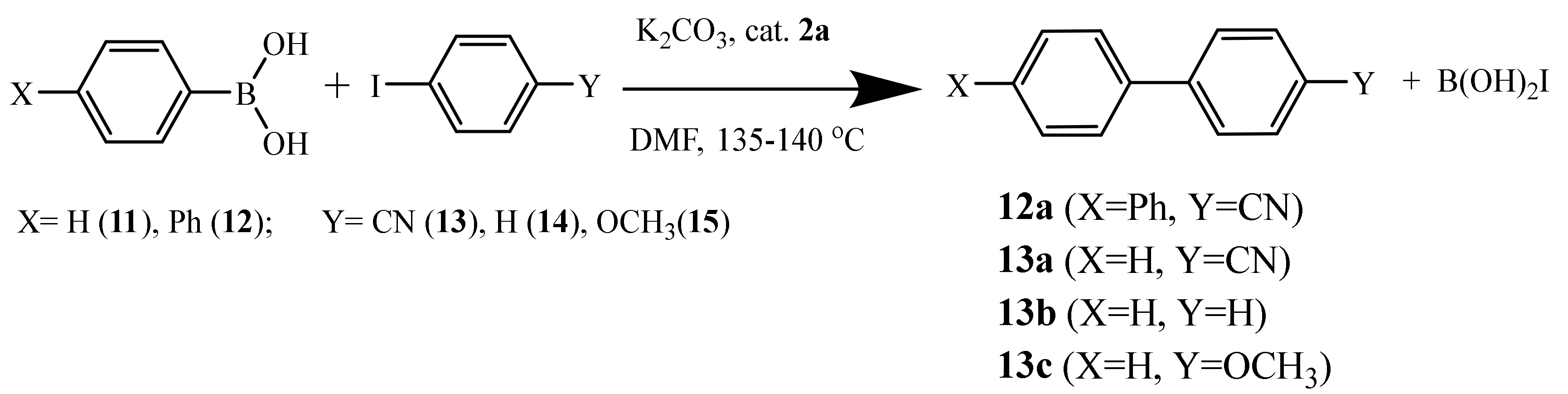
2.4. Recoverable Pd Complex-Catalyzed Suzuki-Miyaura Reaction of Aryl Halides
2.4.1. Aryl Iododes
2.4.2. Aryl Bromides
2.5. Detection of Metal Recovery by ICP-MS
3. Experimental
3.1. General Procedures
3.2. Starting Materials
3.3. Preparation of Palladium Complexes
3.4. Analytical Data for the Ligands and Pd Metal Complexes
3.5. Procedures in Catalytic Stille Reaction and Recovery
3.6. Procedures in Catalytic Suzuki-Miyaura Reaction and Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cordovilla, C.; Bartolomé, C.; Martínez-Ilarduya, J.S.M.; Espinet, P. The Stille reaction, 38 years later. ACS Catal. 2015, 5, 3040–3053. [Google Scholar] [CrossRef] [Green Version]
- Lee, V. Application of copper (i) salt and fluoride promoted Stille coupling reactions in the synthesis of bioactive molecules. Org. Biomol. Chem. 2019, 17, 9095–9123. [Google Scholar] [CrossRef] [PubMed]
- Nikoorazm, M.; Ghorbani-Choghamarani, A.; Khanmoradi, M. Application of Pd-2A3HP-MCM-41 to the Suzuki, Heck and Stille coupling reactions and synthesis of 5-substituted 1H-tetrazoles. Appl. Organomet. Chem. 2016, 30, 705–712. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Kawahata, M.; Yang, Z.-K.; Miyamoto, K.; Komagawa, S.; Yamaguchi, K.; Wang, C.; Uchiyama, M. Stille coupling via C–N bond cleavage. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.J. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Sofiyev, V.; Navarro, G.; Trauner, D. Biomimetic synthesis of the shimalactones. Org. Lett. 2008, 10, 149–152. [Google Scholar] [CrossRef]
- Souris, C.; Frébault, F.; Patel, A.; Audisio, D.; Houk, K.N.; Maulide, N. Stereoselective synthesis of dienyl-carboxylate building blocks: Formal synthesis of inthomycin C. Org. Lett. 2013, 15, 3242–3245. [Google Scholar] [CrossRef] [Green Version]
- Heravi, M.M.; Hashemi, E.; Azimian, F. Recent developments of the Stille reaction as a revolutionized method in total synthesis. Tetrahedron 2014, 70, 7–21. [Google Scholar] [CrossRef]
- Lord, A.M.; Mahon, M.F.; Lloyd, M.D.; Threadgill, M.D. Design, synthesis, and evaluation in vitro of quinoline-8-carboxamides, a new class of poly (adenosine-diphosphate-ribose) polymerase-1 (PARP-1) inhibitor. J. Med. Chem. 2009, 52, 868–877. [Google Scholar] [CrossRef]
- Cullen, M.D.; Deng, B.L.; Hartman, T.L.; Watson, K.M.; Buckheit, R.W.; Pannecouque, C.; Cushman, M. Synthesis and biological evaluation of alkenyldiarylmethane HIV-1 non-nucleoside reverse transcriptase inhibitors that possess increased hydrolytic stability. J. Med. Chem. 2007, 50, 4854–4867. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; King, N.P.; Finlay, M.R.V.; He, Y.; Roschangar, F.; Vourloumis, D.; Hepworth, D. Total synthesis of epothilone E and related side-chain modified analogues via a Stille coupling based strategy. Bioorg. Med. Chem. 1999, 7, 665–697. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille polycondensation for synthesis of functional materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef]
- Choudary, B.M.; Madhi, S.; Chowdari, N.S.; Kantam, M.L.; Sreedhar, B. Layered double hydroxide supported nanopalladium catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-type coupling reactions of chloroarenes. J. Am. Chem. Soc. 2002, 124, 14127–14136. [Google Scholar] [CrossRef]
- Mosleh, I.; Shahsavari, H.R.; Beitle, R.; Beyzavi, M.H. Recombinant Peptide Fusion Protein-templated Palladium Nanoparticles for Suzuki-Miyaura and Stille Coupling Reactions. ChemCatChem 2020, 12, 2942–2946. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Norouzi, M. Palladium supported on modified magnetic nanoparticles: A phosphine-free and heterogeneous catalyst for Suzuki and Stille reactions. Appl. Organomet. Chem. 2016, 30, 140–147. [Google Scholar] [CrossRef]
- Mohazzab, B.F.; Jaleh, B.; Issaabadi, Z.; Nasrollahzadeh, M.; Varma, R.S. Stainless steel mesh-GO/Pd NPs: Catalytic applications of Suzuki–Miyaura and Stille coupling reactions in eco-friendly media. Green Chem. 2019, 21, 3319–3327. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Lofù, A.; Mastrorilli, P.; Mucciante, V.; Nobile, C.F. Stille coupling reactions catalysed by a polymer supported palladium complex. J. Organomet. Chem. 2006, 691, 131–137. [Google Scholar] [CrossRef]
- Yin, X.; Guo, F.; Lalancette, R.A.; Jäkle, F. Luminescent main-chain organoborane polymers: Highly robust, electron-deficient poly (oligothiophene borane) s via Stille coupling polymerization. Macromolecules 2016, 49, 537–546. [Google Scholar] [CrossRef]
- Ryu, S.H.; Choi, S.J.; Seon, J.H.; Jo, B.; Lee, S.M.; Kim, H.J.; Ko, Y.-J.; Ko, K.C.; Ahn, T.K.; Son, S.U. Visible light-driven Suzuki–Miyaura reaction by self-supported Pd nanocatalysts in the formation of Stille coupling-based photoactive microporous organic polymers. Catal. Sci. Technol. 2020, 10, 5535–5543. [Google Scholar] [CrossRef]
- McAfee, S.M.; McCahill, J.S.; Macaulay, C.M.; Hendsbee, A.D.; Welch, G.C. Utility of a heterogeneous palladium catalyst for the synthesis of a molecular semiconductor via Stille, Suzuki, and direct heteroarylation cross-coupling reactions. RSC Adv. 2015, 5, 26097–26106. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Sen, R.; Maity, T.; Koner, S. Anchoring of palladium onto surface of porous metal–organic framework through post-synthesis modification and studies on Suzuki and Stille coupling reactions under heterogeneous condition. Langmuir 2013, 29, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Choghamarani, A.; Nikpour, F.; Ghorbani, F.; Havasi, F. Anchoring of Pd (II) complex in functionalized MCM-41 as an efficient and recoverable novel nano catalyst in C–C, C–O and C–N coupling reactions using Ph3SnCl. RSC Adv. 2015, 5, 33212–33220. [Google Scholar] [CrossRef]
- Khanmoradi, M.; Nikoorazm, M.; Ghorbani-Choghamarani, A. Synthesis and characterization of Pd schiff base complex immobilized onto functionalized nanoporous MCM-41 and its catalytic efficacy in the Suzuki, Heck and Stille coupling reactions. Catal. Lett. 2017, 147, 1114–1126. [Google Scholar] [CrossRef]
- Su, W.; Urgaonkar, S.; McLaughlin, P.A.; Verkade, J.G. Highly active palladium catalysts supported by bulky proazaphosphatrane ligands for Stille cross-coupling: Coupling of aryl and vinyl chlorides, room temperature coupling of aryl bromides, coupling of aryl triflates, and synthesis of sterically hindered biaryls. J. Am. Chem. Soc. 2004, 126, 16433–16439. [Google Scholar] [PubMed]
- Li, X.; Zhu, T.; Shao, Z.; Li, Y.; Chang, H.; Gao, W.; Zhang, Y.; Wei, W. Newly-generated Al(OH)3-supported Pd nanoparticles-catalyzed Stille and Kumada coupling reactions of diazonium salts,(Het) aryl chlorides. Tetrahedron 2016, 72, 69–75. [Google Scholar] [CrossRef]
- Holz, J.; Pfeffer, C.; Zuo, H.; Beierlein, D.; Richter, G.; Klemm, E.; Peters, R. In Situ Generated Gold Nanoparticles on Active Carbon as Reusable Highly Efficient Catalysts for a C–C Stille Coupling. Angew. Chem. Int. Ed. 2019, 58, 10330–10334. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Choghamarani, A.; Naghipour, A.; Heidarizadi, F.; Shirkhani, R.; Notash, B. Bis[(2-methylacetatobenzyl)tri(p-tolyl) phosphonium] hexabromodipalladate (II); Synthesis, characterization, structural study and application as a retrievable heterogeneous catalyst for the amination of aryl halides and Stille cross-coupling reaction. Inorg. Chim. Acta 2016, 446, 97–102. [Google Scholar] [CrossRef]
- Naghipour, A.; Ghorbani-Choghamarani, A.; Heidarizadi, F.; Notash, B. Synthesis, characterization and structural study of a phosphonium salt containing the [Pd2Br6]2−ion and its application as a novel, efficient and renewable heterogeneous catalyst for amination of aryl halides and the Stille cross-coupling reaction. Polyhedron 2016, 105, 18–26. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Liu, L.-J.; Chang, F.-P.; Cheng, Y.-L.; Tsai, F.-Y. A Highly Efficient and Reusable Palladium (II)/Cationic 2, 2′-Bipyridyl-Catalyzed Stille Coupling in Water. Molecules 2016, 21, 1205. [Google Scholar] [CrossRef] [Green Version]
- Ogo, S.; Takebe, Y.; Uehara, K.; Yamazaki, T.; Nakai, H.; Watanabe, Y.; Fukuzumi, S. pH-Dependent C−C Coupling Reactions Catalyzed by Water-Soluble Palladacyclic Aqua Catalysts in Water. Organometallics 2006, 25, 331–338. [Google Scholar] [CrossRef]
- Fareghi-Alamdari, R.; Golestanzadeh, M.; Bagheri, O. An efficient and recoverable palladium organocatalyst for Suzuki reaction in aqueous media. Appl. Organomet. Chem. 2017, 31, e3698. [Google Scholar] [CrossRef]
- Hao, W.; Xi, Z.; Cai, M. A practical synthesis of biaryls and aromatic acetylenes by Stille coupling in room-temperature ionic liquids. Synth. Commun. 2012, 42, 2396–2406. [Google Scholar] [CrossRef]
- Chiappe, C.; Imperato, G.; Napolitano, E.; Pieraccini, D. Ligandless Stille cross-coupling in ionic liquids. Green Chem. 2004, 6, 33–36. [Google Scholar] [CrossRef]
- Baran, T.; Baran, N.Y.; Menteş, A. An easily recoverable and highly reproducible agar-supported palladium catalyst for Suzuki-Miyaura coupling reactions and reduction of o-nitroaniline. Int. J. Biol. Macromol. 2018, 115, 249–256. [Google Scholar] [CrossRef]
- Okamoto, K.; Akiyama, R.; Kobayashi, S. Suzuki–Miyaura Coupling Catalyzed by Polymer-Incarcerated Palladium, a Highly Active, Recoverable, and Reusable Pd Catalyst. Org. Lett. 2004, 6, 1987–1990. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Z.; Liu, M.; Pan, C.; Ding, J.; Wu, H.; Cheng, J. Aminophosphine supported on Al2O3 as recoverable catalyst for the Suzuki coupling. Catal. Commun. 2007, 8, 2150–2152. [Google Scholar] [CrossRef]
- Smith, M.D.; Stepan, A.F.; Ramarao, C.; Brennan, P.E.; Ley, S.V. Palladium-containing perovskites: Recoverable and reuseable catalysts for Suzuki couplings. Chem. Commun. 2003, 21, 2652–2653. [Google Scholar] [CrossRef]
- Guerra, R.R.; Martins, F.C.; Lima, C.G.; Goncalves, R.H.; Leite, E.R.; Pereira-Filho, E.R.; Schwab, R.S. Factorial design evaluation of the Suzuki cross-coupling reaction using a magnetically recoverable palladium catalyst. Tetrahedron Lett. 2017, 58, 903–908. [Google Scholar] [CrossRef]
- Rangraz, Y.; Nemati, F.; Elhampour, A. A novel magnetically recoverable palladium nanocatalyst containing organoselenium ligand for the synthesis of biaryls via Suzuki-Miyaura coupling reaction. J. Phys. Chem. Solids 2020, 138, 109251–109258. [Google Scholar] [CrossRef]
- Lu, N.; Chen, S.-C.; Chen, T.-C.; Liu, L.-K. Palladium-catalyzed Heck reaction under thermomorphic mode. Tetrahedron Lett. 2008, 49, 371–375. [Google Scholar] [CrossRef]
- Li, C.-K.; Ghalwadkar, A.; Lu, N. Recoverable cationic Pd-catalyzed Heck reaction under thermomorphic mode. J. Organomet. Chem. 2011, 696, 3637–3642. [Google Scholar] [CrossRef]
- Lu, N.; Lin, Y.-C.; Chen, J.-Y.; Fan, C.-W.; Liu, L.-K. New bis (fluoro-ponytailed) bipyridine ligands for Pd-catalyzed Heck reactions under fluorous biphasic catalysis condition. Tetrahedron 2007, 63, 2019–2023. [Google Scholar] [CrossRef]
- Lu, N.; Ou, Y.-M.; Feng, T.-Y.; Cheng, W.-J.; Tu, W.-H.; Su, H.-C.; Wang, X.; Liu, L.; Hennek, M.D.; Sayler, T.S. Synthesis and characterization of polyfluorinated 2, 2′-bipyridines and their palladium and platinum complexes [MX2(bis(RfCH2OCH2)-2, 2′-bpy)](X = Cl, Br). J. Fluor. Chem. 2012, 137, 54–63. [Google Scholar] [CrossRef]
- Lu, N.; Lin, K.Y.; Li, C.K.; Kung, C.C.; Yeh, Y.P.; Cheng, Y.Y.; Liu, L.K. Recyclable palladium catalysts for the Heck/Sonogashira reaction under microwave-assisted thermomorphic conditions. J. Chin. Chem. Soc. 2015, 62, 64–72. [Google Scholar] [CrossRef]
- Lu, N.; Chen, Y.-C.; Chen, W.-S.; Chen, T.-L.; Wu, S.-J. Efficient, Recoverable, Copper-Free Sonogashira Reaction under FBS and Thermomorphic Mode. J. Organomet. Chem. 2009, 694, 278–284. [Google Scholar] [CrossRef]
- Lu, N.; Chen, S.C.; Lin, Y.C.; Cheng, Y.Y.; Liu, L.K. High Fluorine Content Bis (fluoro-Ponytailed) Bipyridine Palladium Complexes as Catalyst for Mizoroki-Heck Reactions under Fluorous Biphasic Catalysis Conditions. J. Chin. Chem. Soc. 2008, 55, 89–96. [Google Scholar] [CrossRef]
- Yabe, Y.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Palladium on charcoal-catalyzed ligand-free Stille coupling. Tetrahedron 2010, 66, 8654–8660. [Google Scholar] [CrossRef]
- Wolf, C.; Lerebours, R. Efficient Stille cross-coupling reaction using aryl chlorides or bromides in water. J. Org. Chem. 2003, 68, 7551–7554. [Google Scholar] [CrossRef] [PubMed]
- Milstein, D.; Stille, J.K. A general, selective, and facile method for ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium. J. Am. Chem. Soc. 1978, 100, 3636–3638. [Google Scholar] [CrossRef]
- Lu, N.; Yeh, Y.P.; Wang, G.B.; Feng, T.Y.; Shih, Y.H.; Chen, D. Dye-sensitized TiO2-catalyzed photodegradation of sulfamethoxazole under blue or yellow light. Environ. Sci. Pollut. Res. 2017, 24, 489–499. [Google Scholar] [CrossRef]
- Howell, J.L.; Lu, N.; Friesen, C.M. New derivatives of poly-hexafluoropropylene oxide from the corresponding alcohol. J. Fluor. Chem. 2005, 126, 281–288. [Google Scholar] [CrossRef]
- Lu, N.; Tu, W.H.; Hou, H.C.; Lin, C.T.; Li, C.K.; Liu, L.K. Synthesis, structure and spectroelectrochemical property of (2, 2′-bipyridine)–metal (M = Pt, Pd) dichloride with 4, 4′-bis (fluorous-ponytail) on bipyridine. Polyhedron 2010, 29, 1123–1129. [Google Scholar] [CrossRef]
- Periyanagounder, D.; Wei, T.C.; Li, T.Y.; Lin, C.H.; Gonçalves, T.P.; Fu, H.C.; Tsai, D.S.; Ke, J.J.; Kuo, H.W.; Huang, K.W.; et al. Fast-Response, Highly Air-Stable, and Water-Resistant Organic Photodetectors Based on a Single-Crystal Pt Complex. Adv. Mater. 2020, 32, 1904634. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Chen, J.Y.; Fan, C.W.; Lin, Y.C.; Wen, Y.S.; Liu, L.K. (2, 2′-Bipyridine) palladiumdichloride Derivatives as Recyclable Catalysts in Heck Reactions. J. Chin. Chem. Soc. 2006, 53, 1517–1521. [Google Scholar] [CrossRef]
- Howell, J.L.; Lu, N.; Perez, E.W.; Friesen, C.M.; Novak, I.; Waterfeld, A.; Thrasher, J.S. The preparation of primary poly-hexafluoropropylene oxide halides (poly-HFPO-CF2X where X = I, Br, Cl and F). J. Fluor. Chem. 2004, 125, 1513–1518. [Google Scholar] [CrossRef]






| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON c |
|---|---|---|---|---|
| 1 | 3 | 120 | 100 (99) b | 20 |
| 2 | 3 | 120 | 100 (91) | 20 |
| 3 | 3 | 120 | 100 (95) | 20 |
| 4 | 3 | 120 | 100 | 20 |
| 5 | 4 | 130 | 100 | 20 |
| 6 | 4 | 130 | 100 | 20 |
| 7 | 6 | 130 | 100 | 20 |
| 8 | 6 | 130 | 100 | 20 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 1 | 120 | 100 (98) b | 100 |
| 2 | 1 | 120 | 100 | 100 |
| 3 | 1 | 120 | 100 (95) | 100 |
| 4 | 1 | 120 | 100 | 100 |
| 5 | 1 | 120 | 100 (99) | 100 |
| 6 | 1 | 120 | 100 | 100 |
| 7 | 1 | 120 | 99 | 99 |
| 8 | 1 | 120 | 98 | 98 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 1 | 120 | 100 (98) b | 100 |
| 2 | 1 | 120 | 100 (95) | 100 |
| 3 | 1 | 120 | 100 (97) | 100 |
| 4 | 1 | 120 | 100 | 100 |
| 5 | 1 | 120 | 100 | 100 |
| 6 | 1 | 120 | 100 | 100 |
| 7 | 1 | 120 | 99 | 99 |
| 8 | 1 | 120 | 97 | 97 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 8 | 130 | 100 (98) b | 12.5 |
| 2 | 8 | 130 | 100 (95) | 12.5 |
| 3 | 8 | 130 | 100 (99) | 12.5 |
| 4 | 8 | 130 | 100 | 12.5 |
| 5 | 8 | 130 | 100 | 12.5 |
| 6 | 9 | 140 | 100 | 12.5 |
| 7 | 10 | 150 | 100 | 12.5 |
| 8 | 15 | 150 | 100 | 12.5 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 12 | 140 | 100 | 10 |
| 2 | 12 | 140 | 100 (99) b | 10 |
| 3 | 12 | 140 | 100 | 10 |
| 4 | 14 | 150 | 100 | 10 |
| 5 | 14 | 150 | 100 | 10 |
| 6 | 20 | 150 | 100 (97) | 10 |
| 7 | 24 | 150 | 95 | 9.5 |
| 8 | 30 | 150 | 86 | 8.6 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 4 | 140 | 100 | 10 |
| 2 | 5 | 140 | 100 (98) b | 10 |
| 3 | 5 | 140 | 100 | 10 |
| 4 | 6 | 140 | 100 (98) | 10 |
| 5 | 6 | 140 | 100 | 10 |
| 6 | 7 | 140 | 100 (97) | 10 |
| 7 | 10 | 140 | 100 | 10 |
| 8 | 18 | 140 | 100 | 10 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 4 | 120 | 100 | 10 |
| 2 | 4 | 120 | 100 | 10 |
| 3 | 4 | 120 | 100 (98) b | 10 |
| 4 | 4 | 120 | 100 | 10 |
| 5 | 4 | 120 | 100 (98) | 10 |
| 6 | 5 | 150 | 100 | 10 |
| 7 | 15 | 150 | 100 | 10 |
| 8 | 20 | 150 | 100 | 10 |
| Entry | Cycle | Reactant A (X=) | Reactant B (Y=) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|---|---|
| 1 | 1st | Ph | CN | 135 | 100 | 33.3 |
| 2 | 2nd | Ph | CN | 135 | 100 | 33.3 |
| 3 | 3rd | Ph | CN | 135 | 100 | 33.3 |
| 4 | 1st | H | CN | 140 | 95 | 31.7 |
| 5 | 2nd | H | CN | 140 | 92 | 30.7 |
| 6 | 3rd | H | CN | 140 | 85 | 28.3 |
| 7 | 1st | H | H | 140 | 80 | 26.7 |
| 8 | 2nd | H | H | 140 | 75 | 25 |
| 9 | 1st | H | OCH3 | 140 | 70 | 23.3 |
| 10 | 2nd | H | OCH3 | 140 | 60 | 20 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 8 | 150 | 99 | 33 |
| 2 | 8 | 150 | 99 | 33 |
| 3 | 8 | 150 | 99 (98) b | 33 |
| 4 | 8 | 150 | 92 | 30.7 |
| 5 | 8 | 150 | 81 | 27 |
| 6 | 8 | 150 | 78 (77) | 26 |
| 7 | 8 | 150 | 73 | 24.3 |
| 8 | 8 | 150 | 78 | 26 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 8 | 150 | 99 | 33 |
| 2 | 8 | 150 | 97 | 32.3 |
| 3 | 8 | 150 | 99 (97) b | 33 |
| 4 | 8 | 150 | 99 | 33 |
| 5 | 8 | 150 | 99 | 33 |
| 6 | 8 | 150 | 99 | 33 |
| 7 | 8 | 150 | 99 | 33 |
| 8 | 8 | 150 | 92 | 30.7 |
| Cycle No. | Time (h) | Temp (°C) | Yield (%) a | TON |
|---|---|---|---|---|
| 1 | 8 | 150 | 99 | 33 |
| 2 | 8 | 150 | 98 | 32.7 |
| 3 | 8 | 150 | 99 (98) b | 33 |
| 4 | 8 | 150 | 99 | 33 |
| 5 | 8 | 150 | 98 | 32.7 |
| 6 | 8 | 150 | 99 | 33 |
| 7 | 8 | 150 | 99 | 33 |
| 8 | 8 | 150 | 92 (89) | 30.7 |
| 9 | 8 | 150 | 83 | 27.7 |
| Table No.-Cycle No. | Pd Detected from ICP-MS (In ppm) a | Wt of the Catalyst Used (mg) | Pd Leaching (%) | Pd Recovery (%) |
|---|---|---|---|---|
| 1–1 | 2.932 | 80 | 0.11 | 99.89 |
| 1–8 | 5.953 | 80 | 0.21 | 99.79 |
| 2–1 | 4.109 | 16 | 0.72 | 99.28 |
| 2–5 | 1.372 | 16 | 0.27 | 99.73 |
| 3–2 | 0.37 | 16 | 0.06 | 99.94 |
| 3–6 | 0.393 | 16 | 0.08 | 99.92 |
| 4–3 | 2.673 | 128 | 0.07 | 99.93 |
| 4–7 | 21.86 | 128 | 0.46 | 99.54 |
| Table No.-Cycle No. | Pd Detected from ICP-MS (In ppm) a | Wt of the Catalyst Used (mg) | Pd Leaching (%) | Pd Recovery (%) |
|---|---|---|---|---|
| 5–1 | 2.514 | 160 | 0.05 | 99.95 |
| 5–3 | 4.398 | 160 | 0.08 | 99.92 |
| 6–2 | 34.89 | 160 | 1.18 | 98.82 |
| 6–4 | 15.08 | 160 | 0.63 | 99.37 |
| 7–5 | 0.62 | 160 | 0.03 | 99.97 |
| 7–8 | 21.76 | 160 | 0.76 | 99.24 |
| Table No.-Cycle No. | Pd Detected from ICP-MS (In ppm) a | Wt of the Catalyst Used (mg) | Pd Leaching (%) | Pd Recovery (%) |
|---|---|---|---|---|
| 8–1 | 5.12 | 47 | 0.40 | 99.6 |
| 8–3 | 7.69 | 47 | 0.60 | 99.4 |
| 9–1 | 4.08 | 44 | 0.31 | 99.69 |
| 9–5 | 12.32 | 44 | 0.99 | 99.01 |
| 10–3 | 3.33 | 41 | 0.26 | 99.74 |
| 10–5 | 13.03 | 41 | 1.06 | 98.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessema, E.; Elakkat, V.; Chiu, C.-F.; Tsai, Z.-L.; Chan, K.L.; Shen, C.-R.; Su, H.-C.; Lu, N. Recoverable Palladium-Catalyzed Carbon-Carbon Bond Forming Reactions under Thermomorphic Mode: Stille and Suzuki-Miyaura Reactions. Molecules 2021, 26, 1414. https://doi.org/10.3390/molecules26051414
Tessema E, Elakkat V, Chiu C-F, Tsai Z-L, Chan KL, Shen C-R, Su H-C, Lu N. Recoverable Palladium-Catalyzed Carbon-Carbon Bond Forming Reactions under Thermomorphic Mode: Stille and Suzuki-Miyaura Reactions. Molecules. 2021; 26(5):1414. https://doi.org/10.3390/molecules26051414
Chicago/Turabian StyleTessema, Eskedar, Vijayanath Elakkat, Chiao-Fan Chiu, Zong-Lin Tsai, Ka Long Chan, Chia-Rui Shen, Han-Chang Su, and Norman Lu. 2021. "Recoverable Palladium-Catalyzed Carbon-Carbon Bond Forming Reactions under Thermomorphic Mode: Stille and Suzuki-Miyaura Reactions" Molecules 26, no. 5: 1414. https://doi.org/10.3390/molecules26051414
APA StyleTessema, E., Elakkat, V., Chiu, C. -F., Tsai, Z. -L., Chan, K. L., Shen, C. -R., Su, H. -C., & Lu, N. (2021). Recoverable Palladium-Catalyzed Carbon-Carbon Bond Forming Reactions under Thermomorphic Mode: Stille and Suzuki-Miyaura Reactions. Molecules, 26(5), 1414. https://doi.org/10.3390/molecules26051414






