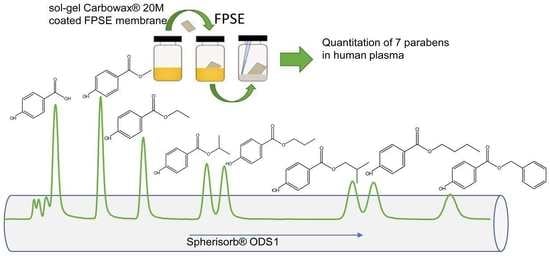
Determination of Intact Parabens in the Human Plasma of Cancer and Non-Cancer Patients Using a Validated Fabric Phase Sorptive Extraction Reversed-Phase Liquid Chromatography Method with UV Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.1.1. Chromatography Optimization
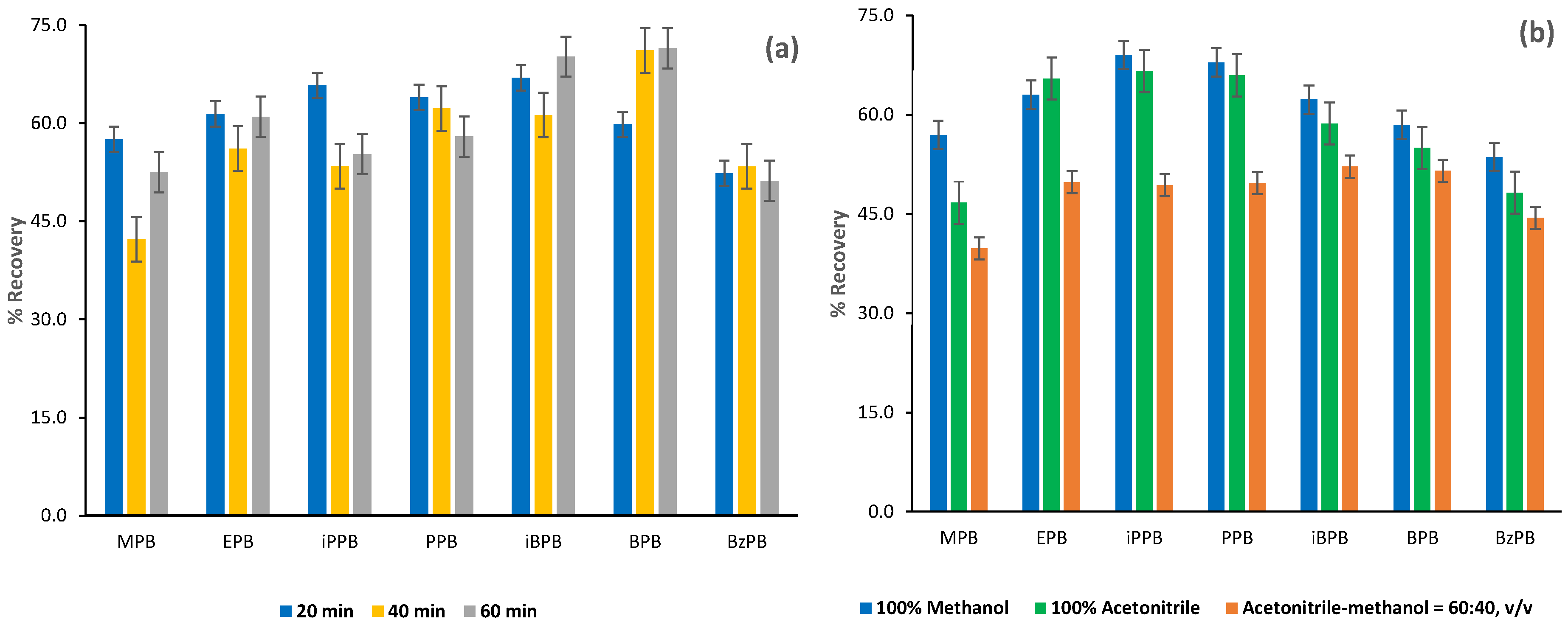
2.1.2. Optimization of the FPSE Procedure
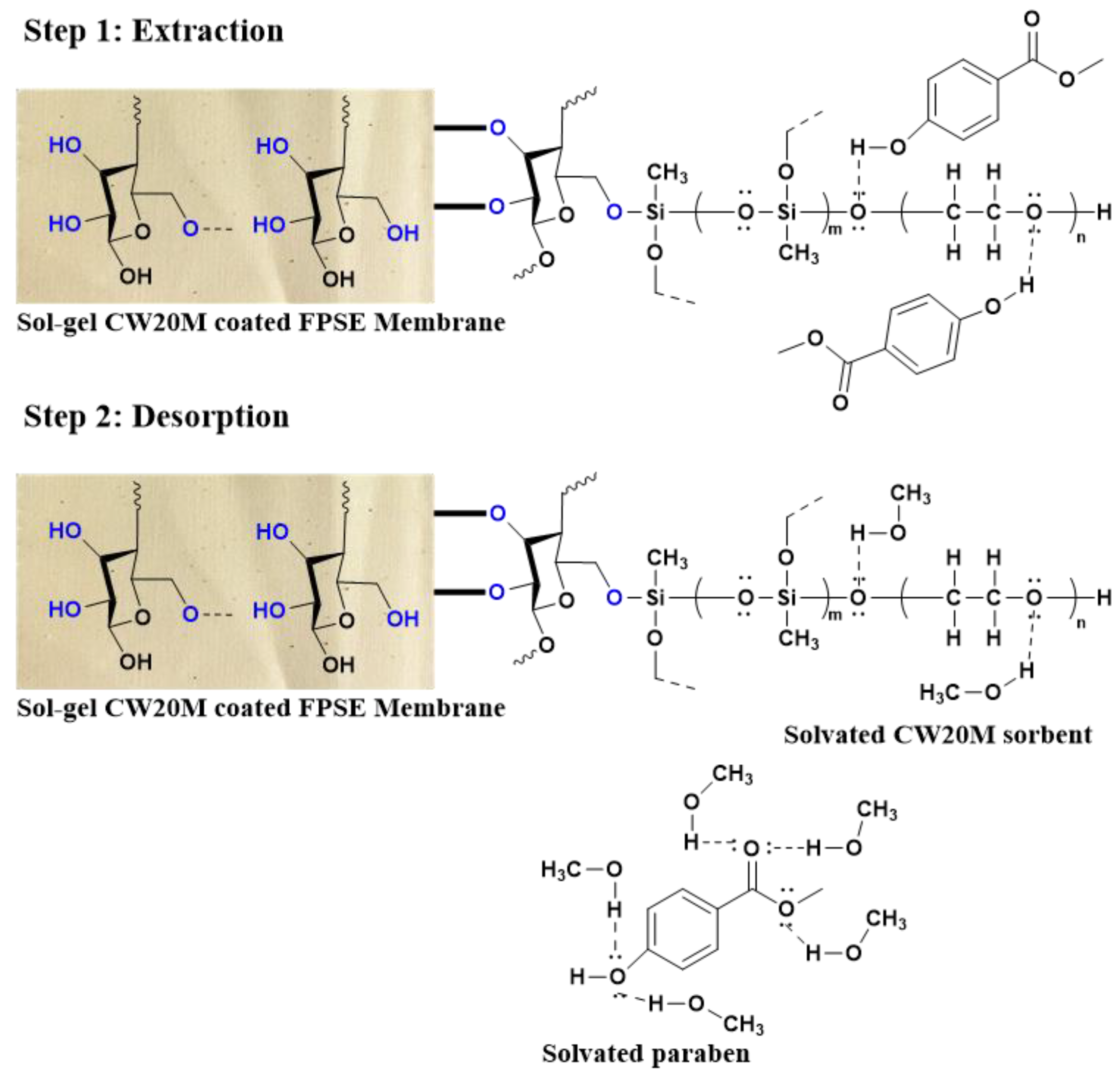
2.1.3. Mechanism of Extraction in FPSE Membrane
2.1.4. Green Attributes of Fabric Phase Sorptive Extraction
2.2. Statistical Analysis of Method Validation Data
2.2.1. Selectivity and Specificity
2.2.2. Linearity, Precision, and Accuracy
2.2.3. Recovery
2.2.4. Stability
2.3. Application to Real Human Plasma Samples
2.4. Comparison with Other Analytical Methods
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Human Plasma Samples
3.3. Instrumentation
3.4. Stock and Working Standard Solutions
3.5. Calibration Standards and Quality Control Samples
3.6. Sample Preparation Procedure
3.7. Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liao, C.; Chen, L.; Kannan, K. Occurrence of parabens in foodstuffs from China and its implications for human dietary exposure. Environ. Int. 2013, 57–58, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Greige-Gerges, H.; Karam, N.; Piet, M.H.; Netter, P.; Magdalou, J. Metabolism of parabens (4-hydroxybenzoic acid esters) by hepatic esterases and UDP-glucuronosyltransferases in Man. Drug Metabol. Pharmacokinet. 2010, 25, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Hagedornleweke, U.; Lippold, B.C. Absorption of sunscreens and other compounds through human skin in-vivo—Derivation of a method to predict maximum fluxes. Pharm. Res. 1995, 12, 1354–1360. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Parabens can enable hallmarks and characteristics of cancer in human breast epithelial cells: A review of the literature with reference to new exposure data and regulatory status. J. Appl. Toxicol. 2014, 34, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.L. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben, and butylparaben. J. Am. Coll. Toxicol. 1984, 3, 147–209. [Google Scholar]
- Fransway, A.F.; Fransway, P.J.; Belsito, D.V.; Yiannias, J.A. Paraben Toxicology. Dermatitis 2019, 30, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Byford, J.R.; Shaw, L.E.; Drew, M.G.B.; Pope, G.S.; Sauer, M.J.; Darbre, P.D. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 80, 49–60. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef]
- Sun, L.; Yu, T.; Guo, J.; Zhang, Z.; Hu, Y.; Xiao, X.; Sun, Y.; Xiao, H.; Li, J.; Zhu, D.; et al. The estrogenicity of methylparaben and ethylparaben at doses close to the acceptable daily intake in immature Sprague-Dawley rats. Sci. Rep. 2016, 6, 25173. [Google Scholar] [CrossRef]
- Darbre, P.D.; Byford, J.R.; Shaw, L.E.; Hall, S.; Coldham, N.G.; Pope, G.S.; Sauer, M.J. Oestrogenic activity of benzylparaben. J. Appl. Toxicol. 2003, 23, 43–51. [Google Scholar] [CrossRef]
- Okubo, T.; Yokoyama, Y.; Kano, K.; Kano, I. ER-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ER alpha and PR. Food Chem. Toxicol. 2001, 39, 1225–1232. [Google Scholar] [CrossRef]
- Janjua, N.R.; Mortensen, G.K.; Andersson, A.M.; Kongshoj, B.; Skakkebaek, N.E.; Wulf, H.C. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ. Sci. Technol. 2007, 41, 5564–5570. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jørgensen, N.; Andersson, A.M. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS). J. Expo. Sci. Environ. Epidemiol. 2011, 21, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Barr, L.; Metaxas, G.; Harbach, C.A.J.; Savoy, L.A.; Darbre, P.D. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J. Appl. Toxicol. 2012, 32, 219–232. [Google Scholar] [CrossRef] [PubMed]
- JECFA. Evaluation of certain food additives and contaminants. In 67th Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 940; Rome, Italy, 2006; pp. 1–104. Available online: https://apps.who.int/iris/bitstream/handle/10665/43592/WHO_TRS_940_eng.pdf?sequence=1&isAllowed=y (accessed on 17 January 2021).
- SCCS/1514/13 Scientific Committee on Consumer Safety (SCCS). Opinion on parabens; Updated request for a scientific opinion on propyl-and butylparaben, COLIPA No 23P82. In European Commission 2013, Report No SCCS/1514/13; Luxembourg, 2013; pp. 1–50. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_132.pdf (accessed on 17 January 2021).
- COMMISSION REGULATION (EU) No 358/2014 of 9 April 2014 Amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; Annex II the Following Entries 1374 to 1378. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32014R0358&from=EN#d1e32-7-1 (accessed on 17 January 2021).
- SCCS/1623/20 Scientific Committee on Consumer Safety (SCCS). Preliminary Opinion on Propylparaben. In European Commission 2020, Report No. SCCS/1623/20; Luxembourg, 2020; pp. 1–57. Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_243.pdf (accessed on 17 January 2021).
- Kolatorova, L.; Vitku, J.; Hampl, R.; Adamcova, K.; Skodova, T.; Simkova, M.; Parizek, A.; Starka, P.; Duskova, M. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res. 2018, 163, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Tongur, T.; Erkaymaz, T.; Erdogan, G.; Unal, B.; Sik, B.; Simsek, T. Traces of intact paraben molecules in endometrial carcinoma. Environ. Sci. Poll. Res. 2019, 26, 31158–31165. [Google Scholar] [CrossRef]
- Shirai, S.; Suzuki, Y.; Yoshinaga, J.; Shiraishi, H.; Mizumoto, Y. Urinary excretion of parabens in pregnant Japanese women. Reprod. Toxicol. 2013, 35, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Ünüvar, T.; Büyükgebiz, A. Fetal and Neonatal Endocrine Disruptors. J. Clin. Res. Pediatr. Endocrinol. 2012, 4, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, I.; Vela-Soria, F.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Ballesterosa, O.; Navalón, A. Analytical methods for the assessment of endocrine disrupting chemical exposure during human fetal and lactation stages: A review. Anal. Chim. Acta 2015, 892, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Raza, N.; Kim, K.H.; Abdullah, M.; Raza, W.; Brown, R.J.W. Recent developments in analytical quantitation approaches for parabens in human-associated samples. TrAC Trends Anal. Chem. 2018, 98, 161–173. [Google Scholar] [CrossRef]
- Grecco, C.F.; Souza, I.D.; Queiroz, M.E.C. Recent development of chromatographic methods to determine parabens in breast milk samples: A review. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1093–1094, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Sandanger, T.M.; Huber, S.; Moe, M.K.; Braathen, T.; Leknes, H.; Lund, E. Plasma concentrations of parabens in postmenopausal women and self-reported use of personal care products: The NOWAC postgenome study. J. Exp. Sci. Environ. Epidemiol. 2011, 21, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Grzeskowiak, T.; Czarczynska-Goslinska, B.; Zgoła-Grzeskowiak, A. Current approaches in sample preparation for trace analysis of selected endocrine disrupting compounds: Focus on polychlorinated biphenyls, alkylphenols, and parabens. TrAC Trends Anal. Chem. 2016, 75, 209–226. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Wang, L.; Thomaidis, N.S.; Kannan, K. A multi-class bioanalytical methodology for the determination of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters, benzophenone-type ultraviolet filters, triclosan, and triclocarban in human urine by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1324, 141–148. [Google Scholar]
- Kolatorova Sosvorova, L.; Chlupacova, T.; Vitku, J.; Vlk, M.; Heracek, J.; Starka, L.; Saman, D.; Simkova, M.; Hampl, R. Determination of selected bisphenols, parabens and estrogens in human plasma using LC-MS/MS. Talanta 2017, 174, 21–28. [Google Scholar] [CrossRef]
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2016, 119, 16–26. [Google Scholar] [CrossRef]
- Melo, L.P.; Queiroz, M.E.C. A molecularly imprinted polymer for microdisc solid phase extraction of parabens from human milk samples. Anal. Methods 2013, 5, 3538. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, R.; Zafra-Gómez, A.; Camino-Sánchez, F.J.; Ballesteros, O.; Navalón, A. Gas chromatography and ultra-high performance liquid chromatography tandem mass spectrometry methods for the determination of selected endocrine disrupting chemicals in human breast milk after stir-bar sorptive extraction. J. Chromatogr. A 2014, 1349, 69–79. [Google Scholar] [CrossRef]
- Fotouhi, M.; Seidi, S.; Shanehsaz, M.; Naseri, M.T. Magnetically assisted matrix solid phase dispersion for extraction of parabens from M. breast milks. J. Chromatogr. A 2017, 1504, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Iribarne-Durán, L.M.; Mustielesa, V.; Jiménez-Díaz, I.; Fernández, M.F.; Olea, N. QuEChERS and ultra-high performance liquid chromatography–tandem mass spectrometry method for the determination of parabens and ultraviolet filters in human milk samples. J. Chromatogr. A 2018, 1546, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Gallardo-Torres, M.E.; Ballesteros, O.; Díaz, C.; Pérez, J.; Navalón, A.; Fernández, M.F.; Olea, N. Assessment of parabens and ultraviolet filters in human placenta tissue by ultrasound-assisted extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 153–161. [Google Scholar] [CrossRef]
- Tartaglia, A.; Kabir, A.; Ulusoy, S.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Furton, K.G.; Locatelli, M. FPSE-HPLC-PDA analysis of seven paraben residues in human whole blood, plasma, and urine. J. Chromatogr. B 2019, 1125, 121707. [Google Scholar] [CrossRef]
- Rigkos, G.; Alampanos, V.; Kabir, A.; Furton, K.G.; Roje, Ž.; Vrček, I.V.; Panderi, I.; Samanidou, V. An improved fabric-phase sorptive extraction protocol for the determination of seven parabens in human urine by HPLC-DAD. Biomed. Chromatogr. 2020, e4974. [Google Scholar] [CrossRef]
- Alampanos, V.; Kabir, A.; Furton, K.G.; Roje, Ž.; Vrček, I.V.; Samanidou, V. Fabric phase sorptive extraction combined with high-performance-liquid chromatography-photodiode array analysis for the determination of seven parabens in human breast tissues: Application to cancerous and non-cancerous samples. J. Chromatogr. A 2020, 1630, 461530. [Google Scholar] [CrossRef]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractors (FPSE). United States Patent Application Publication US 2014/0274660A1, 18 September 2014. Available online: https://patentimages.storage.googleapis.com/c2/36/06/2787b6030e4008/US20140274660A1.pdf (accessed on 17 January 2021).
- Locatelli, M.; Tinari, N.; Grassadonia, A.; Tartaglia, A.; Macerola, D.; Piccolantonio, S.; Sperandio, E.; D’Ovidio, C.; Carradori, S.; Ulusoy, H.I.; et al. FPSE-HPLC-DAD method for the quantitation of anticancer drugs in human whole blood, plasma, and urine. J. Chromatogr. B 2018, 1095, 204–213. [Google Scholar] [CrossRef]
- Locatelli, M.; Kabir, A.; Innosa, D.; Lopatriello, T.; Furton, K.G. A fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for the determination of twelve azole antimicrobial drug residues in human plasma and urine. J. Chromatogr. B 2017, 1040, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Lioupi, A.; Kabir, A.; Furton, K.G.; Samanidou, V. Fabric phase sorptive extraction for the isolation of five common antidepressants from human urine prior to HPLC-DAD analysis. J. Chromatogr. B 2019, 1118–1119, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.F.; Kabir, A.; Marcé, R.M.; Fontanals, N. Dynamic fabric phase sorptive extraction for a group of pharmaceuticals and personal care products from environmental waters. J. Chromatogr. A 2016, 1456, 19–26. [Google Scholar] [CrossRef]
- Valkova, N.; Lépine, F.; Valeanu, L.; Dupont, M.; Labrie, L.; Bisaillon, J.G.; Beaudet, R.; Shareck, F.; Richard, V. Hydrolysis of 4-Hydroxybenzoic Acid Esters (Parabens) and Their Aerobic Transformation into Phenol by the Resistant Enterobacter cloacae Strain EM. Appl. Environ. Microbiol. 2001, 67, 2404–2409. [Google Scholar] [CrossRef] [Green Version]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K. Fabric Phase Sorptive Extraction Explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Seethapathy, S.; Gorecki, T. Applications of polydimethylsiloxane in analytical chemistry: A review. Anal. Chim. Acta 2012, 750, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Galuszka, A.; Migaszewski, Z.; Namiesnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Bioanalytical Method Validation, Committee for Medicinal Products for Human Use (CHMP); EMEA/CHMP/EWP/192217/2009 Rev. 1; European Medical Agency: London, UK, 2015. [Google Scholar]
- Ye, X.Y.; Wong, L.Y.; Jia, L.T.; Needham, L.L.; Calafat, A.M. Stability of the conjugated species of environmental phenols and parabens in human serum. Environ. Int. 2009, 35, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, A.; Locatelli, M.; Kabir, A.; Furton, K.W.; Macerola, D.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Maroni, F.; Bruni, P.; et al. Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules 2019, 24, 382. [Google Scholar] [CrossRef] [Green Version]





| Chemical Structures/Chemical Names | Physicochemical Parameters 1 |
|---|---|
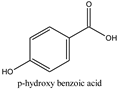 | Log P: 1.2 CLogP: 1.5572 pKa: 4.109, 9.685 |
 | Log P: 1.46 ± 0.47 CLogP: 1.9846 pKa: 9.007 |
 | Log P: 1.83 ± 0.49 CLogP: 2.5136 pKa: 8.981 |
 | Log P: 2.12 ± 0.47 CLogP: 2.8226 pKa: 8.955 |
 | Log P: 2.29 ± 0.47 CLogP: 3.0426 pKa: 8.970 |
 | Log P: 2.69 ± 0.47 CLogP: 3.4416 pKa: 8.959 |
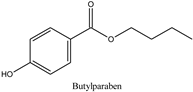 | Log P: 2.71 ± 0.47 CLogP: 3.5716 pKa: 8.965 |
 | Log P: 3.20 ± 0.47 CLogP: 3.8126 pKa: 8.931 |
| Compound 1 | Matrix | Regression Equations 2 | r 3 | Standard Deviation | Sr 4 | |
|---|---|---|---|---|---|---|
| Slope | Intercept | |||||
| MPB | Water | SMPB = 113.9 × CMPB −795 | 0.998 | 2.6 | 92 | 0.05 |
| Human plasma | SMPB = 61.2 × CMPB −598 | 0.996 | 2.4 | 78 | 0.08 | |
| EPB | Water | SEPB = 122.1 × CEPB −805 | 0.9994 | 1.8 | 65 | 0.04 |
| Human plasma | SEPB = 70.4 × CEPB −477 | 0.998 | 2.2 | 70 | 0.06 | |
| iPPB | Water | SiPPB = 96.4 × CiPPB −774 | 0.991 | 4.1 | 145 | 0.08 |
| Human plasma | SiPPB = 63.1 × CiPPB −479 | 0.993 | 3.4 | 121 | 0.11 | |
| PPB | Water | SPPB = 97.5 × CPPB −669 | 0.995 | 4.4 | 164 | 0.08 |
| Human plasma | SPPB = 59.1 × CPPB −429 | 0.993 | 3.1 | 116 | 0.10 | |
| iBPB | Water | SiBPB = 98.1 × CiBPB −789 | 0.998 | 2.8 | 99 | 0.08 |
| Human plasma | SiBPB = 61.0 × CiBPB −557 | 0.995 | 2.6 | 86 | 0.09 | |
| BPB | Water | SBPB = 97.5 × CBPB – 907 | 0.9991 | 2.2 | 72 | 0.05 |
| Human plasma | SBPB = 60.7 × CBPB – 566 | 0.995 | 2.5 | 82 | 0.09 | |
| BzPB | Water | SBzPB = 92.5 × CBzPB −682 | 0.998 | 2.4 | 87 | 0.05 |
| Human plasma | SBzPB = 46.7 × CBzPB −318 | 0.998 | 1.2 | 45 | 0.05 | |
| Compound 1 | Concentration (ng mL−1) | ||
|---|---|---|---|
| Added Concentration | 20 | 100 | 500 |
| MPB | |||
| Overall mean | 20.88 ± 0.45 | 98.4 ± 3.5 | 494.2 ± 9.6 |
| Intraday CV(%) 2 | 1.93 | 3.60 | 1.95 |
| Total precision CV (%) 2 | 2.29 | 3.54 | 1.93 |
| Total accuracy Er% 3 | 104.4 | 98.4 | 98.8 |
| EPB | |||
| Overall mean | 20.94 ± 0.82 | 100.5 ± 4.9 | 504.6 ± 9.1 |
| Intraday CV(%) 2 | 4.30 | 4.33 | 1.98 |
| Total precision CV (%) 2 | 3.76 | 5.15 | 1.73 |
| Total accuracy Er% 3 | 104.3 | 100.5 | 100.9 |
| iPPB | |||
| Overall mean | 20.84 ± 0.46 | 100.2 ± 2.8 | 499.2 ± 8.9 |
| Intraday CV(%) 2 | 6.54 | 3.05 | 1.60 |
| Total precision CV (%) 2 | 5.90 | 2.66 | 1.86 |
| Total accuracy Er%3 | 104.2 | 100.2 | 98.8 |
| PPB | |||
| Overall mean | 20.4 ± 1.7 | 101.5 ± 3.2 | 500.2 ± 7.9 |
| Intraday CV(%) 2 | 8.61 | 3.34 | 1.56 |
| Total precision CV (%) 2 | 8.29 | 2.97 | 1.61 |
| Total accuracy Er%3 | 102.0 | 101.5 | 100.1 |
| iBPB | |||
| Overall mean | 21.1 ± 1.8 | 98.2 ± 3.8 | 501.5 ± 9.6 |
| Intraday CV(%) 2 | 9.05 | 4.17 | 2.83 |
| Total precision CV (%) 2 | 8.73 | 3.76 | 2.58 |
| Total accuracy Er% 3 | 105.1 | 98.2 | 100.3 |
| BBP | |||
| Overall mean | 19.39 ± 0.55 | 101.2 ± 1.6 | 498.8 ± 6.3 |
| Intraday CV(%) 2 | 2.71 | 1.67 | 1.27 |
| Total precision CV (%) 2 | 2.87 | 1.52 | 1.26 |
| Total accuracy Er% 3 | 96.9 | 101.2 | 99.8 |
| BzBP | |||
| Overall mean | 21.09 ± 0.55 | 103.9 ± 1.6 | 502 ± 11 |
| Intraday CV(%) 2 | 2.82 | 1.56 | 2.50 |
| Total precision CV (%) 2 | 2.53 | 1.50 | 2.33 |
| Total accuracy Er% 3 | 105.4 | 103.9 | 100.4 |
| Woman No. | Surgery 1 | Age | BMI 2 | Compound 3,4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MPB | EPB | iPPB | PPB | iBPB | BPB | BzPB | ||||
| 1 | BC | 43 | 25.0 | 110 | 40 | 50 | <LOD | <LOD | <LOD | <LOD |
| 2 | BC | 59 | 34.3 | 40 | <LOD | 30 | <LOD | 40 | <LOD | <LOD |
| 3 | BC | 34 | 28 | 150 | <LOD | detected | <LOD | 80 | <LOD | 70 |
| 4 | BC | 56 | 25.5 | 60 | <LOD | 40 | <LOD | <LOD | <LOD | <LOD |
| 5 | BC | 76 | 26 | 170 | <LOD | detected | <LOD | <LOD | <LOD | <LOD |
| 6 | BC | 45 | 20.9 | 30 | detected | <LOD | detected | <LOD | <LOD | <LOD |
| 7 | BC | 45 | 20.4 | 20 | 30 | <LOD | 30 | 30 | <LOD | <LOD |
| 8 | BC | 83 | 27.5 | 140 | <LOD | <LOD | 10 | 300 | <LOD | 90 |
| 9 | BC | 65 | 23 | 20 | <LOD | <LOD | detected | <LOD | <LOD | <LOD |
| 10 | BC | 63 | 26.2 | 30 | detected | <LOD | <LOD | <LOD | <LOD | <LOD |
| 11 | AE | 46 | 20.3 | 150 | 80 | 30 | <LOD | 50 | <LOD | <LOD |
| 12 | AE | 51 | 28.3 | 80 | detected | 70 | <LOD | <LOD | <LOD | 50 |
| 13 | AE | 48 | 18.4 | detected | 60 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 14 | AE | 33 | 25.1 | detected | 20 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 15 | AE | 47 | 27.6 | detected | 70 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 16 | AE | 38 | 25.9 | 30 | detected | <LOD | <LOD | <LOD | <LOD | <LOD |
| 17 | AE | 59 | 24.9 | 60 | 40 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 18 | AE | 55 | 35.4 | detected | 30 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 19 | AE | 48 | 26.6 | 20 | detected | <LOD | <LOD | <LOD | <LOD | <LOD |
| 20 | AE | 49 | 22.6 | 22 | 50 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Compound 1 | MPB | EPB | iPPB | PPB | iBPB | BPB | BzPB |
|---|---|---|---|---|---|---|---|
| Percentage of the human plasma samples in which quantified and detected | 100 | 60 | 30 | 30 | 25 | Not detected | 15 |
| Percentage of the human plasma samples in which quantified | 80 | 45 | 25 | 10 | 25 | Not detected | 15 |
| Percentage of the human plasma samples from healthy women in which quantified | 60 | 70 | 20 | 0 | 10 | Not detected | 10 |
| Percentage of the human plasma samples from cancerous cases in which quantified | 100 | 20 | 30 | 20 | 40 | Not detected | 20 |
| Mean plasma concentration in heathy women (ng mL−1) | 60.3 | 50.0 | 50.0 | - | 50.0 | Not detected | 50.0 |
| Mean plasma concentration in cancerous cases (ng mL−1) | 77.0 | 35.0 | 40.0 | 20.0 | 112.5 | Not detected | 80.0 |
| Mean plasma concentration in all the samples (ng mL−1) | 70.8 | 46.7 | 44.0 | 20.0 | 100.0 | Not detected | 70.0 |
| Matrix | Analytes | Analytical Method; Column; Flow Rate | Run Time | Sample Preparation/Extraction Time | Sample Volume | %Recovery | Repeatability (%CV) | Linearity Range | LOQ; LOD | Application to Real Samples | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human plasma | MPB, EPB, iPPB, PPB, iBPB, BPB, BzPB | RPHPLC-UV; Spherisorb ODS1 (150 × 2.0 mm, 3 μm); 0.25 mL/min isocratic elution | 22 min | FPSE/ 40 min | 50 μL | 50.1–65.8% | 1.3 to 9.0% (7 parabens) | 20–500 ng/mL | LOQ: 20 ng/mL, LOD: 7 ng/mL | 20 samples from healthy and cancerous patients (women) | Current method |
| Human plasma, whole blood, human urine | MPB, EPB, iPPB, PPB, iBPB, BPB, BzPB | RPHPLC-DAD; Spherisorb C18 (150 × 4.6 mm, 5 μm); 1.0 mL/min Isocratic elution | 25 min | FPSE/ 60 min | 450 μL | − | 1.2 to 10.1% (7 parabens) | 0.1–10 μg/mL | LOQ: 100 ng/mL, LOD: 30 ng/mL | 6 samples | [38] |
| Human plasma | MPB, EPB, PPB, BPB, BzPB Bisphenols Estrogens | LC-MS/MS; Kinetex C18 (150 × 3.0 mm, 1.7 μm) 0.4 mL/min Gradient elution | 11 min | LLE, derivatization with dansyl chloride | 500 μL | 103.6–112.7% | 1.3 to 6% (5 parabens) | MPB: 0.25–32 ng/mL EPB, PPB, BPB, BzPB: 0.094–12 ng/mL | LOQ: 0.134 to 0.202 ng/mL | 58 samples from men of reproductive age; 27 maternal and cord plasma samples | [21,31] |
| Human urine, serum, seminal plasma | MPB, EPB, PPB, BPB, BzPB | LC–MS/MS; Synergi™ Fusion-RP 80 Å (75 × 2.0 mm; 4 μm); 0.3 mL/min Gradient elution | 17 min | Automated SPE/time not specified | 500 μL | 98.3–101.5% | 2.8 to 29.2% (5 parabens) | 0.5–500 ng/mL | LODs < 0.41 ng/mL | 60 samples from young Danish men | [15] |
| Human plasma | MPB, EPB, PPB, BPB, BzPB | LC-TOF/MS; Waters® Acquity BEH Phenyl (100 mm × 2.1 mm, 1.7 μm) 0.45 mL/min Gradient elution | 10 min | SPE on OASIS HLB cartridges | 500 μL | - | < 12% (5 parabens) | 50 to 600 pg injected on column | LODs: MPB 7 ng/mL EPB 3 ng/mL PPB 2 ng/mL | 332 samples from postmenopausal women | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parla, A.; Zormpa, E.; Paloumpis, N.; Kabir, A.; Furton, K.G.; Roje, Ž.; Samanidou, V.; Vinković Vrček, I.; Panderi, I. Determination of Intact Parabens in the Human Plasma of Cancer and Non-Cancer Patients Using a Validated Fabric Phase Sorptive Extraction Reversed-Phase Liquid Chromatography Method with UV Detection. Molecules 2021, 26, 1526. https://doi.org/10.3390/molecules26061526
Parla A, Zormpa E, Paloumpis N, Kabir A, Furton KG, Roje Ž, Samanidou V, Vinković Vrček I, Panderi I. Determination of Intact Parabens in the Human Plasma of Cancer and Non-Cancer Patients Using a Validated Fabric Phase Sorptive Extraction Reversed-Phase Liquid Chromatography Method with UV Detection. Molecules. 2021; 26(6):1526. https://doi.org/10.3390/molecules26061526
Chicago/Turabian StyleParla, Anthi, Eirini Zormpa, Nikolaos Paloumpis, Abuzar Kabir, Kenneth G. Furton, Željka Roje, Victoria Samanidou, Ivana Vinković Vrček, and Irene Panderi. 2021. "Determination of Intact Parabens in the Human Plasma of Cancer and Non-Cancer Patients Using a Validated Fabric Phase Sorptive Extraction Reversed-Phase Liquid Chromatography Method with UV Detection" Molecules 26, no. 6: 1526. https://doi.org/10.3390/molecules26061526
APA StyleParla, A., Zormpa, E., Paloumpis, N., Kabir, A., Furton, K. G., Roje, Ž., Samanidou, V., Vinković Vrček, I., & Panderi, I. (2021). Determination of Intact Parabens in the Human Plasma of Cancer and Non-Cancer Patients Using a Validated Fabric Phase Sorptive Extraction Reversed-Phase Liquid Chromatography Method with UV Detection. Molecules, 26(6), 1526. https://doi.org/10.3390/molecules26061526