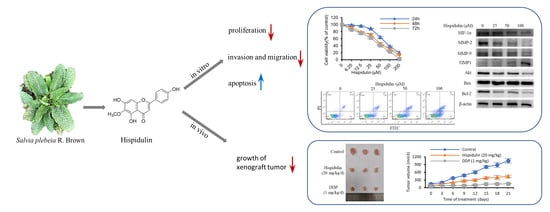
The Effect of Hispidulin, a Flavonoid from Salvia plebeia, on Human Nasopharyngeal Carcinoma CNE-2Z Cell Proliferation, Migration, Invasion, and Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Isolation of Hispidulin from the Bioassay-Guided Fractionation of the EtOH Extract of S. plebeia
2.2. Hispidulin Inhibits the Proliferation and Clonogenic Ability of CNE-2Z Cells
2.3. Hispidulin Promotes the Apoptosis of CNE-2Z Cells
2.4. Hispidulin Inhibits CNE-2Z Cells Migration and Invasion
2.5. Hispidulin Suppresses Tumour Growth in Xenografted Nude Mice Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Material and Extraction
4.3. Cell Line and Cell Culture
4.4. Cell Viability Assay
4.5. Colony Formation Assay
4.6. Flow Cytometry Analysis of Apoptosis
4.7. Western Blotting Analysis
4.8. Scratch Wound, Transwell Migration, and Invasion Assays
4.9. Xenograft Model
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Mahdavifar, N.; Ghoncheh, M.; Mohammadian-Hafshejani, A.; Khosravi, B.; Salehiniya, H. Epidemiology and inequality in the incidence and mortality of nasopharynx cancer in Asia. Osong Public Health Res. Perspect. 2016, 7, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.I.; Mok, V.W. The management of neck metastases in nasopharyngeal cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 99–102. [Google Scholar] [CrossRef]
- Yang, G.C. Observation on the therapeutic effect of Salvia plebeia R. Br. on acute nephritis and hematuria in children. J. Hubei Univ. Chin Med. 2007, 9, 65. [Google Scholar]
- Kong, Q.X.; Dong, F.; Li, S.Y.; Qian, J.; Qu, Z.Y.; Zou, X.; Jiang, X.; Li, X. Study on the effect and mechanism of extract of Salvia plebeia R.Br. on chronic pharyngitis. Nat. Prod. Res. 2018, 30, 109–113. [Google Scholar]
- Ding, P.; Tian, Y.Q. Study Progress on the Chinese Salvia plebeia R. Br. J. Anhui Agric. Sci. 2008, 36, 4133–4144. [Google Scholar]
- Ren, J.; Pan, S.S.; Lu, X.Z.; Zhou, M.; Hu, K. Antitumor activity of dichloromethane extract from Salvia plebeia and induction of apoptosis on K562 cells. Chin. Herb. Med. 2010, 3, 36–40. [Google Scholar]
- Kim, H.A.; Lee, J.M. Effect of antioxidant activities and apoptosis induction of Salvia plebeia R. Br. in human breast cancer MCF-7 Cells. Korean Soc. Community Living Sci. 2018, 29, 197–205. [Google Scholar] [CrossRef]
- Chen, Y.T.; Zheng, R.L.; Jia, Z.J.; Ju, Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef]
- Salah, S.M.; Jäger, A.K. Two flavonoids from Artemisia herba-alba Asso with in vitro GABAA-benzodiazepine receptor activity. J. Ethnopharmacol. 2005, 99, 145–146. [Google Scholar] [CrossRef]
- Yin, Y.; Gong, F.Y.; Wu, X.X.; Sun, Y.; Li, Y.H.; Chen, T.; Xu, Q. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J. Ethnopharmacol. 2008, 120, 1–6. [Google Scholar] [CrossRef]
- Jin, X.F.; Qian, J.; Lu, Y.H. The role of hepatoprotective effect of a flavonoid-rich extract of Salvia plebeia R.Br. on carbon tetrachloride-induced acute hepatic injury in mice. J. Med. Plants Res. 2011, 5, 1558–1563. [Google Scholar]
- Liu, K.; Zhao, F.; Yan, J.; Xia, Z.; Jiang, D.; Ma, P. Hispidulin: A promising flavonoid with diverse anti-cancer properties. Life Sci. 2020, 259, 118395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Mabry, T.J. Flavonoids from Artemisia frigida. Phytochemistry 1981, 20, 1389–1395. [Google Scholar] [CrossRef]
- Llanos, L.; Moreu, R.; Ortin, T.; Peiró, A.M.; Pascual, S.; Bellot, P.; Barquero, C.; Francés, R.; Such, J.; Pérez-Mateo, M.; et al. The existence of a relationship between increased serum alanine aminotransferase levels detected in premarketing clinical trials and postmarketing published hepatotoxicity case reports. Aliment. Pharmacol. Ther. 2010, 31, 1337–1345. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Wan, X.H.; Niu, F.J.; Xie, S.M.; Guo, H.; Yang, Y.Y.; Guo, L.Y.; Zhou, C.Z. Salvia plebeia R. Br.: An overview about its traditional uses, chemical constituents, pharmacology and modern applications. Biomed. Pharmacother. 2020, 121, 109589. [Google Scholar] [CrossRef]
- Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, hispidulin, and cirsimaritin identified in Tamarix ramosissima Barks from Southern Xinjiang and their antioxidant and antimicrobial activities. Molecules 2019, 24, 390. [Google Scholar] [CrossRef] [Green Version]
- Srisook, K.; Srisook, E.; Nachaiyo, W.; Chan-In, M.; Thongbai, J.; Wongyoo, K.; Chawsuanthong, S.; Wannasri, K.; Intasuwan, S.; Watcharanawee, K. Bioassay-guided isolation and mechanistic action of anti-inflammatory agents from Clerodendrum inerme leaves. J. Ethnopharmacol. 2015, 165, 94–102. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Z.; Ma, C. Hispidulin exerts anti-osteoporotic activity in ovariectomized mice via activating AMPK signaling pathway. Cell Biochem. Biophys. 2014, 69, 311–317. [Google Scholar] [CrossRef]
- Bourdillat, B.; Delautier, D.; Labat, C.; Benveniste, J.; Potier, P.; Brink, C. Hispidulin, a natural flavone, inhibits human platelet aggregation by increasing cAMP levels. Eur. J. Pharmacol. 1988, 147, 1–6. [Google Scholar] [CrossRef]
- An, P.; Xie, J.; Qiu, S.; Liu, Y.; Wang, J.; Xiu, X.; Li, L.; Tang, M. Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci. 2019, 232, 116599. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Gao, H.; Xie, J.; Yuan, Y.P.; Yuan, Q.; Gao, M.Q.; Liu, K.L.; Chen, X.H.; Han, Y.T.; Han, Z.W. Hispidulin induces ER stress-mediated apoptosis in human hepatocellular carcinoma cells in vitro and in vivo by activating AMPK signaling pathway. Acta Pharmacol. Sin. 2019, 40, 666–676. [Google Scholar] [CrossRef]
- He, L.; Wu, Y.; Lin, L.; Wang, J.; Wu, Y.; Chen, Y.; Yi, Z.; Liu, M.; Pang, X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011, 102, 219–225. [Google Scholar] [CrossRef]
- Gao, H.; Gao, M.Q.; Peng, J.J.; Han, M.; Liu, K.L.; Han, Y.T. Hispidulin mediates apoptosis in human renal cell carcinoma by inducing ceramide accumulation. Acta Pharmacol. Sin. 2017, 38, 1618–1631. [Google Scholar] [CrossRef] [Green Version]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. PI3K: Downstream AKTion blocks apoptosis. Cell 1997, 88, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Yi, S.M.; Yun, J.W.; Jung, J.H.; Kim, D.H.; Kim, H.J.; Chang, S.H.; Kim, G.; Ryu, C.H.; Shin, S.C.; et al. Polyphenols isolated from Allium cepa L. induces apoptosis by induction of p53 and suppression of Bcl-2 through inhibiting PI3K/Akt signaling pathway in AGS human cancer cells. J. Cancer Prev. 2014, 19, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Agani, F.; Jiang, B.H. Oxygen-independent regulation of HIF-1: Novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr. Cancer Drug Targets 2013, 13, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, L.; Zhao, P.; Yao, L.; Jin, H.; Liang, S.; Wang, Y.; Zhang, D.; Pang, Y.; Shi, Y.; et al. Hypoxia promotes metastasis in human gastric cancer by up-regulating the 67-kDa laminin receptor. Cancer Sci. 2010, 101, 1653–1660. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Sun, X.; Li, B.; Ma, H.; Wu, P.; Zhang, Y.; Zhu, M.; Li, H.-M.; Qin, M.; Wu, C.-Z. The Effect of Hispidulin, a Flavonoid from Salvia plebeia, on Human Nasopharyngeal Carcinoma CNE-2Z Cell Proliferation, Migration, Invasion, and Apoptosis. Molecules 2021, 26, 1604. https://doi.org/10.3390/molecules26061604
Dai Y, Sun X, Li B, Ma H, Wu P, Zhang Y, Zhu M, Li H-M, Qin M, Wu C-Z. The Effect of Hispidulin, a Flavonoid from Salvia plebeia, on Human Nasopharyngeal Carcinoma CNE-2Z Cell Proliferation, Migration, Invasion, and Apoptosis. Molecules. 2021; 26(6):1604. https://doi.org/10.3390/molecules26061604
Chicago/Turabian StyleDai, Yiqun, Xiaolong Sun, Bohan Li, Hui Ma, Pingping Wu, Yingping Zhang, Meilin Zhu, Hong-Mei Li, Minjian Qin, and Cheng-Zhu Wu. 2021. "The Effect of Hispidulin, a Flavonoid from Salvia plebeia, on Human Nasopharyngeal Carcinoma CNE-2Z Cell Proliferation, Migration, Invasion, and Apoptosis" Molecules 26, no. 6: 1604. https://doi.org/10.3390/molecules26061604
APA StyleDai, Y., Sun, X., Li, B., Ma, H., Wu, P., Zhang, Y., Zhu, M., Li, H.-M., Qin, M., & Wu, C.-Z. (2021). The Effect of Hispidulin, a Flavonoid from Salvia plebeia, on Human Nasopharyngeal Carcinoma CNE-2Z Cell Proliferation, Migration, Invasion, and Apoptosis. Molecules, 26(6), 1604. https://doi.org/10.3390/molecules26061604