Mutual Support of Ligand- and Structure-Based Approaches—To What Extent We Can Optimize the Power of Predictive Model? Case Study of Opioid Receptors
Abstract
1. Introduction
2. Methods
2.1. Dataset Preparation
2.2. ML-Based Predictions
2.3. Molecular Docking
3. Results and Discussion
3.1. Dataset Analysis
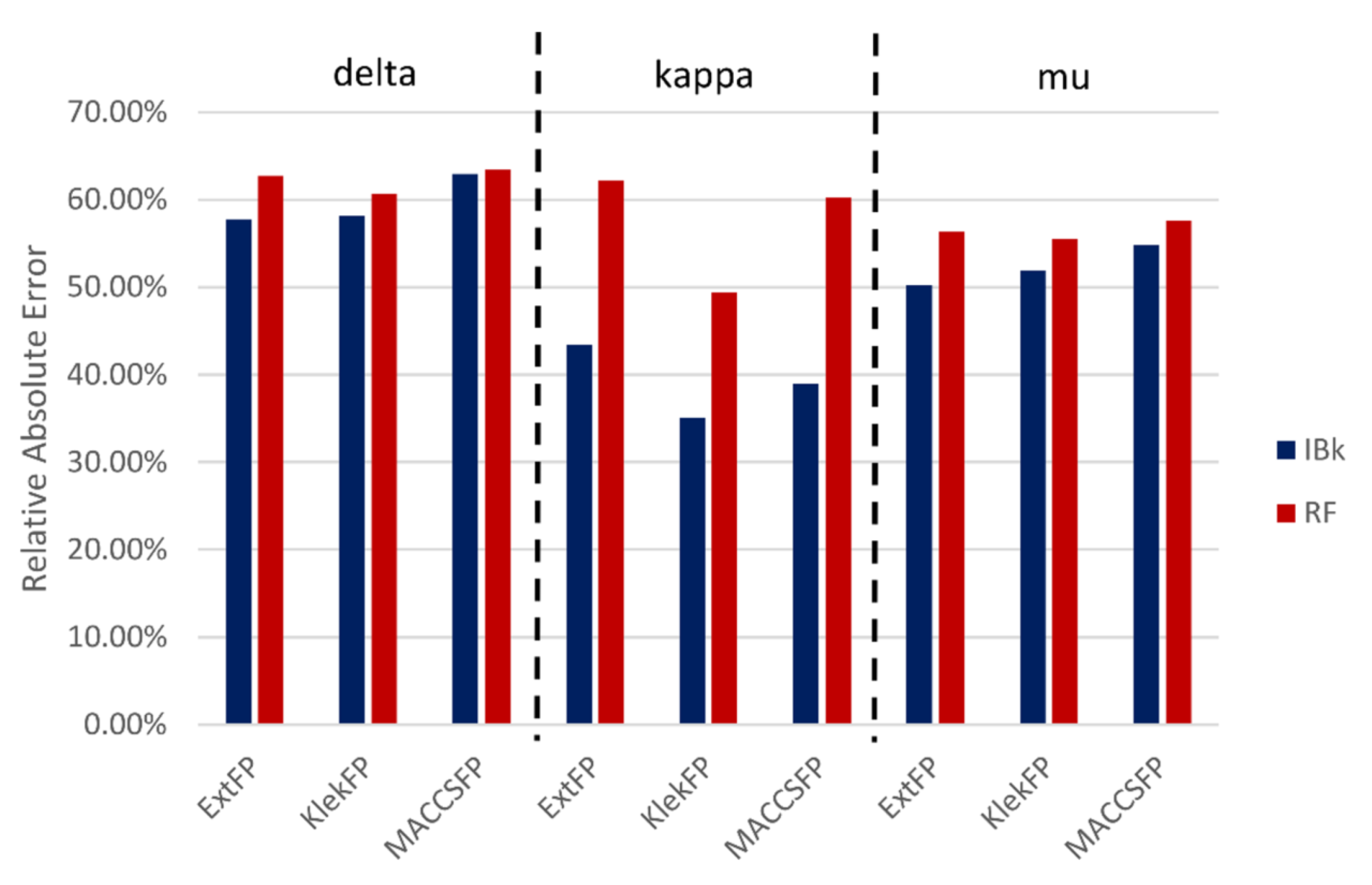
3.2. Global Effectiveness of ML Methods Predictions (Regression Experiments)
3.2.1. Ligand-Based Analysis
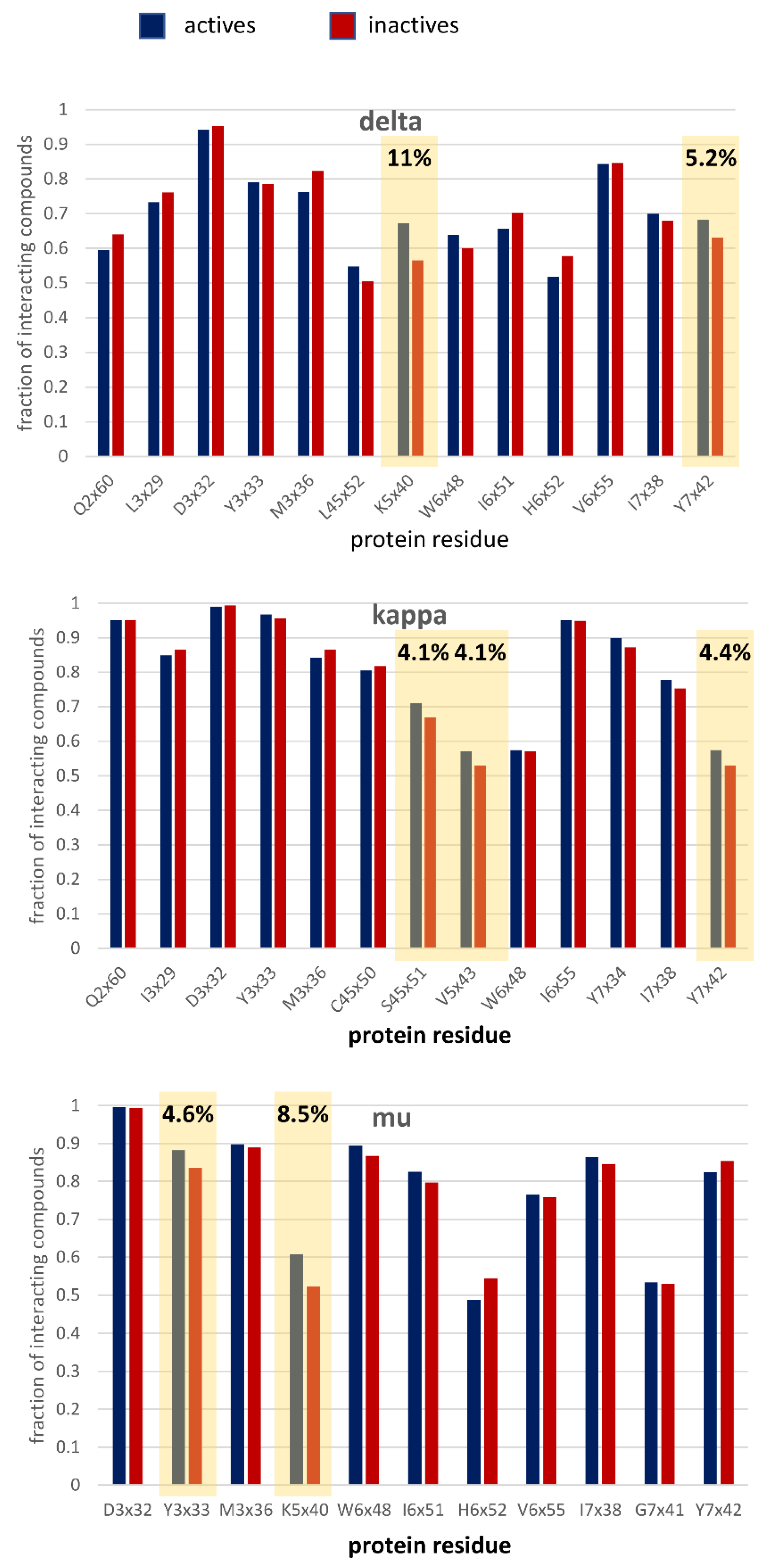
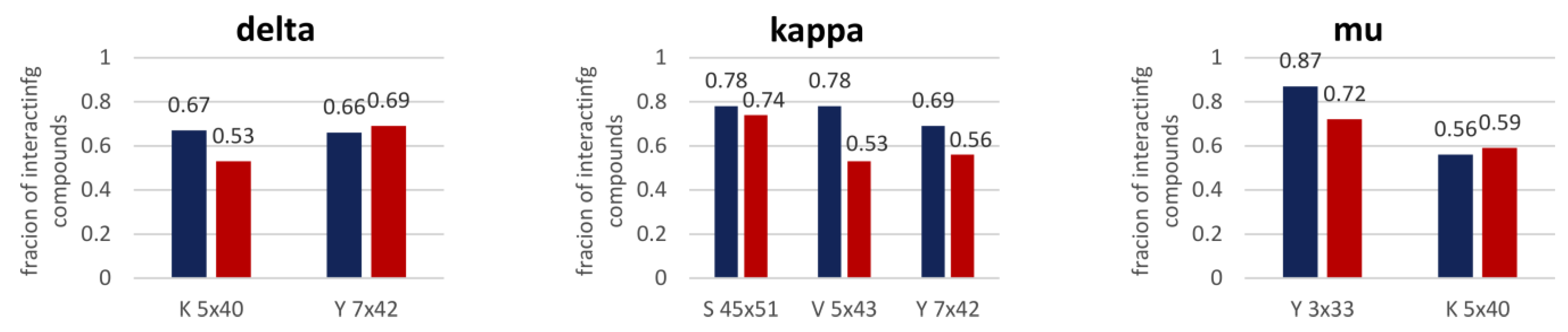
3.2.2. Structure-Based Analysis
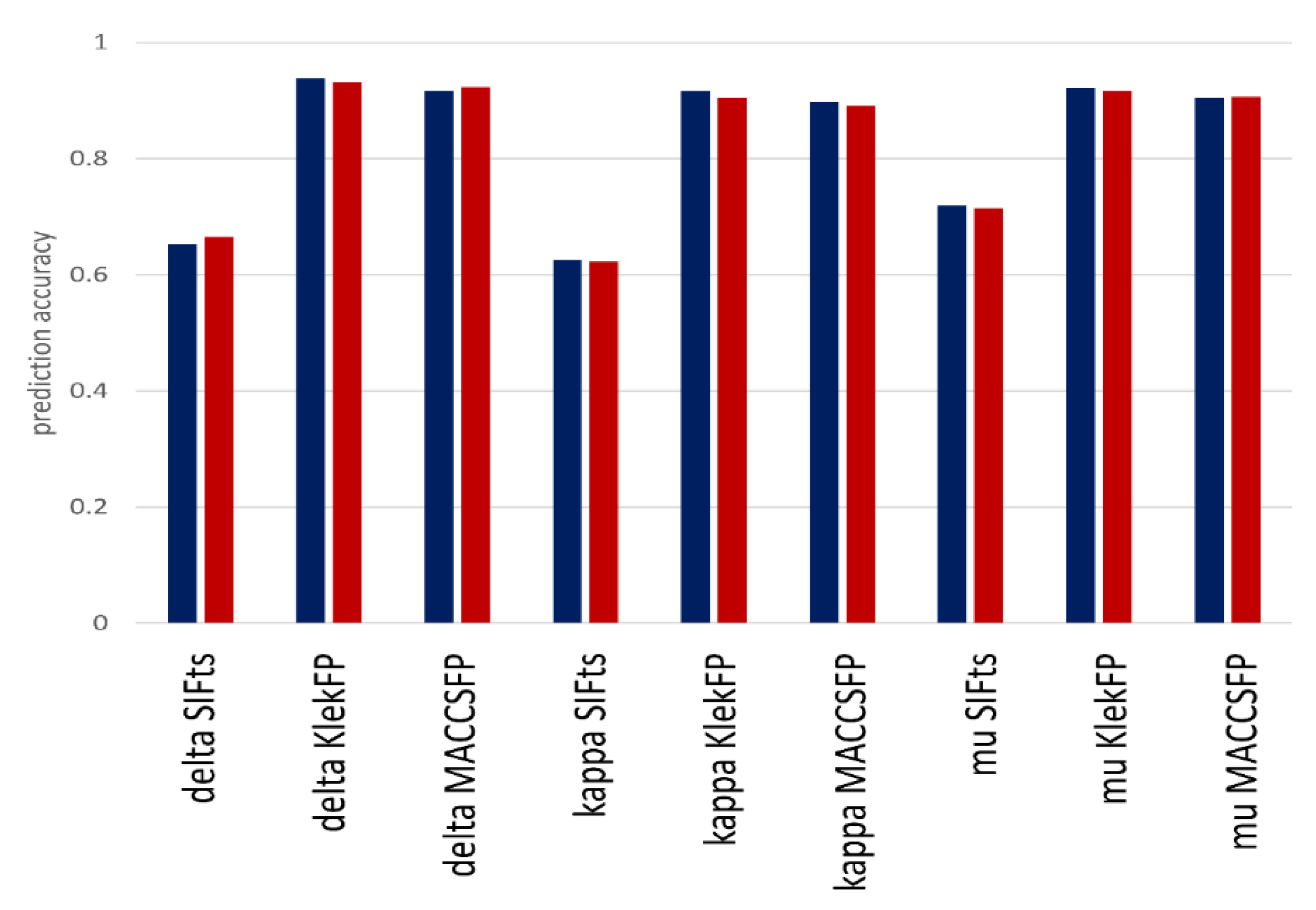
3.3. Classification Experiments
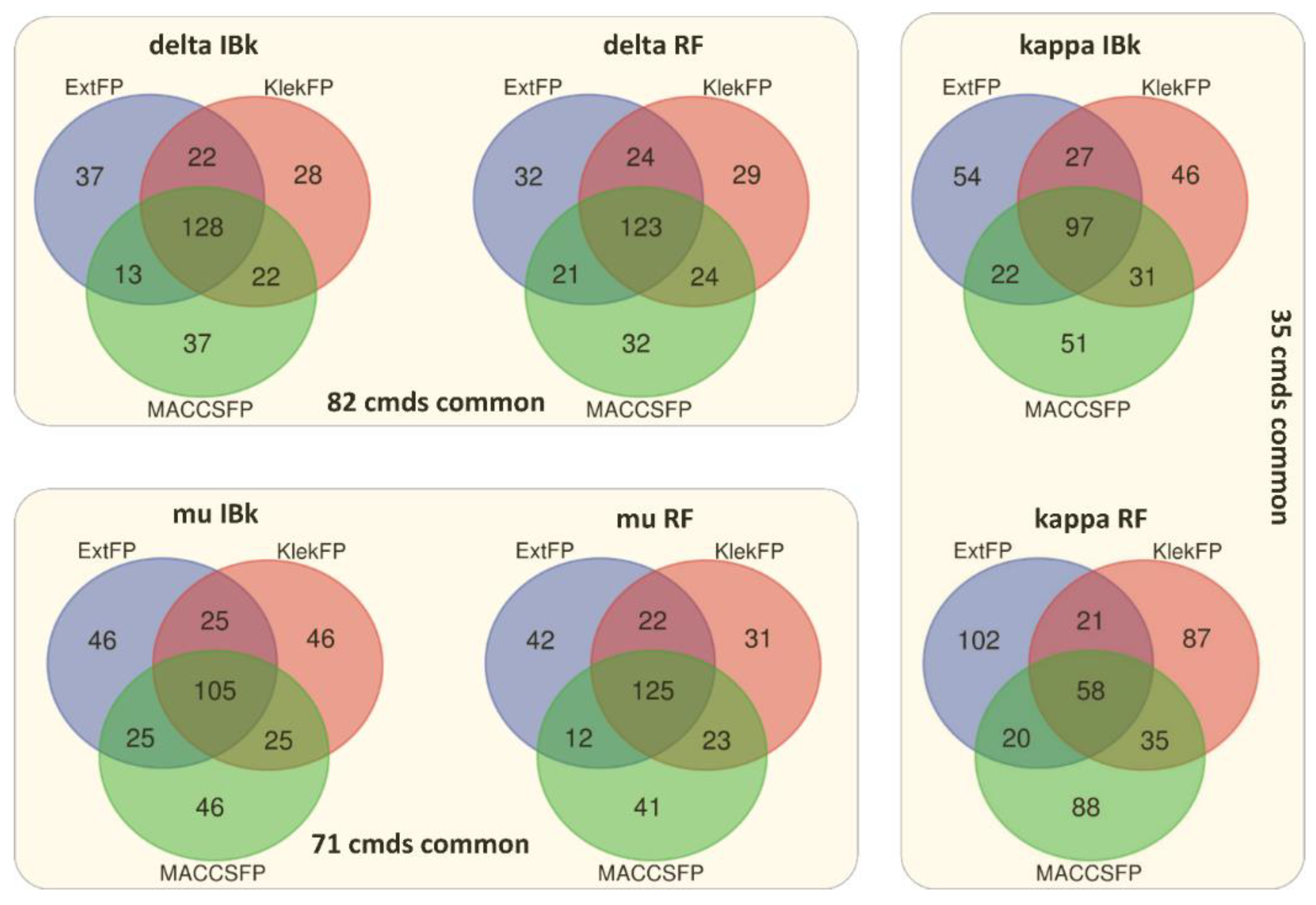
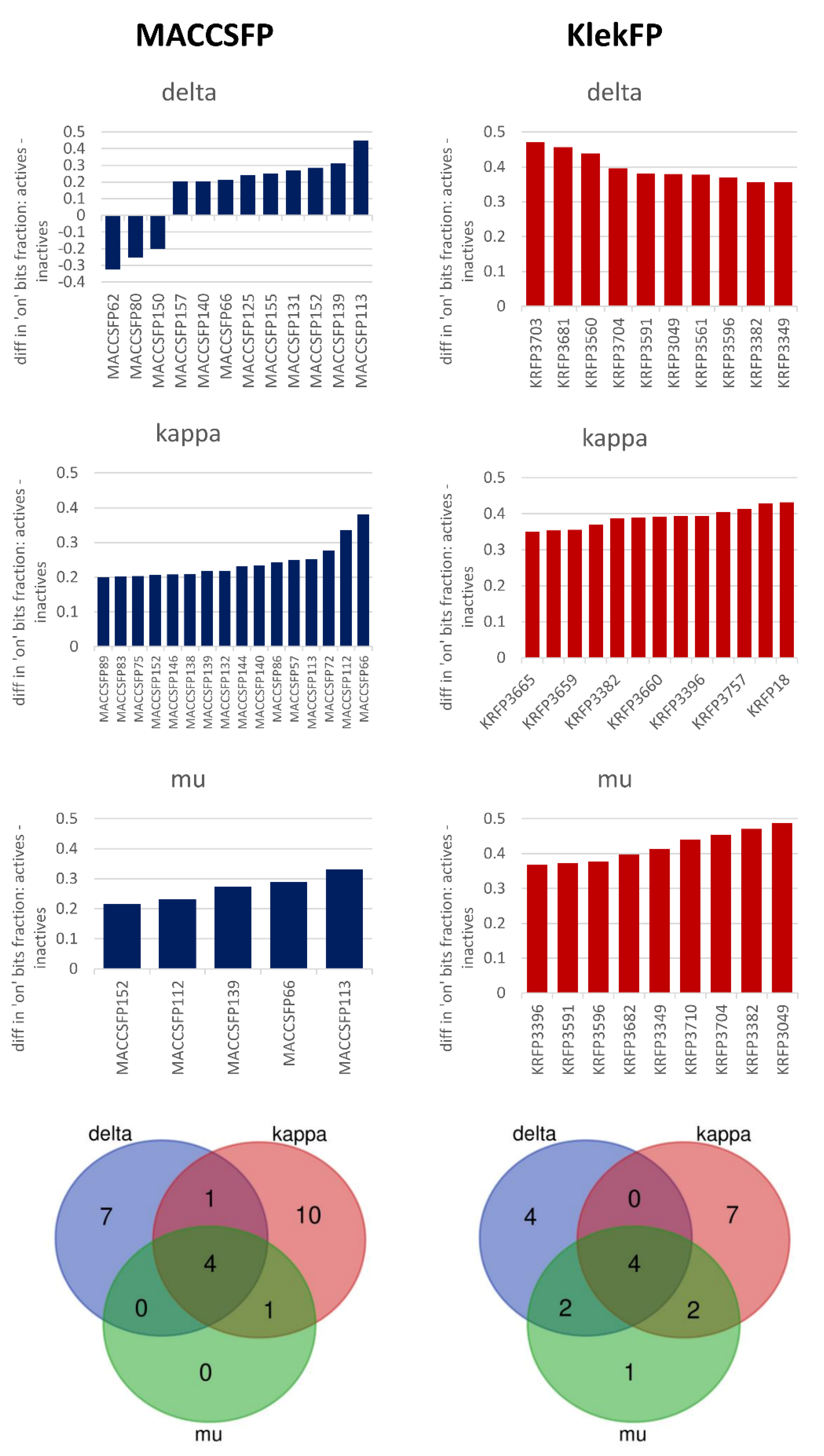
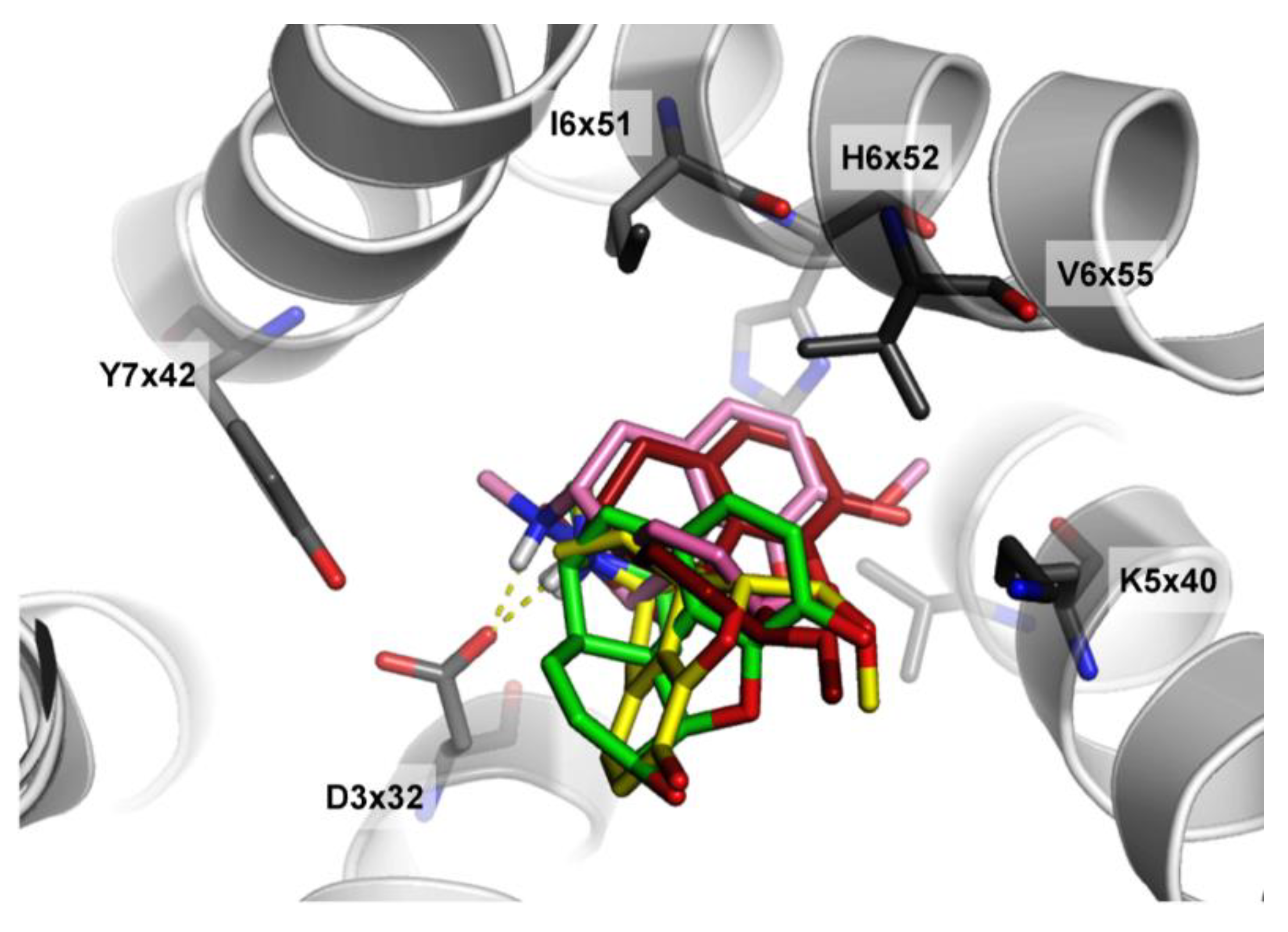
3.4. Case Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| QSAR | Quantitative structure–activity relationship |
| ML | Machine learning |
| CADD | Computer-aided drug design |
| MCC | Matthews correlation coefficient |
| MSE | Mean squared error |
| GPCRs | G-protein-coupled receptors |
| ExtFP | Extended fingerprint |
| KlekFP | Klekota–Roth fingerprint |
| MACCSFP | MACCS fingerprint |
| IBk | k-nearest neighbor algorithm |
| RF | Random forest |
| SIFts | Structural interaction fingerprint |
References
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W. Computational methods in drug discovery. Pharmacol. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Srinivas, K. Modern drug discovery process: An in silico approach. J. Bioinform. Seq. Anal. 2011, 2, 89–94. [Google Scholar]
- Koeppen, H.; Kriegl, J.; Lessel, U.; Tautermann, C.S.; Wellenzohn, B. Ligand-Based Virtual Screening. In Virtual Screening: Principles, Challenges, and Practical Guidelines; Mannhold, R., Kubinyi, H., Folkers, G., Sotriffer, C., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Vidal, D.; Garcia-Serna, R.; Mestres, J. Ligand-Based Approaches to In Silico Pharmacology. Methods Mol. Biol. 2011, 672, 489–502. [Google Scholar] [CrossRef]
- Bacilieri, M.; Moro, S. Ligand-Based Drug and Design Methodologies and in Drug and Discovery Process and An and Overview. Curr. Drug Discov. Technol. 2006, 3, 155–165. [Google Scholar] [CrossRef]
- Douguet, D. Ligand-Based Approaches in Virtual Screening. Curr. Comput. Aided Drug Des. 2008, 4, 180–190. [Google Scholar] [CrossRef]
- Skoda, P.; Hoksza, D. Benchmarking platform for ligand-based virtual screening. In Proceedings of the Proceedings–2016 IEEE International Conference on Bioinformatics and Biomedicine, BIBM 2016, Shenzhen, China, 15–18 December 2016. [Google Scholar]
- Ewing, T.; Baber, J.C.; Feher, M. Novel 2D fingerprints for ligand-based virtual screening. J. Chem. Inf. Model. 2006, 46, 2423–2431. [Google Scholar] [CrossRef]
- Emsley, P.; Debreczeni, J.É.; Ferguson, A.D.; Sources, X.; Collection, H.D.; Tari, L.; Maddaford, S.P.; Bensen, D.C.; Hoffman, I.D. Structure-Based Drug Discovery. In Structure-Based Drug Discovery, Methods in Molecular Biology; Tari, L.W., Ed.; Humana Press: Totowa, NJ, USA, 2012. [Google Scholar]
- Lyne, P.D. Structure-based virtual screening: An overview. Drug Discov. Today 2002, 7, 1047–1055. [Google Scholar] [CrossRef]
- Ghosh, S.; Nie, A.; An, J.; Huang, Z. Structure-based virtual screening of chemical libraries for drug discovery. Curr. Opin. Chem. Biol. 2006, 10, 194–202. [Google Scholar] [CrossRef]
- Jhoti, H.; Leach, A.R. Structure-Based Drug Discovery; Springer: Dordrecht, The Netherlands, 2007; ISBN1 1402044062. ISBN2 9781402044069. [Google Scholar]
- Anderson, A.C.; Laboratories, B. The Process of Structure-Based Drug Design. Chem. Biol. 2003, 10, 787–797. [Google Scholar] [CrossRef]
- Kroemer, R.T. Structure-Based Drug Design: Docking and Scoring. Curr. Protein Pept. Sci. 2007, 8, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Mobarec, J.C.; Sanchez, R.; Filizola, M. Modern homology modeling of G-protein coupled receptors: Which structural template to use? J. Med. Chem. 2009, 52, 5207–5216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wallach, I.; Heifets, A. Most Ligand-Based Classi fi cation Benchmarks Reward Memorization Rather than Generalization. J. Chem. Inf. Model. 2018, 58, 916–932. [Google Scholar] [CrossRef]
- Leśniak, D.; Podlewska, S.; Jastrzȩbski, S.; Sieradzki, I.; Bojarski, A.J.; Tabor, J. Development of New Methods Needs Proper Evaluation-Benchmarking Sets for Machine Learning Experiments for Class A GPCRs. J. Chem. Inf. Model. 2019, 59, 4974–4992. [Google Scholar] [CrossRef]
- Kurczab, R.; Smusz, S.; Bojarski, A.J. The influence of training actives/inactives ratio on machine learning performance. J. Cheminform. 2013, 5, P30. [Google Scholar] [CrossRef]
- Kirchmair, J.; Markt, P.; Distinto, S.; Wolber, G.; Langer, T. Evaluation of the performance of 3D virtual screening protocols: RMSD comparisons, enrichment assessments, and decoy selection—what can we learn from earlier mistakes? J. Comput. Aided. Mol. Des. 2008, 22, 213–228. [Google Scholar] [CrossRef]
- Smusz, S.; Kurczab, R.; Bojarski, A.J. A multidimensional analysis of machine learning methods performance in the classification of bioactive compounds. Chemom. Intell. Lab. Syst. 2013, 128, 89–100. [Google Scholar] [CrossRef]
- Ma, X.H.; Wang, R.; Yang, S.Y.; Li, Z.R.; Xue, Y.; Wei, Y.C.; Low, B.C.; Chen, Y.Z. Evaluation of virtual screening performance of support vector machines trained by sparsely distributed active compounds. J. Chem. Inf. Model. 2008, 48, 1227–1237. [Google Scholar] [CrossRef]
- Smusz, S.; Kurczab, R.; Bojarski, A.J. The influence of the inactives subset generation on the performance of machine learning methods. J. Cheminform. 2013, 5, 17. [Google Scholar] [CrossRef]
- Vihinen, M. How to evaluate performance of prediction methods? Measures and their interpretation in variation effect analysis. BMC Genom. 2012, 13, S2. [Google Scholar] [CrossRef]
- Stefanowski, J.; Pachocki, M. Comparing Performance of Committee Based Approaches to Active Learning. Inf. Syst. 2001, 457–470. [Google Scholar]
- Schwaighofer, A.; Schroeter, T.; Mika, S.; Blanchard, G. How wrong can we get? A review of machine learning approaches and error bars. Comb. Chem. High. Throughput Screen. 2009, 12, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Heilker, R.; Wolff, M.; Tautermann, C.S.; Bieler, M. G-protein-coupled receptor-focused drug discovery using a target class platform approach. Drug Discov. Today 2009, 14, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Katritch, V.; Cherezov, V.; Stevens, R.C. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol. Sci. 2011, 33, 17–27. [Google Scholar] [CrossRef]
- Kobilka, B.K. G protein coupled receptor structure and activation. Biochim. Biophys. Acta 2007, 1768, 794–807. [Google Scholar] [CrossRef]
- Pasternak, G.W. Opiate pharmacology and relief of pain. J. Clin. Oncol. 2014, 32, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef]
- Groer, C.E.; Tidgewell, K.; Moyer, R.A.; Harding, W.W.; Rothman, R.B.; Prisinzano, T.E.; Bohn, L.M. An opioid agonist that does not induce mu-opioid receptor--arrestin interactions or receptor internalization. Mol. Pharmacol. 2007, 71, 549–557. [Google Scholar] [CrossRef]
- Wu, H.; Wacker, D.; Mileni, M.; Katritch, V.; Han, G.W.; Vardy, E.; Liu, W.; Thompson, A.A.; Huang, X.P.; Carroll, F.I.; et al. Structure of the human κ-opioid receptor in complex with JDTic. Nature 2012, 485, 327–332. [Google Scholar] [CrossRef]
- Quock, R.M.; Burkey, T.H.; Varga, E.; Hosohata, Y.; Hosohata, K.; Cowell, S.M.; Slate, C.A.; Ehlert, F.J.; Roeske, W.R.; Yamamura, H.I. The delta-opioid receptor: Molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol. Rev. 1999, 51, 503–532. [Google Scholar] [PubMed]
- Pasternak, G.W. Insights into mu opioid pharmacology: The role of mu opioid receptor subtypes. Life Sci. 2001, 68, 2213–2219. [Google Scholar] [CrossRef]
- Hert, J.; Willett, P.; Wilton, D.J.; Acklin, P.; Azzaoui, K.; Jacoby, E.; Schuffenhauer, A. Comparison of topological descriptors for similarity-based virtual screening using multiple bioactive reference structures. Org. Biomol. Chem. 2004, 2, 3256–3266. [Google Scholar] [CrossRef]
- Plewczynski, D. Brainstorming: Weighted voting prediction of inhibitors for protein targets. J. Mol. Model. 2011, 17, 2133–2141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Truchon, J.F.; Bayly, C.I. Evaluating virtual screening methods: Good and bad metrics for the “early recognition” problem. J. Chem. Inf. Model. 2007, 47, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2011, 40, D1100-7. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.W.E.I. Software News and Update PaDEL-Descriptor: An Open Source Software to Calculate Molecular Descriptors and Fingerprints. J. Comput. Chem. 2010, 32, 1466–1474. [Google Scholar] [CrossRef]
- Steinbeck, C.; Han, Y.; Kuhn, S.; Horlacher, O.; Luttmann, E.; Willighagen, E. The Chemistry Development Kit (CDK): An open-source Java library for Chemo- and Bioinformatics. J. Chem. Inf. Comput. Sci. 2003, 43, 493–500. [Google Scholar] [CrossRef]
- Klekota, J.; Roth, F.P. Chemical substructures that enrich for biological activity. Bioinformatics 2008, 24, 2518–2525. [Google Scholar] [CrossRef]
- MACCS Structural Keys, Accelrys; San Diego, USA. Available online: https://docs.eyesopen.com/toolkits/python/graphsimtk/fingerprint.html (accessed on 2 July 2020).
- Cunningham, P.; Delany, S.J. k-Nearest Neighbour Classifiers. Mult. Classif. Syst. 2007, 34, 1–17. [Google Scholar] [CrossRef]
- Svetnik, V.; Liaw, A.; Tong, C.; Culberson, J.C.; Sheridan, R.P.; Feuston, B.P. Random forest: A classification and regression tool for compound classification and QSAR modeling. J. Chem. Inf. Comput. Sci. 2003, 43, 1947–1958. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software: An update. SIGKDD Explor. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Glide, Schrödinger Release 2019-3. LLC: New York, NY, USA, 2019. Available online: https://www.schrodinger.com (accessed on 2 July 2020).
- Small-Molecule Drug Discovery Suite 2020-2, Schrödinger. LLC: New York, NY, USA, 2017. Available online: https://www.schrodinger.com (accessed on 2 July 2020).
- Deng, Z.; Chuaqui, C.; Singh, J. Structural interaction fingerprint (SIFt): A novel method for analyzing three-dimensional protein-ligand binding interactions. J. Med. Chem. 2004, 47, 337–344. [Google Scholar] [CrossRef] [PubMed]
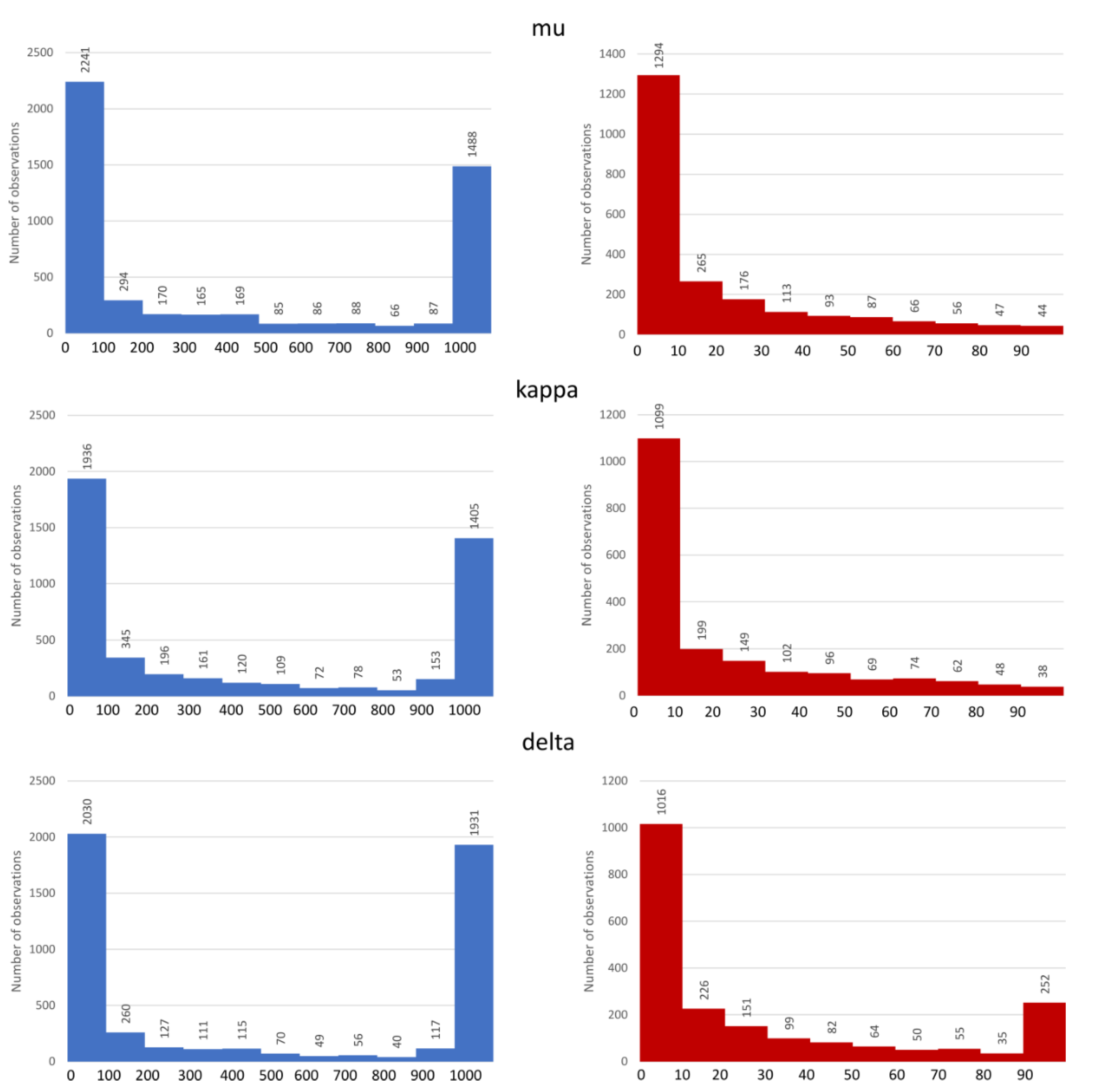

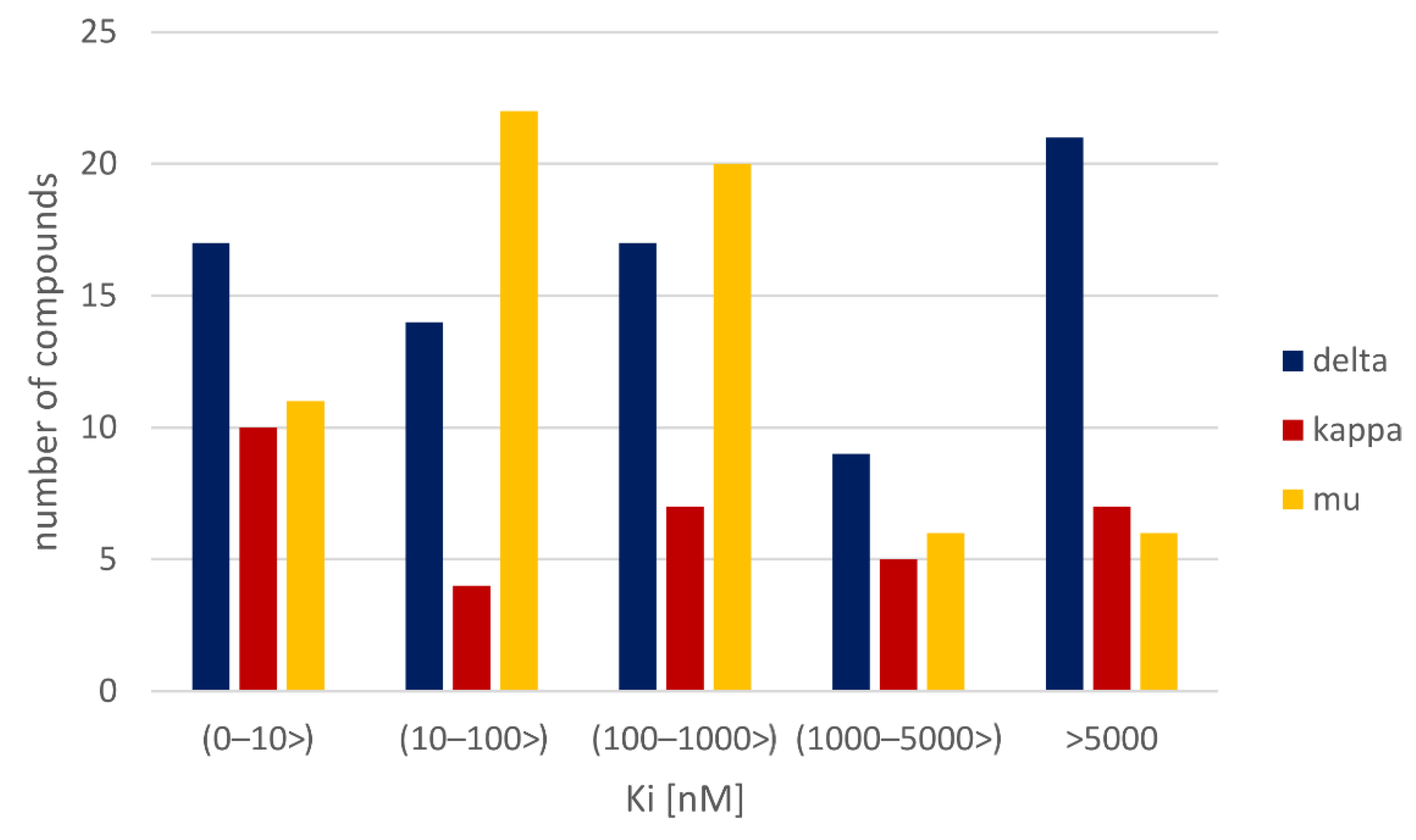

| Target | PDB ID | Resolution (Å) | Co-Crystallized Ligand Type | Receptor State |
|---|---|---|---|---|
| Mu opioid receptor | 4DKL | 2.8 | Antagonist | Inactive |
| Delta opioid receptor | 4RWD | 2.7 | Antagonist | Inactive |
| Kappa opioid receptor | 6B73 | 3.1 | Agonist | Active |
| Target | Total Number of Common Compounds with the Highest Error | Number of Common Compounds Belonging to the Set of Active Molecules (Fraction of All Common) | Number of Common Compounds Belonging to the Set of Inactive Molecules (Fraction of All Common) |
|---|---|---|---|
| Delta opioid receptor | 82 | 31 (38%) | 31 (38%) |
| Kappa opioid receptor | 35 | 14 (40%) | 12 (34%) |
| Mu opioid receptor | 71 | 37 (52%) | 13 (18%) |
| ChEMBL ID | Chemical Structure | Ki (nM) | Docking Score | Tanimoto Coefficient towards ChEMBL358043 |
|---|---|---|---|---|
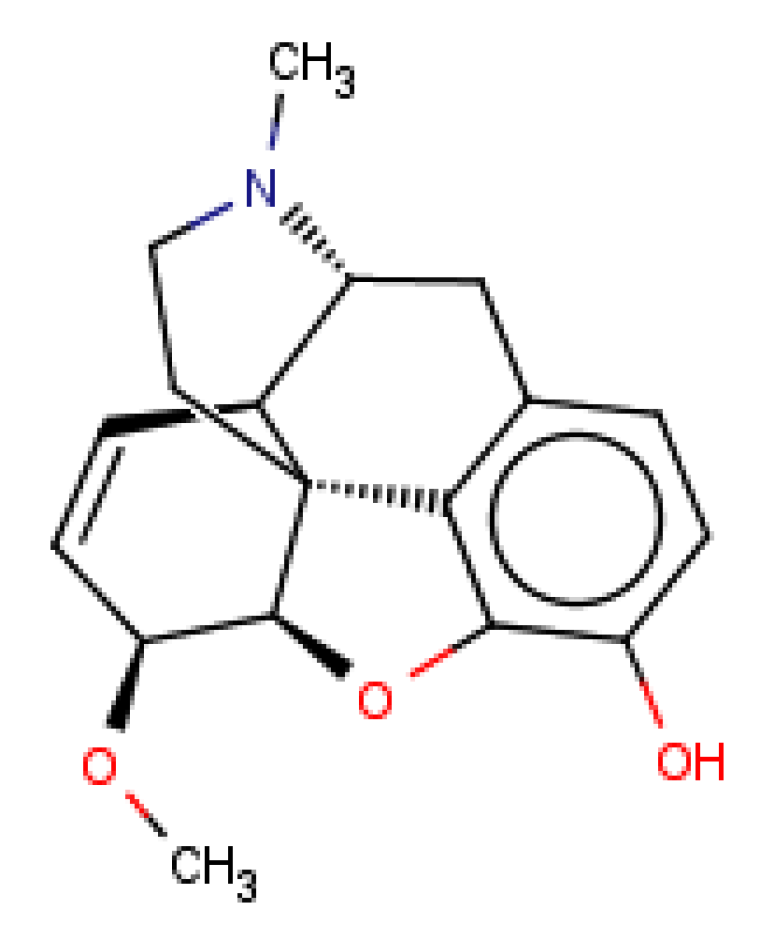
| CHEMBL3923831 | 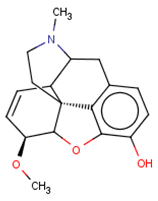 | 4297 | −5.23 | 1.0 |
| CHEMBL412301 | 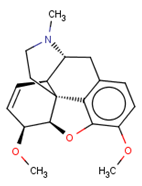 | 4410 | −3.93 | 0.996 |
| CHEMBL409938 |  | 11 | −4.14 | 0.988 |
| CHEMBL369475 | 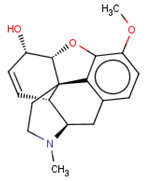 | 5000 | −5.16 | 0.984 |
| CHEMBL485 | 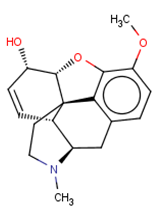 | 28,438 | −4.13 | 0.984 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podlewska, S.; Kurczab, R. Mutual Support of Ligand- and Structure-Based Approaches—To What Extent We Can Optimize the Power of Predictive Model? Case Study of Opioid Receptors. Molecules 2021, 26, 1607. https://doi.org/10.3390/molecules26061607
Podlewska S, Kurczab R. Mutual Support of Ligand- and Structure-Based Approaches—To What Extent We Can Optimize the Power of Predictive Model? Case Study of Opioid Receptors. Molecules. 2021; 26(6):1607. https://doi.org/10.3390/molecules26061607
Chicago/Turabian StylePodlewska, Sabina, and Rafał Kurczab. 2021. "Mutual Support of Ligand- and Structure-Based Approaches—To What Extent We Can Optimize the Power of Predictive Model? Case Study of Opioid Receptors" Molecules 26, no. 6: 1607. https://doi.org/10.3390/molecules26061607
APA StylePodlewska, S., & Kurczab, R. (2021). Mutual Support of Ligand- and Structure-Based Approaches—To What Extent We Can Optimize the Power of Predictive Model? Case Study of Opioid Receptors. Molecules, 26(6), 1607. https://doi.org/10.3390/molecules26061607







