Occurrence of Peptide-Peptide Interactions during the Purification of Self-Assembling Peptide f1-8 from a β-Lactoglobulin Tryptic Hydrolysate
Abstract
1. Introduction
2. Results
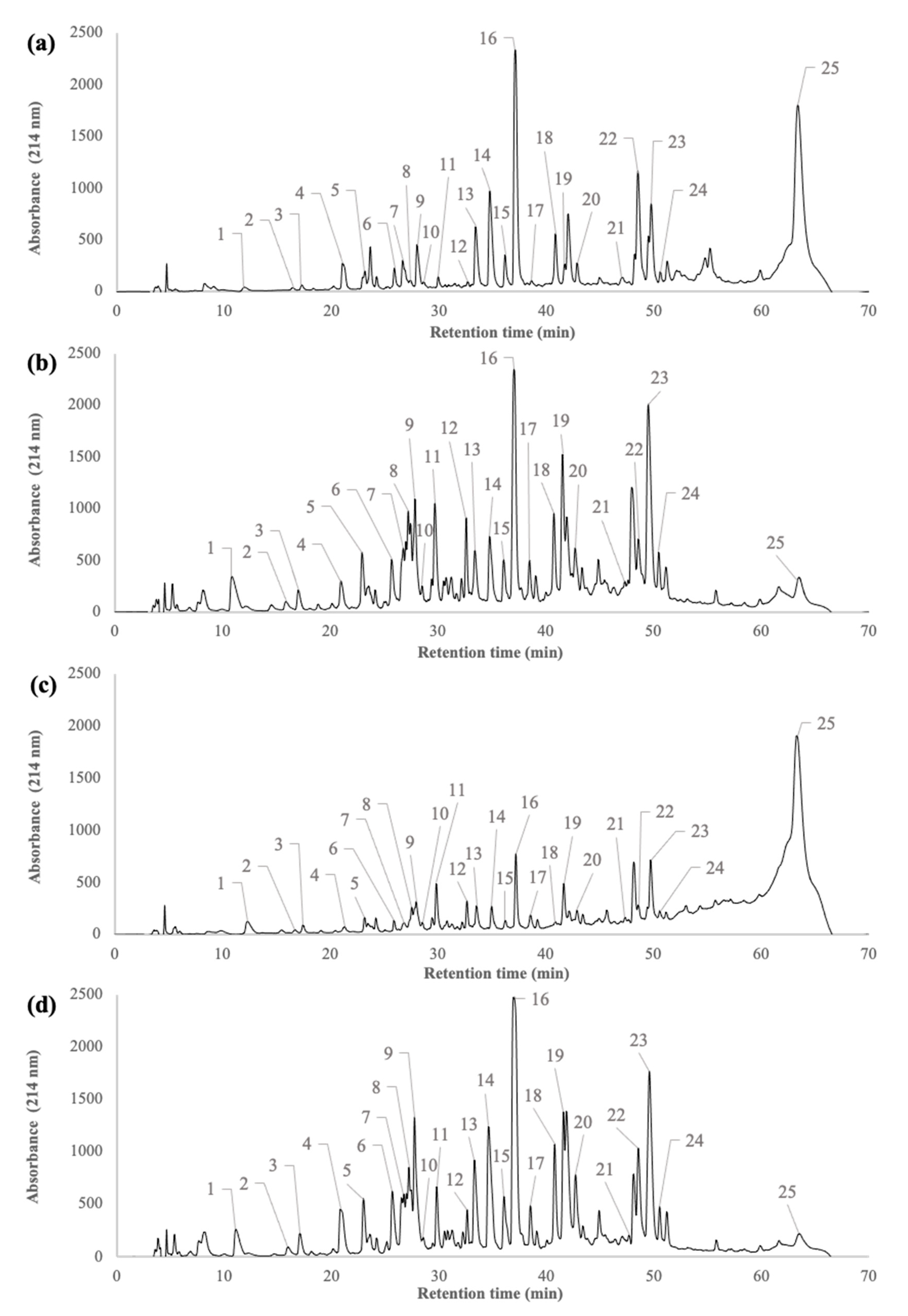
2.1. Peptide Profiles of β-Lactoglobulin Tryptic Hydrolysate during the Filtration Processes
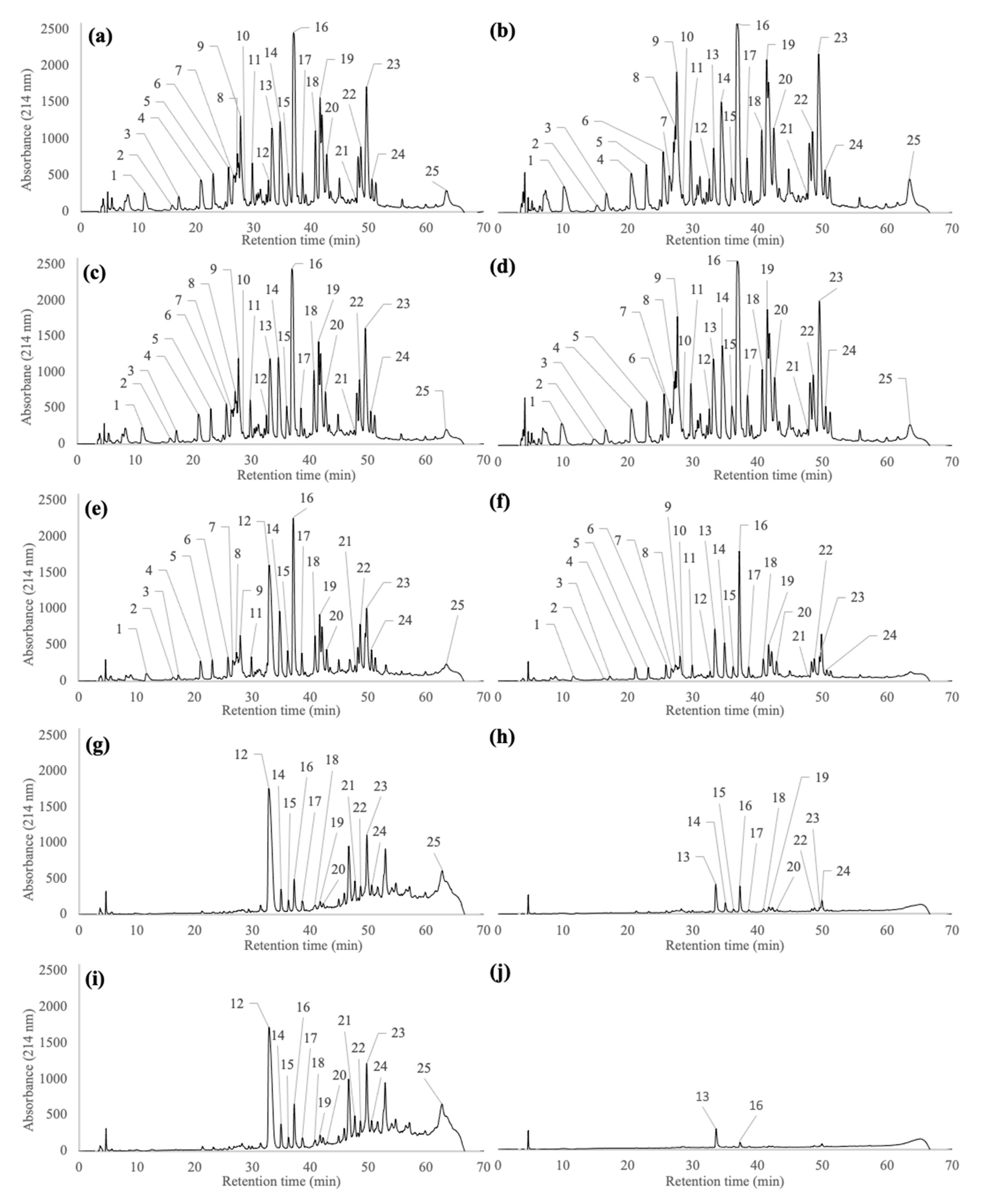
2.2. Peptide Profiles of Pellets and Supernatants Generated during the Wash and Centrifugation Steps of Pf1-8 Purification
2.3. Proportions and Concentrations of Pf1-8, Pf15-20 and Pf41-60 during the Purification Step of Pf1-8
2.4. Gelation Properties of Pellet Solutions Obtained during Pf1-8 Purification
3. Discussion
3.1. Composition of β-Lactoglobulin Tryptic Hydrolysate during Filtration Processes
3.2. Impact of the Wash Steps on Fractionation, Recovery and Purity of Pf1-8
3.3. Gelation Capacities of Peptide Fractions
4. Materials and Methods
4.1. Preparation of Whey Protein Solutions
4.2. Production of Tryptic Hydrolysate of Whey Proteins
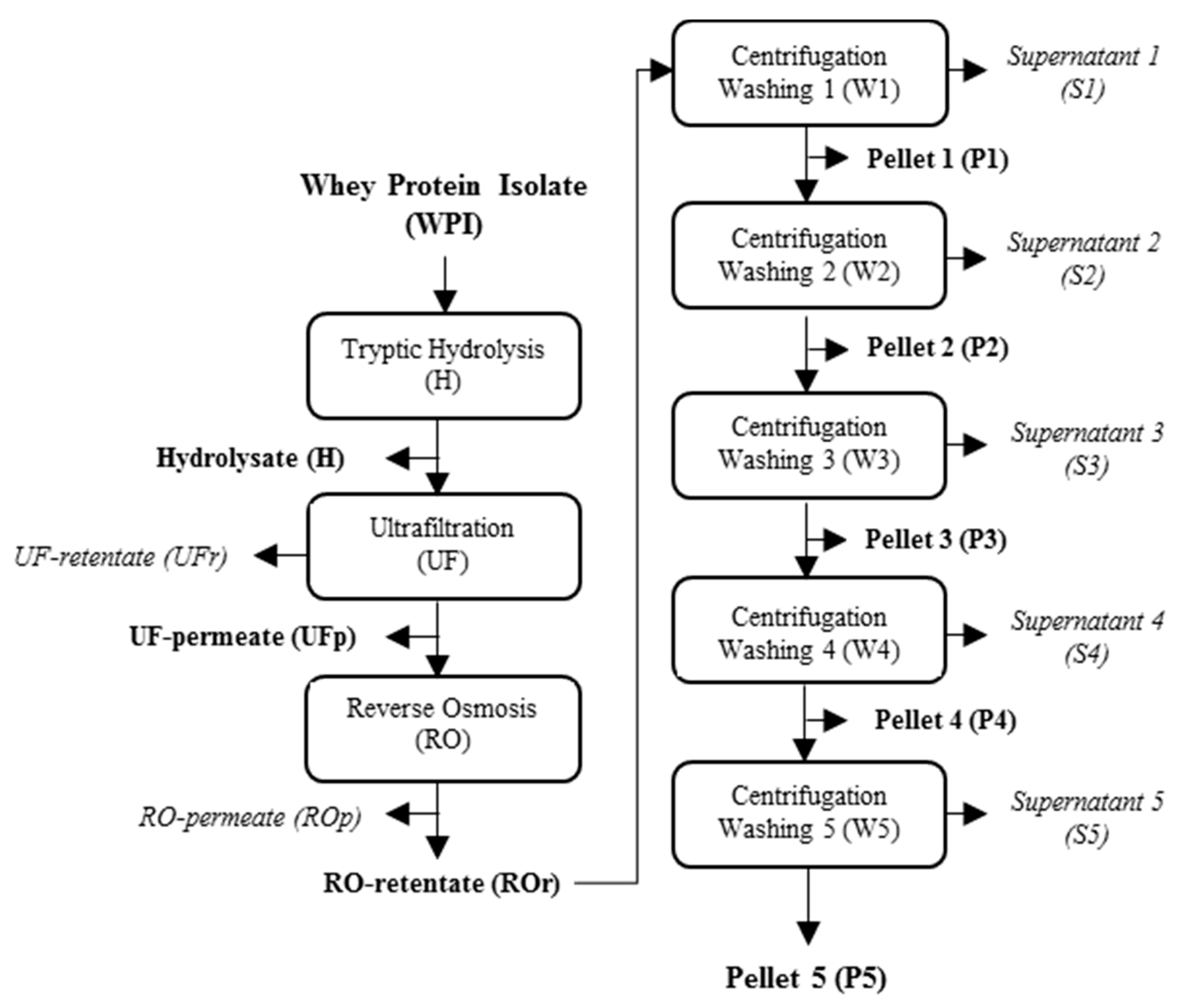
4.3. Production, Concentration and Purification of Tryptic Peptides of Whey Proteins with Gelation Properties
4.4. Peptide Composition
4.4.1. Protein Content
4.4.2. Peptide Characterization
4.5. Gelation Properties of Whey Peptide Fractions
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- El-Agamy, E. The challenge of cow milk protein allergy. Small Rumin. Res. 2007, 68, 64–72. [Google Scholar] [CrossRef]
- Mann, B.; Athira, S.; Sharma, R.; Kumar, R.; Sarkar, P. Bioactive Peptides from Whey Proteins. In Whey Proteins; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 519–547. [Google Scholar]
- Jeewanthi, R.K.C.; Lee, N.-K.; Paik, H.-D. Improved Functional Characteristics of Whey Protein Hydrolysates in Food Industry. Food Sci. Anim. Resour. 2015, 35, 350–359. [Google Scholar] [CrossRef]
- Lacou, L.; Léonil, J.; Gagnaire, V. Functional properties of peptides: From single peptide solutions to a mixture of peptides in food products. Food Hydrocoll. 2016, 57, 187–199. [Google Scholar] [CrossRef]
- Otte, J.; Lomholt, S.B.; Ipsen, R.; Stapelfeldt, H.; Bukrinsky, J.T.; Qvist, K.B. Aggregate Formation during Hydrolysis of β-Lactoglobulin with a Glu and Asp Specific Protease from Bacillus licheniformis. J. Agric. Food Chem. 1997, 45, 4889–4896. [Google Scholar] [CrossRef]
- Doucet, D.; Gauthier, S.F.; Otter, D.E.; Foegeding, E.A. Enzyme-Induced Gelation of Extensively Hydrolyzed Whey Proteins by Alcalase: Comparison with the Plastein Reaction and Characterization of Interactions. J. Agric. Food Chem. 2003, 51, 6036–6042. [Google Scholar] [CrossRef]
- Malekshahi, M.R.-; Lempsink, L.; Amidi, M.; Hennink, W.E.; Mastrobattista, E. Biomedical Applications of Self-Assembling Peptides. Bioconjugate Chem. 2015, 27, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef]
- Rajagopal, K.; Schneider, J.P. Self-assembling peptides and proteins for nanotechnological applications. Curr. Opin. Struct. Biol. 2004, 14, 480–486. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.; Zhao, X. Comparative studies on the self-assembling behaviors of cationic and catanionic surfactant-like peptides. J. Colloid Interface Sci. 2009, 336, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Qiu, F.; Zhao, X. Molecular design and applications of self-assembling surfactant-like peptides. J. Nanomater. 2013, 2013, 469261. [Google Scholar] [CrossRef]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W. Self-assembling amphiphilic peptides. J. Pept. Sci. 2014, 20, 453–467. [Google Scholar] [CrossRef]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Discovery and design of self-assembling peptides. Interface Focus 2017, 7, 20170028. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef]
- Yuan, C.; Levin, A.; Chen, W.; Xing, R.; Zou, Q.; Herling, T.W.; Challa, P.K.; Knowles, T.P.J.; Yan, X. Nucleation and Growth of Amino Acid and Peptide Supramolecular Polymers through Liquid–Liquid Phase Separation. Angew. Chem. 2019, 131, 18284–18291. [Google Scholar] [CrossRef]
- Otte, J.; Lomholt, S.B.; Halkier, T.; Qvist, K.B. Identification of Peptides in Aggregates Formed during Hydrolysis of β-Lactoglobulin B with a Glu and Asp Specific Microbial Protease. J. Agric. Food Chem. 2000, 48, 2443–2447. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, R.; Otte, J.; Qvist, K.B. Molecular self-assembly of partially hydrolysed α-lactalbumin resulting in strong gels with a novel microstructure. J. Dairy Res. 2001, 68, 277–286. [Google Scholar] [CrossRef]
- Otte, J.; Ipsen, R.; Ladefoged, A.M.; Sørensen, J. Protease-induced aggregation of bovine α-lactalbumin: Identification of the primary associating fragment. J. Dairy Res. 2004, 71, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.; Ipsen, R.; Bauer, R.; Bjerrum, M.J.; Waninge, R. Formation of amyloid-like fibrils upon limited proteolysis of bovine α-lactalbumin. Int. Dairy J. 2005, 15, 219–229. [Google Scholar] [CrossRef]
- Creusot, N.; Gruppen, H. Hydrolysis of Whey Protein Isolate with Bacillus licheniformis Protease: Fractionation and Identification of Aggregating Peptides. J. Agric. Food Chem. 2007, 55, 9241–9250. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, R.; Otte, J. Self-assembly of partially hydrolysed α-lactalbumin. Biotechnol. Adv. 2007, 25, 602–605. [Google Scholar] [CrossRef]
- Creusot, N.; Gruppen, H. Hydrolysis of Whey Protein Isolate with Bacillus licheniformis Protease: Aggregating Capacities of Peptide Fractions. J. Agric. Food Chem. 2008, 56, 10332–10339. [Google Scholar] [CrossRef] [PubMed]
- Ting, B.P.C.P.; Gauthier, S.F.; Pouliot, Y. Fractionation of β-Lactoglobulin Tryptic Peptides using Spiral Wound Nanofiltration Membranes. Sep. Sci. Technol. 2007, 42, 2419–2433. [Google Scholar] [CrossRef]
- Pouliot, Y.; Guy, M.-M.; Tremblay, M.; Gaonac’H, A.-C.; Ting, B.P.C.P.; Gauthier, S.F.; Voyer, N. Isolation and Characterization of an Aggregating Peptide from a Tryptic Hydrolysate of Whey Proteins. J. Agric. Food Chem. 2009, 57, 3760–3764. [Google Scholar] [CrossRef]
- Guy, M.-M.; Tremblay, M.; Voyer, N.; Gauthier, S.F.; Pouliot, Y. Formation and Stability of Nanofibers from a Milk-Derived Peptide. J. Agric. Food Chem. 2011, 59, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Guy, M.-M.; Voyer, N. Structure and hydrogel formation studies on homologs of a lactoglobulin-derived peptide. Biophys. Chem. 2012, 163–164, 1–10. [Google Scholar] [CrossRef]
- Suwal, S.; Oliveira, R.S.P.; Pimont-Farge, M.; Marciniak, A.; Brisson, G.; Pouliot, Y.; Doyen, A. Formation of Stable Supramolecular Structure with β-Lactoglobulin-Derived Self-Assembling Peptide f1-8 and Bovine Micellar Caseins. J. Agric. Food Chem. 2019, 67, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Groleau, P.E.; Morin, P.; Gauthier, S.F.; Pouliot, Y. Effect of Physicochemical Conditions on Peptide−Peptide Interactions in a Tryptic Hydrolysate of β-Lactoglobulin and Identification of Aggregating Peptides. J. Agric. Food Chem. 2003, 51, 4370–4375. [Google Scholar] [CrossRef] [PubMed]
- Causserand, C.; Nyström, M.; Aimar, P. Study of streaming potentials of clean and fouled ultrafiltration membranes. J. Membr. Sci. 1994, 88, 211–222. [Google Scholar] [CrossRef]
- Pouliot, Y.; Gauthier, S.F. Effect of peptide distribution on the fractionation of whey protein hydrolysates by nanofiltration membranes. Le Lait 2000, 80, 113–120. [Google Scholar] [CrossRef]
- Groleau, P.E.; Jimenez-Flores, R.; Gauthier, S.F.; Pouliot, Y. Fractionation of β-Lactoglobulin Tryptic Peptides by Ampholyte-Free Isoelectric Focusing. J. Agric. Food Chem. 2002, 50, 578–583. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Influence of ultrafiltration membrane characteristics on adsorptive fouling with dextrans. J. Membr. Sci. 2005, 266, 132–142. [Google Scholar] [CrossRef]
- Gurgel, P.V.; Carbonell, R.G.; Swaisgood, H.E. Studies of the Binding of α-Lactalbumin to Immobilized Peptide Ligands. J. Agric. Food Chem. 2001, 49, 5765–5770. [Google Scholar] [CrossRef] [PubMed]
- Noiseux, I.; Gauthier, S.F.; Turgeon, S.L. Interactions between Bovine β-Lactoglobulin and Peptides under Different Physicochemical Conditions. J. Agric. Food Chem. 2002, 50, 1587–1592. [Google Scholar] [CrossRef]
- Caessens, P.W.J.R.; Visser, S.; Gruppen, H.; Voragen, A.G.J. β-Lactoglobulin Hydrolysis. 1. Peptide Composition and Functional Properties of Hydrolysates Obtained by the Action of Plasmin, Trypsin, and Staphylococcus aureus V8 Protease. J. Agric. Food Chem. 1999, 47, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Groleau, P.E.; Lapointe, J.-F.; Gauthier, S.F.; Pouliot, Y. Effect of aggregating peptides on the fractionation of β-LG tryptic hydrolysate by nanofiltration membrane. J. Membr. Sci. 2004, 234, 121–129. [Google Scholar] [CrossRef]
- Ratté, G. Interaction Entre un Peptide de ß-Lactoglobuline Bovine et les Protéines du Lactosérum. Le cas de L’alpha-Lactalbumine. Ph.D. Thesis, Université Laval, Québec, QC, Canada, 2013. [Google Scholar]
- Lapointe, J.-F.; Gauthier, S.F.; Pouliot, Y.; Bouchard, C. Characterization of interactions between β-lactoglobulin tryptic peptides and a nanofiltration membrane: Impact on the surface membrane properties as determined by contact angle measurements. J. Membr. Sci. 2005, 261, 36–48. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Gauthier, S.F.; Molle, D.; Leonil, J. Interfacial properties of tryptic peptides of beta-lactoglobulin. J. Agric. Food Chem. 1992, 40, 669–675. [Google Scholar] [CrossRef]
- Hamada, D.; Dobson, C.M. A kinetic study of β-lactoglobulin amyloid fibril formation promoted by urea. Protein Sci. 2009, 11, 2417–2426. [Google Scholar] [CrossRef]
- Jansens, K.J.; Rombouts, I.; Grootaert, C.; Brijs, K.; Van Camp, J.; Van Der Meeren, P.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Rational Design of Amyloid-Like Fibrillary Structures for Tailoring Food Protein Techno-Functionality and Their Potential Health Implications. Compr. Rev. Food Sci. Food Saf. 2018, 18, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Doyen, A.; Husson, E.; Bazinet, L. Use of an electrodialytic reactor for the simultaneous β-lactoglobulin enzymatic hydrolysis and fractionation of generated bioactive peptides. Food Chem. 2013, 136, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
| Peak a | Peptide Sequence | Amino Acid Composition | MW b (g/mol) | pI c | Hydrophobicity d (kcal/Residue) |
|---|---|---|---|---|---|
| 1 | 139–141 | ALK | 331.2 | 8.80 | 1.55 |
| 2 | 146–148 | HIR | 424.3 | 9.76 | 1.23 |
| 3 | 136–138 | FDK | 409.2 | 5.84 | 1.38 |
| 4 | 41–42 | VY | 280.2 | 5.49 | 2.28 |
| 5 | 71–75 | IIAEK | 573.4 | 6.00 | 1.63 |
| 6 | 33–40 | DAQSAPLR | 856.5 | 5.84 | 0.91 |
| 7 | 84–91 | IDALNENK | 916.5 | 4.37 | 0.95 |
| 8 | 83–91 | KIDALNENK | 915.5 | 6.07 | 1.18 |
| 9 | 33–39 | DAQSAPL | 700.3 | 3.80 | 0.93 |
| 10 | 9–14 | GLDIQK | 673.4 | 5.84 | 1.14 |
| 11 | 125–135 | TPEVDDEALEK | 1245.6 | 3.83 | 0.85 |
| 12 | 1–8 | LIVTQTMK | 933.5 | 8.75 | 1.34 |
| 13 | 142–148 | ALPMHIR | 837.5 | 9.80 | 1.54 |
| 14 | 92–101 | VLVLDTDYKK | 1193.2 | 5.93 | 1.45 |
| 15 | 125–138 | TPEVDDEALEKFDK | 1635.4 | 4.02 | 0.97 |
| 16 | 15–20 | VAGTWY | 694.5 | 5.49 | 1.46 |
| 17 | 92–100 | VLVLDTDYK | 1065.6 | 4.21 | 1.44 |
| 18 | 61–70 + 149–162 | WENGECAQKK + LSFNPTQLEEQCHI | 2928.4 | 4.57 | 0.89 |
| 19 | 76–82 | TLIPAVFK | 774.4 | 8.41 | 1.88 |
| 20 | 78–82 | IPAVF | 545.3 | 5.52 | 2.13 |
| 21 | 43–60 | VEELKPTPEGDLEILLQK | 2052.6 | 4.25 | 1.13 |
| 22 | 21–40 | SLAMAASDISLLDAQSAPLR | 2029.7 | 4.21 | 1.05 |
| 23 | 41–60 | VYVEELKPTPEGDLEILLQK | 2323.3 | 4.25 | 1.24 |
| 24 | 21–32 | SLAMAASDISLL | 1190.7 | 3.80 | 1.14 |
| 25 | α-lactalbumin | - | 14,176.2 | - | - |
| Pf1-8 | Pf15-20 | Pf41-60 | ||||
|---|---|---|---|---|---|---|
| Relative Proportion (%) | Concentration (mg/mL) | Relative Proportion (%) | Concentration (mg/mL) | Relative Proportion (%) | Concentration (mg/mL) | |
| P1 | 0.85 ± 0.09 a | 0.07 ± 0.00 a | 15.1 ± 0.06 a | 1.36 ± 0.01 a | 9.21 ± 0.23 a | 0.83 ± 0.01 a |
| S1 | 0.72 ± 0.00 a | ND * | 14.1 ± 0.00 a | ND * | 9.48 ± 0.00 a | ND * |
| P2 | 0.81 ± 0.15 a | 0.07 ± 0.00 a | 15.1 ± 1.87 a | 1.34 ± 0.01 a | 9.21 ± 0.48 a | 0.82 ± 0.01 a |
| S2 | 0.88 ± 0.05 a | ND * | 12.6 ± 1.76 a,b | ND * | 8.63 ± 0.48 a | ND * |
| P3 | 15.4 ± 4.43 b | 1.30 ± 0.01 b | 15.6 ± 0.04 a | 1.41 ± 0.01 b | 5.80 ± 5.24 a | 0.52 ± 0.00 b |
| S3 | 0.79 ± 0.16 a | ND * | 20.2 ± 0.98 a,c | ND * | 6.44 ± 5.58 a | ND * |
| P4 | 35.9 ± 3.15 c | 3.29 ± 0.02 c | 4.15 ± 0.37 b | 0.38 ± 0.00 c | 11.1 ± 0.50 a | 1.02 ± 0.01 c |
| S4 | - | ND * | 27.1 ± 3.49 c | ND * | 13.4 ± 0.73 a | ND * |
| P5 | 38.9 ± 4.09 c | 3.57 ± 0.06 d | 3.99 ± 1.61 b | 0.37 ± 0.01 c | 10.8 ± 1.39 a | 0.99 ± 0.02 c |
| S5 | - | ND * | 25.5 ± 5.24 c | ND * | - | ND * |
| Peptide Concentration (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | 50 | 75 | |
| P1 | − | − | − | + | + | + |
| P2 | − | − | + | ND | ND | ND |
| P3 | − | − | + | ND | ND | ND |
| P4 | − | + | ND | ND | ND | ND |
| P5 | − | + | ND | ND | ND | ND |
| Pellet Fraction Concentration (mg/mL) | Pf1-8 Concentration (mg/mL) | Pf15-20 Concentration (mg/mL) | Pf41-60 Concentration (mg/mL) |
|---|---|---|---|
| P1 5 | 0.04 ± 0.01 a | 0.76 ± 0.00 a | 0.46 ± 0.01 a |
| P1 10 | 0.09 ± 0.01 a | 1.51 ± 0.01 b | 0.92 ± 0.02 a,b |
| P1 20 | 0.17 ± 0.02 a | 3.03 ± 0.01 c | 1.84 ± 0.05 b |
| P1 40 * | 0.34 ± 0.04 a | 6.05 ±0.02 e | 3.69 ± 0.09 c |
| P1 50 * | 0.43 ± 0.05 a,b | 7.56 ± 0.03 f | 4.61 ± 0.11 c |
| P1 75 * | 0.64 ± 0.07 a,b,c | 11.3 ± 0.04 g | 6.91 ± 0.17 d |
| P2 5 | 0.04 ± 0.01 a | 0.76 ± 0.09 a | 0.46 ± 0.02 a |
| P2 10 | 0.08 ± 0.02 a | 1.51 ± 0.19 b | 0.92 ± 0.05 a,b |
| P2 20 * | 0.16 ± 0.03 a | 3.02 ± 0.37 c | 1.84 ± 0.1 b |
| P3 5 | 0.77 ± 0.22 a,b,c | 0.78 ± 0.00 a | 0.29 ± 0.26 a |
| P3 10 | 1.54 ± 0.44 b,c,d | 1.56 ± 0.00 b | 0.58 ± 0.52 a |
| P3 20 * | 3.08 ± 0.89 e,f | 3.12 ± 0.01 c | 1.16 ± 1.05 a,b |
| P4 5 | 1.79 ± 0.16 c,d | 0.21 ± 0.02 d | 0.55 ± 0.03 a |
| P4 10 * | 3.59 ± 0.32 f | 0.42 ± 0.04 a,d | 1.11 ± 0.05 a,b |
| P5 5 | 1.94 ± 0.20 d,e | 0.20 ± 0.08 d | 0.54 ± 0.07 a |
| P5 10 * | 3.88 ± 0.41 f | 0.40 ± 0.16 a,d | 1.08 ± 0.14 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimont-Farge, M.; Bérubé, A.; Perreault, V.; Brisson, G.; Suwal, S.; Pouliot, Y.; Doyen, A. Occurrence of Peptide-Peptide Interactions during the Purification of Self-Assembling Peptide f1-8 from a β-Lactoglobulin Tryptic Hydrolysate. Molecules 2021, 26, 1432. https://doi.org/10.3390/molecules26051432
Pimont-Farge M, Bérubé A, Perreault V, Brisson G, Suwal S, Pouliot Y, Doyen A. Occurrence of Peptide-Peptide Interactions during the Purification of Self-Assembling Peptide f1-8 from a β-Lactoglobulin Tryptic Hydrolysate. Molecules. 2021; 26(5):1432. https://doi.org/10.3390/molecules26051432
Chicago/Turabian StylePimont-Farge, Mathilde, Amélie Bérubé, Véronique Perreault, Guillaume Brisson, Shyam Suwal, Yves Pouliot, and Alain Doyen. 2021. "Occurrence of Peptide-Peptide Interactions during the Purification of Self-Assembling Peptide f1-8 from a β-Lactoglobulin Tryptic Hydrolysate" Molecules 26, no. 5: 1432. https://doi.org/10.3390/molecules26051432
APA StylePimont-Farge, M., Bérubé, A., Perreault, V., Brisson, G., Suwal, S., Pouliot, Y., & Doyen, A. (2021). Occurrence of Peptide-Peptide Interactions during the Purification of Self-Assembling Peptide f1-8 from a β-Lactoglobulin Tryptic Hydrolysate. Molecules, 26(5), 1432. https://doi.org/10.3390/molecules26051432







