Atmospheric Pressure Plasma Surface Treatment of Polymers and Influence on Cell Cultivation
Abstract
:1. Introduction
2. Atmospheric Pressure Plasma Technology
2.1. Plasma Generation
- Local thermodynamic equilibrium (LTE) (or thermal) plasma, and
- Non-local thermodynamic equilibrium (non-LET) (or non-thermal) plasma.
2.2. Classification
- DC and low-frequency discharges such as corona discharges and dielectric barrier discharges,
- Radiofrequency discharges such as atmospheric pressure plasma jets, and
- Microwave induced plasmas.
- A microwave power source;
- microwave equipment for example waveguide;
- an ignition system; and
- gas injection system. As shown in Figure 3.
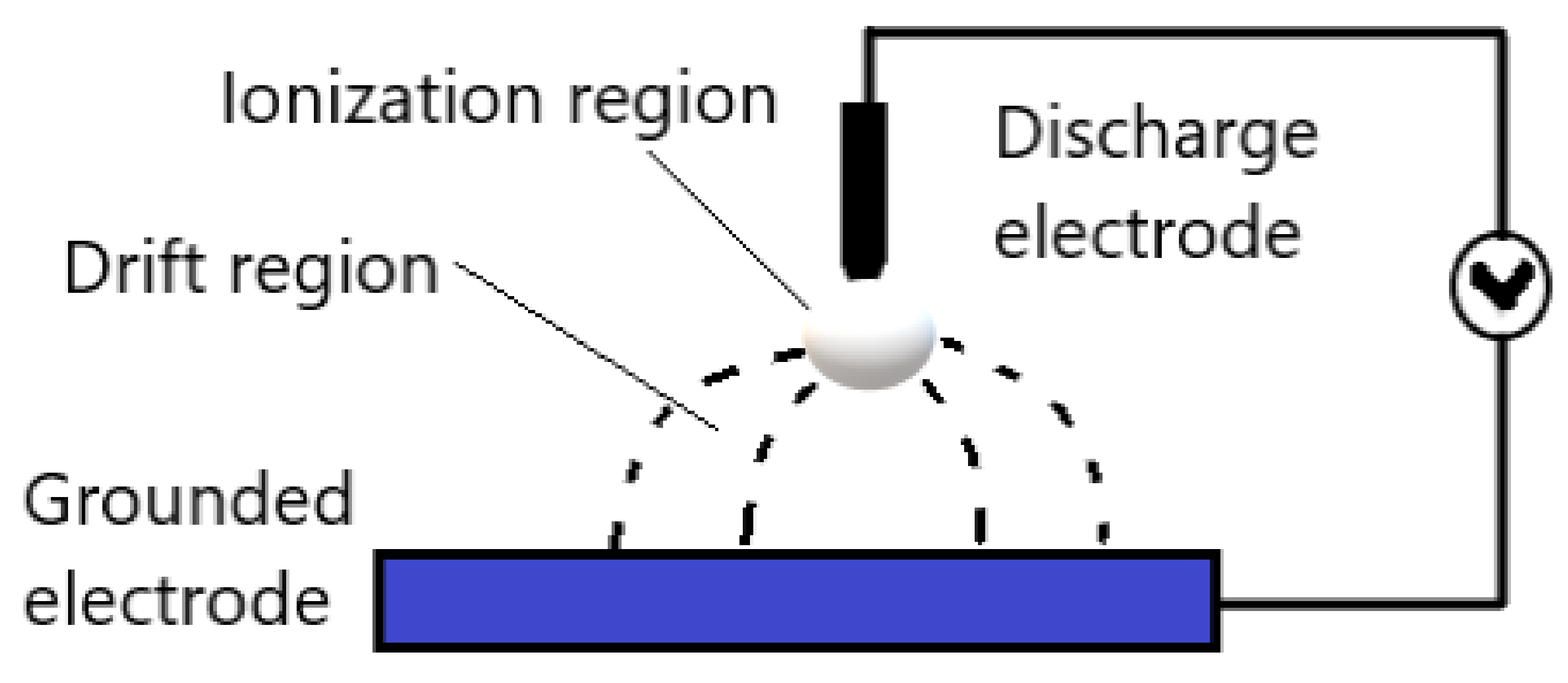
2.2.1. Corona Discharges
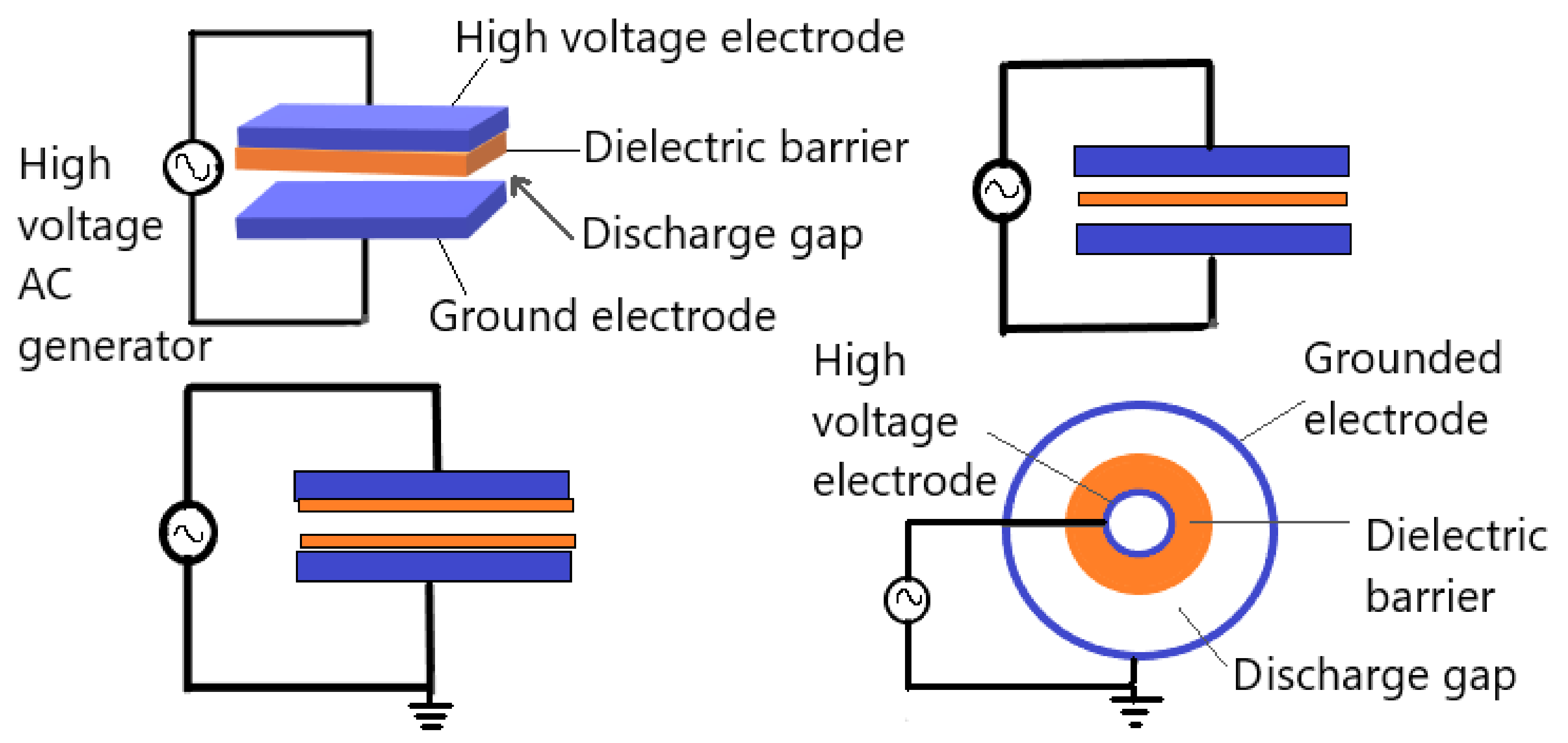
2.2.2. Dielectric Barrier Discharges
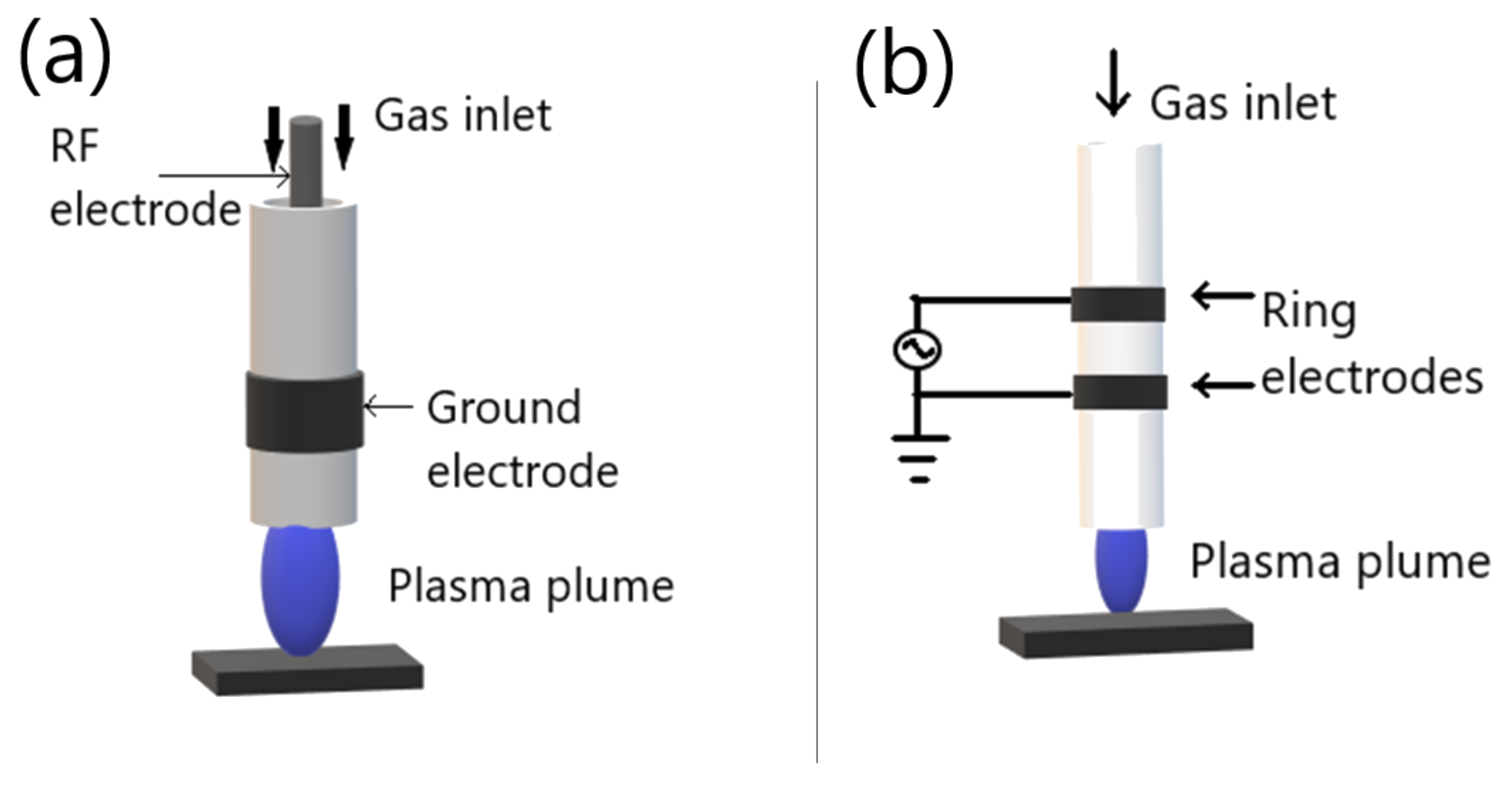
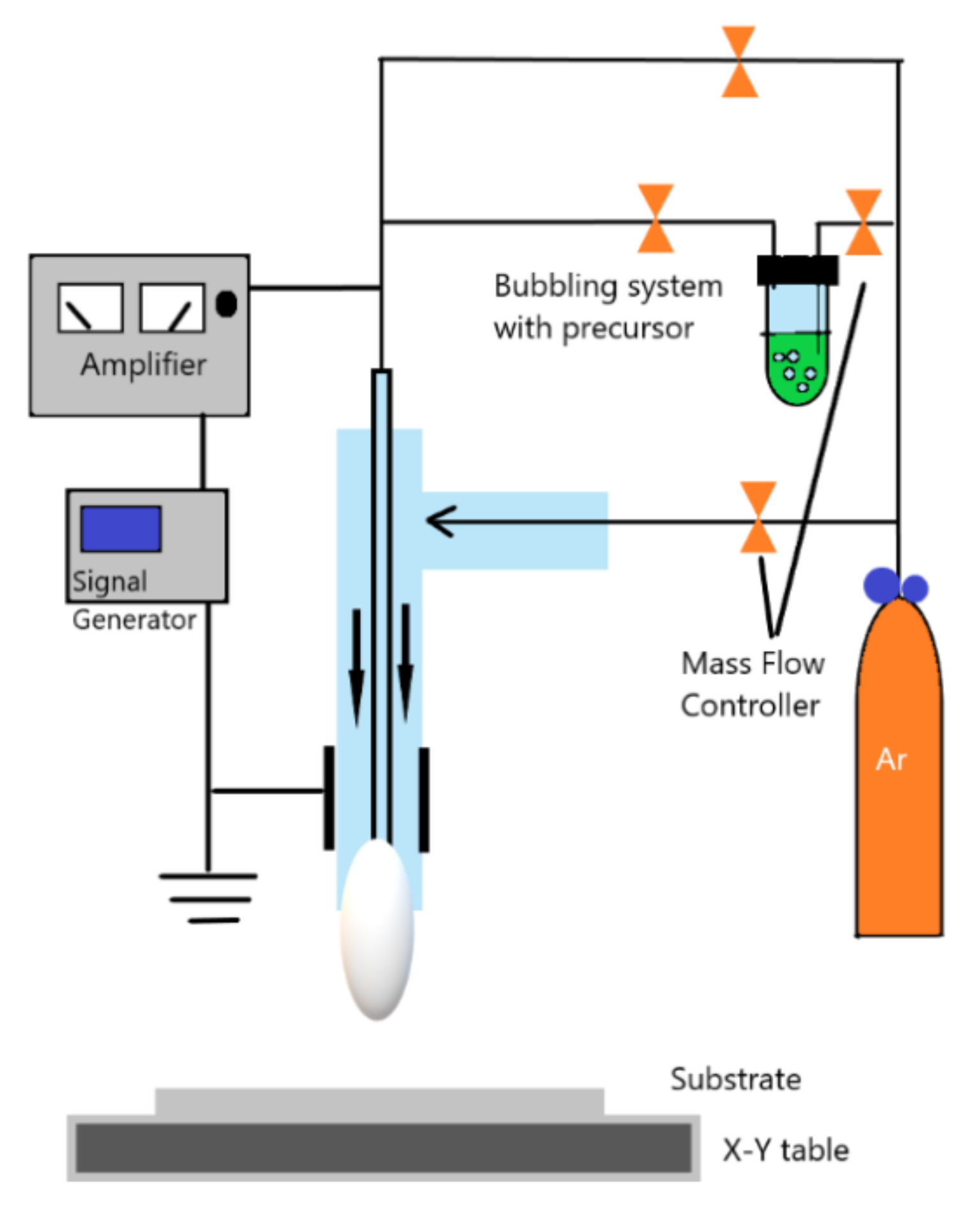
2.2.3. Atmospheric Pressure Plasma Jets
2.3. Active Species
- Oxidizing gases such as O2, air, N2O, and H2O;
- Reducing gases such as H2;
- Nitrogen containing gases such as NH3,
- Fluorine containing gases such as CF4 and SF6, and
- Polymerizing gases which use monomer gases to direct polymerize or graft a layer onto a substrate.
3. Modification of Polymeric Surfaces by Atmospheric Pressure Plasma
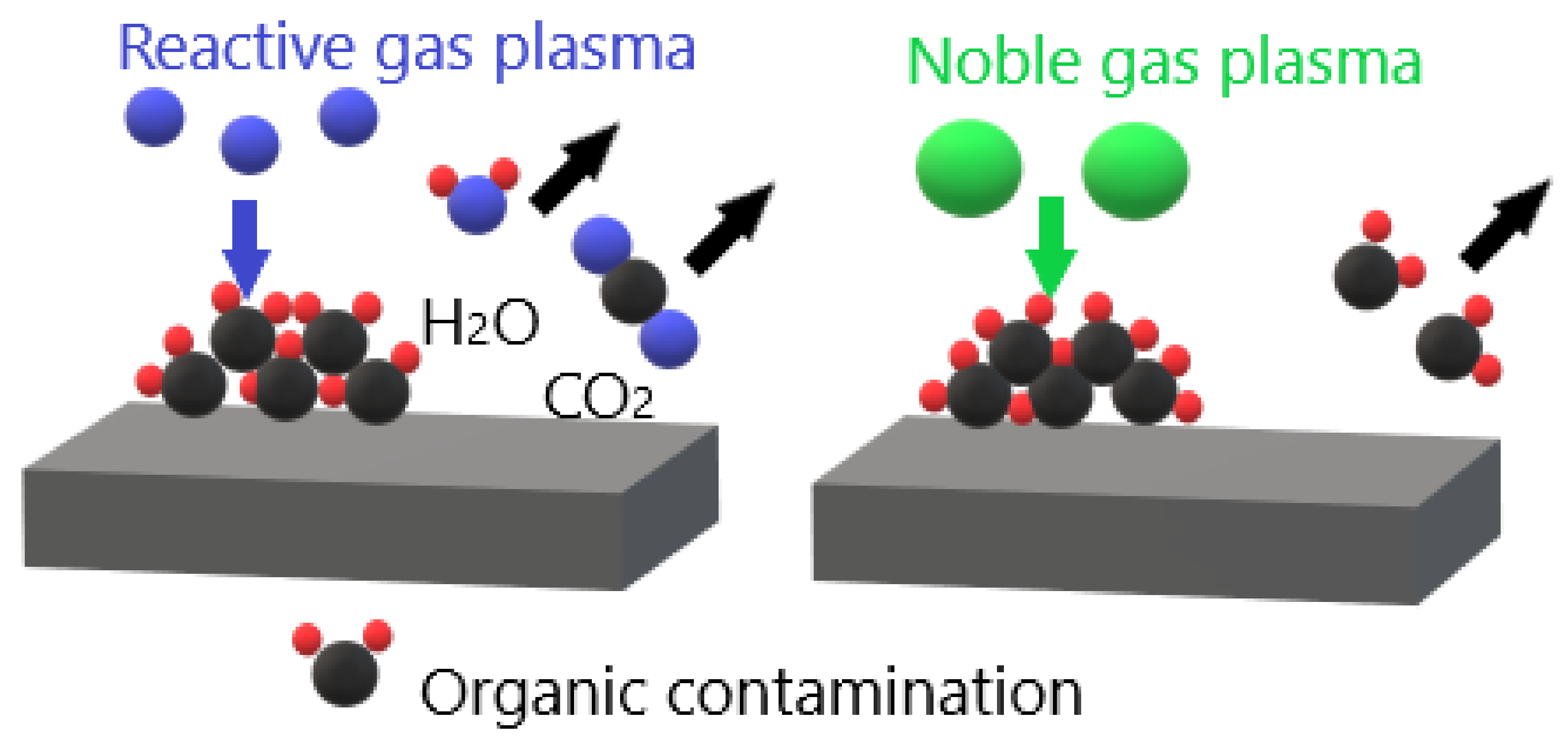
3.1. Removal of Surface Contamination
3.2. Etching
3.3. Substitution of Functional Group
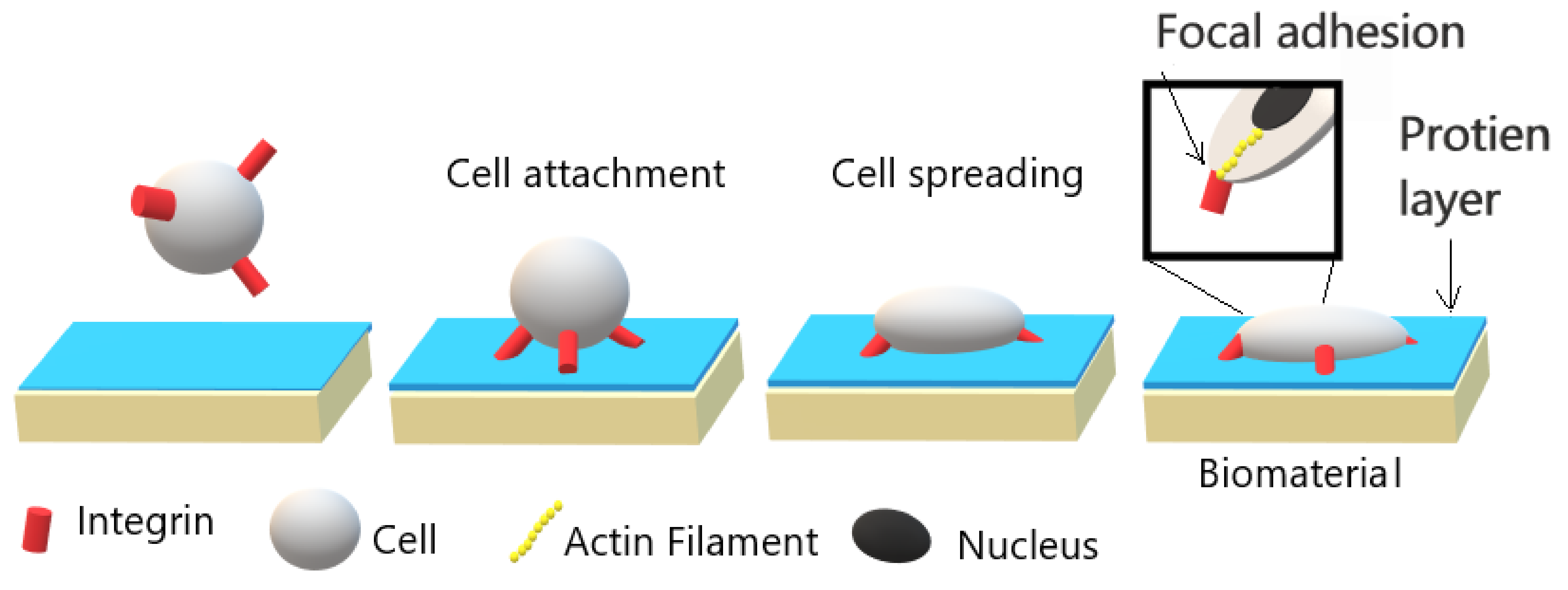
4. Cell Cultivation Verification of Atmospheric Pressure Plasma Treatment
4.1. Fibroblasts
4.2. Osteoblast and Osteosarcoma
4.3. Endothelial
4.4. Other Cell Lines
5. Challenges and Future Perspective
6. Conclusions
- Polymers poor surface properties limit their utilization in medical applications. Over the last decade, extensive effort has been made in polymer surface modification techniques. One of the promising surface modification techniques is atmospheric pressure plasma treatment.
- Atmospheric plasma treatment has significant benefits in comparison with traditional surface modification techniques including wet chemistry technique. For instance, short treatment duration, cost-effectiveness due to avoiding the cost of vacuum equipment, and simplicity are advantages of the atmospheric pressure plasma surface modification technique. Furthermore, the high density of reactive species in this technique, which enhances the formation of functional groups on polymer surface, such as carbonyl group -C=O, carboxyl group -C-O-OH, and hydroxyl group -C-OH, enhance cell-polymer interaction.
- Cell-polymer interaction depends on surface topography, wettability, and cleanliness besides chemical properties.
- As indicated in the review, that atmospheric pressure plasma surface modification technique of polymers has a satisfactory effect on enhancing cell-polymer interaction. Different types of cell lines exhibit enhancement in cell viability, adhesion, and proliferation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue Engineering: Strategies, Stem Cells and Scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pinson, J.; Thiry, D. Surface Modification of Polymers: Methods and Applications; John Wiley & Sons: Weinheim, Germany, 2020; ISBN 978-3-527-34541-0. [Google Scholar]
- Surucu, S.; Turkoglu Sasmazel, H. Development of Core-Shell Coaxially Electrospun Composite PCL/Chitosan Scaffolds. Int. J. Biol. Macromol. 2016, 92, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Primc, G. Recent Advances in Surface Activation of Polytetrafluoroethylene (PTFE) by Gaseous Plasma Treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef]
- García, A.J. Get a Grip: Integrins in Cell–Biomaterial Interactions. Biomaterials 2005, 26, 7525–7529. [Google Scholar] [CrossRef]
- Ferreira, P.; Alves, P.; Coimbra, P.; Gil, M.H. Improving Polymeric Surfaces for Biomedical Applications: A Review. J. Coat. Technol. Res. 2015, 12, 463–475. [Google Scholar] [CrossRef]
- Horbett, T.A.; Latour, R.A. 2.1.2—Adsorbed Proteins on Biomaterials. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Massachusetts, MA, USA, 2020; pp. 645–660. ISBN 978-0-12-816137-1. [Google Scholar]
- Jaganathan, S.K.; Balaji, A.; Vellayappan, M.V.; Subramanian, A.P.; John, A.A.; Asokan, M.K.; Supriyanto, E. Review: Radiation-Induced Surface Modification of Polymers for Biomaterial Application. J. Mater. Sci. 2015, 50, 2007–2018. [Google Scholar] [CrossRef]
- Suggs, A.E. Kr-F Laser Surface Treatment of Poly(Methyl Methacrylate), Glycol-Modified Poly(Ethylene Terephthalate), and Polytetrafluoroethylene for Enhanced Adhesion of Escherichia Coli K-12. Master’s Thesis, Virginia Tech, Blacksburg, WV, USA, 2002. [Google Scholar]
- Olbrich, M.; Punshon, G.; Frischauf, I.; Salacinski, H.J.; Rebollar, E.; Romanin, C.; Seifalian, A.M.; Heitz, J. UV Surface Modification of a New Nanocomposite Polymer to Improve Cytocompatibility. J. Biomater. Sci. Polym. Ed. 2007, 18, 453–468. [Google Scholar] [CrossRef]
- Švorčík, V.; Makajová, Z.; Slepičková Kasálková, N.; Kolská, Z.; Žáková, P.; Karpíšková, J.; Stibor, I.; Slepička, P. Cytocompatibility of Polymers Grafted by Activated Carbon Nano-Particles. Carbon 2014, 69, 361–371. [Google Scholar] [CrossRef]
- Sharma, R.; Sims, R.A.; Mazumder, M.K. Modification of Surface Properties of Polymeric Materials. J. Ark. Acad. Sci. 2002, 56, 6. [Google Scholar]
- Xu, W.; Liu, X. Surface Modification of Polyester Fabric by Corona Discharge Irradiation. Eur. Polym. J. 2003, 39, 199–202. [Google Scholar] [CrossRef]
- Liu, C.; Cui, N.; Brown, N.M.D.; Meenan, B.J. Effects of DBD Plasma Operating Parameters on the Polymer Surface Modification. Surf. Coat. Technol. 2004, 185, 311–320. [Google Scholar] [CrossRef]
- Walsh, J.L.; Iza, F.; Janson, N.B.; Law, V.J.; Kong, M.G. Three Distinct Modes in a Cold Atmospheric Pressure Plasma Jet. J. Phys. Appl. Phys. 2010, 43, 075201. [Google Scholar] [CrossRef]
- Dolci, L.S.; Quiroga, S.D.; Gherardi, M.; Laurita, R.; Liguori, A.; Sanibondi, P.; Fiorani, A.; Calzà, L.; Colombo, V.; Focarete, M.L. Carboxyl Surface Functionalization of Poly(L-Lactic Acid) Electrospun Nanofibers through Atmospheric Non-Thermal Plasma Affects Fibroblast Morphology. Plasma Process. Polym. 2014, 11, 203–213. [Google Scholar] [CrossRef]
- Yildirim, E.D.; Ayan, H.; Vasilets, V.N.; Fridman, A.; Guceri, S.; Sun, W. Effect of Dielectric Barrier Discharge Plasma on the Attachment and Proliferation of Osteoblasts Cultured over Poly(ε-Caprolactone) Scaffolds. Plasma Process. Polym. 2008, 5, 58–66. [Google Scholar] [CrossRef]
- Benedikt, J. Plasma-Chemical Reactions: Low Pressure Acetylene Plasmas. J. Phys. Appl. Phys. 2010, 43, 043001. [Google Scholar] [CrossRef]
- Piel, A. Plasma Physics: An Introduction to Laboratory, Space, and Fusion Plasmas; Springer: Berline, Germany, 2017; ISBN 978-3-319-63427-2. [Google Scholar]
- Vitchuli Gangadharan, N. Atmospheric Pressure Plasma-Electrospin Hybrid Process for Protective Applications. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2011. [Google Scholar]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.-D. Low Temperature Atmospheric Pressure Plasma Sources for Microbial Decontamination. J. Phys. Appl. Phys. 2010, 44, 013002. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Reuter, S.; Laroussi, M.; Liu, D. Nonequilibrium Atmospheric Pressure Plasma Jets: Fundamentals, Diagnostics, and Medical Applications; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-62287-8. [Google Scholar]
- Razzak, M.A.; Takamura, S.; Uesugi, Y. Effects of Radio-Frequency Driving Power, Gas Pressure, and Nitrogen Seeding on the Transition Dynamics in Argon Inductively Coupled Plasmas. J. Appl. Phys. 2004, 96, 4771–4776. [Google Scholar] [CrossRef] [Green Version]
- Çınar, K. Design and Construction of a Microwave Plasma Ion Source. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2011. [Google Scholar]
- Mizeraczyk, J.; Jasiński, M.; Zakrzewski, Z. Hazardous Gas Treatment Using Atmospheric Pressure Microwave Discharges. Plasma Phys. Control. Fusion 2005, 47, B589. [Google Scholar] [CrossRef]
- Cogollo, M.; Balsalobre, M.P.; Diaz Lantada, A.; Puago, H. Design and Experimental Evaluation of Innovative Wire-to-Plane Fins’ Configuration for Atmosphere Corona-Discharge Cooling Devices. Appl. Sci. 2020, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Riba, J.-R.; Morosini, A.; Capelli, F. Comparative Study of AC and Positive and Negative DC Visual Corona for Sphere-Plane Gaps in Atmospheric Air. Energies 2018, 11, 2671. [Google Scholar] [CrossRef] [Green Version]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric Pressure Plasmas: A Review. Spectrochim. Acta B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Dobslaw, C.; Glocker, B. Plasma Technology and Its Relevance in Waste Air and Waste Gas Treatment. Sustainability 2020, 12, 8981. [Google Scholar] [CrossRef]
- Popelka, A.; Khanam, P.N.; AlMa’adeed, M. Surface Modification of Polyethylene/Graphene Composite Using Corona Discharge. J. Phys. Appl. Phys. 2018, 51. [Google Scholar] [CrossRef]
- Pothanamkandathil, V.; Singh, R.; Philip, L.; Ramanujam, S. Effect of Recycling Overhead Gases on Pollutants Degradation Efficiency in Gas-Phase Pulsed Corona Discharge Treatment. J. Environ. Chem. Eng. 2018, 6, 923–929. [Google Scholar] [CrossRef]
- Ajo, P.; Kornev, I.; Preis, S. Pulsed Corona Discharge in Water Treatment: The Effect of Hydrodynamic Conditions on Oxidation Energy Efficiency. Ind. Eng. Chem. Res. 2015, 54, 7452–7458. [Google Scholar] [CrossRef]
- Pontiga, F.; Hadji, K.; Guemou, M.; Yanallah, K.; Fernández-Rueda, A.; Moreno, H. Ozone Production by Corona Discharge Using Ahollow Needle-Plate Electrode System. In Proceedings of the 20th International Conference on Gas Discharges and their Application, Orleans, France, 6–11 July 2014. [Google Scholar]
- Bussiahn, R.; Brandenburg, R.; Gerling, T.; Kindel, E.; Lange, H.; Lembke, N.; Weltmann, K.-D.; von Woedtke, T.; Kocher, T. The Hairline Plasma: An Intermittent Negative Dc-Corona Discharge at Atmospheric Pressure for Plasma Medical Applications. Appl. Phys. Lett. 2010, 96, 143701. [Google Scholar] [CrossRef]
- Rotan, M.; Zhuk, M.; Glaum, J. Activation of Ferroelectric Implant Ceramics by Corona Discharge Poling. J. Eur. Ceram. Soc. 2020, 40, 5402–5409. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric Barrier Discharges: Progress on Plasma Sources and on the Understanding of Regimes and Single Filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-Barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Subedi, D.P.; Joshi, U.M.; Wong, C.S. Dielectric Barrier Discharge (DBD) Plasmas and Their Applications. In Plasma Science and Technology for Emerging Economies: An AAAPT Experience; Rawat, R.S., Ed.; Springer: Singapore, Singapore, 2017; pp. 693–737. ISBN 978-981-10-4217-1. [Google Scholar]
- Xu, X. Dielectric Barrier Discharge—Properties and Applications. Thin Solid Films 2001, 390, 237–242. [Google Scholar] [CrossRef]
- Massines, F.; Gherardi, N.; Naudé, N.; Ségur, P. Recent Advances in the Understanding of Homogeneous Dielectric Barrier Discharges. Eur. Phys. J. Appl. Phys. 2009, 47, 22805. [Google Scholar] [CrossRef] [Green Version]
- Ráhel\textquotesingle, J.; Sherman, D.M. The Transition from a Filamentary Dielectric Barrier Discharge to a Diffuse Barrier Discharge in Air at Atmospheric Pressure. J. Phys. Appl. Phys. 2005, 38, 547–554. [Google Scholar] [CrossRef]
- Liu, C.; Park, D.; Friedman, G.; Fridman, A.; Dobrynin, D. Uniform Dielectric Barrier Discharge Generation in Atmospheric Air Using Nano- Second Pulses: Fast Imaging, Spectroscopy, and Production of Active Species. In Proceedings of the 21st International Symposium on Plasma Chemistry (ISPC 21), Queensland, Australia, 4–9 August 2013. [Google Scholar]
- Borin, D.; Sbaizero, O.; Scuor, N. Application of a Dielectric Barrier Discharge Plasma for Heating Plastic Materials. Plasma Res. Express 2019, 1, 025009. [Google Scholar] [CrossRef]
- Wagner, H.-E.; Brandenburg, R.; Kozlov, K.V.; Sonnenfeld, A.; Michel, P.; Behnke, J.F. The Barrier Discharge: Basic Properties and Applications to Surface Treatment. Vacuum 2003, 71, 417–436. [Google Scholar] [CrossRef] [Green Version]
- Ghomi, H.; Navab Safa, N.; Ghasemi, S. Investigation on a DBD Plasma Reactor. IEEE Trans. Plasma Sci. 2011, 39, 2104–2105. [Google Scholar] [CrossRef]
- Feizollahi, E.; Misra, N.N.; Roopesh, M.S. Factors Influencing the Antimicrobial Efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in Food Processing Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Martines, E. Special Issue “Plasma Technology for Biomedical Applications”. Appl. Sci. 2020, 10, 1524. [Google Scholar] [CrossRef] [Green Version]
- Sohbatzadeh, F.; Samadi, O.; Siadati, S.; Etaati, G.; Asadi, E.; Safari, R. Development of a Radio Frequency Atmospheric Pressure Plasma Jet for Diamond-like Carbon Coatings on Stainless Steel Substrates. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.-D. Atmospheric Pressure Plasma Jets: An Overview of Devices and New Directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Fanelli, F.; Fracassi, F. Atmospheric Pressure Non-Equilibrium Plasma Jet Technology: General Features, Specificities and Applications in Surface Processing of Materials. Surf. Coat. Technol. 2017, 322, 174–201. [Google Scholar] [CrossRef]
- Gomathi, N.; Chanda, A.K.; Neogi, S. Atmospheric Plasma Treatment of Polymers for Biomedical Applications. In Atmospheric Pressure Plasma Treatment of Polymers; John Wiley & Sons: Weinheim, Germany, 2013; pp. 199–215. ISBN 978-1-118-74730-8. [Google Scholar]
- Penkov, O.V.; Khadem, M.; Lim, W.-S.; Kim, D.-E. A Review of Recent Applications of Atmospheric Pressure Plasma Jets for Materials Processing. J. Coat. Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, P.; Pinto, S.; Ferreira, P.; Kaiser, J.-P.; Bruinink, A.; de Sousa, H.C.; Gil, M.H. Improving Cell Adhesion: Development of a Biosensor for Cell Behaviour Monitoring by Surface Grafting of Sulfonic Groups onto a Thermoplastic Polyurethane. J. Mater. Sci. Mater. Med. 2014, 25, 2017–2026. [Google Scholar] [CrossRef] [Green Version]
- Ebnesajjad, S. Chapter 9—Plasma Treatment of Polymeric Materials. In Surface Treatment of Materials for Adhesive Bonding, 2nd ed.; Ebnesajjad, S., Ed.; William Andrew Publishing: Oxford, NY, USA, 2014; pp. 227–269. ISBN 978-0-323-26435-8. [Google Scholar]
- Gomathi, N.; Sureshkumar, A.; Neogi, S. RF Plasma-Treated Polymers for Biomedical Applications. Curr. Sci. 2008, 94, 1478–1486. [Google Scholar]
- Calori, I.R.; Braga, G.; de Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Chapman, C.A.R.; Cuttaz, E.A.; Goding, J.A.; Green, R.A. Actively Controlled Local Drug Delivery Using Conductive Polymer-Based Devices. Appl. Phys. Lett. 2020, 116, 010501. [Google Scholar] [CrossRef] [Green Version]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable Polymer Nanocomposites for Ligament/Tendon Tissue Engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, O.; Sasmazel, H.T. Antibacterial Performance of PCL-Chitosan Core-Shell Scaffolds. J. Nanosci. Nanotechnol. 2018, 18, 2415–2421. [Google Scholar] [CrossRef]
- Cusola, O.; Tabary, N.; Belgacem, M.N.; Bras, J. Cyclodextrin Functionalization of Several Cellulosic Substrates for Prolonged Release of Antibacterial Agents. J. Appl. Polym. Sci. 2013, 129, 604–613. [Google Scholar] [CrossRef]
- Nawalakhe, R.; Shi, Q.; Vitchuli, N.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.A.; Zhang, X.; McCord, M.G. Novel Atmospheric Plasma Enhanced Chitosan Nanofiber/Gauze Composite Wound Dressings. J. Appl. Polym. Sci. 2013, 129, 916–923. [Google Scholar] [CrossRef]
- Sengupta, P.; Ghosh, A.; Bose, N.; Mukherjee, S.; Chowdhury, A.R.; Datta, P. A Comparative Assessment of Poly(Vinylidene Fluoride)/Conducting Polymer Electrospun Nanofiber Membranes for Biomedical Applications. J. Appl. Polym. Sci. 2020, 137, 49115. [Google Scholar] [CrossRef]
- Dubey, N.; Kushwaha, C.S.; Shukla, S.K. A Review on Electrically Conducting Polymer Bionanocomposites for Biomedical and Other Applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 709–727. [Google Scholar] [CrossRef]
- Gilliam, M. Polymer Surface Treatment and Coating Technologies. In Handbook of Manufacturing Engineering and Technology; Springer: London, UK, 2015; pp. 99–124. ISBN 978-1-4471-4669-8. [Google Scholar]
- Bowlin, G. Encyclopedia of Biomaterials and Biomedical Engineering; CRC Press: Boca Rotan, FL, USA, 2008; ISBN 978-0-429-15406-5. [Google Scholar]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y. Atmospheric Pressure Plasma Processing for Polymer Adhesion: A Review. J. Adhes. 2014, 90. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface Modification of Polymers by Plasma Treatments for the Enhancement of Biocompatibility and Controlled Drug Release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Khelifa, F.; Ershov, S.; Habibi, Y.; Snyders, R.; Dubois, P. Free-Radical-Induced Grafting from Plasma Polymer Surfaces. Chem. Rev. 2016, 116, 3975–4005. [Google Scholar] [CrossRef]
- Gozutok, M.; Basar, A.O.; Sasmazel, H.T. Development of Antibacterial Composite Electrospun Chitosan-Coated Polypropylene Materials. J. Nanosci. Nanotechnol. 2018, 18, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Fricke, K.; Koban, I.; Tresp, H.; Jablonowski, L.; Schröder, K.; Kramer, A.; Weltmann, K.-D.; von Woedtke, T.; Kocher, T. Atmospheric Pressure Plasma: A High-Performance Tool for the Efficient Removal of Biofilms. PLoS ONE 2012, 7, e42539. [Google Scholar] [CrossRef] [PubMed]
- Puliyalil, H.; Cvelbar, U. Selective Plasma Etching of Polymeric Substrates for Advanced Applications. Nanomaterials 2016, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Kim, S. Organic Contaminants Removal by Oxygen ECR Plasma. Appl. Surf. Sci. 2007, 253, 3658–3663. [Google Scholar] [CrossRef]
- Abourayana, H.M.; Dowling, D.P. Plasma Processing for Tailoring the Surface Properties of Polymers. Surf. Energy 2015. [Google Scholar] [CrossRef] [Green Version]
- Dimitrakellis, P.; Gogolides, E. Atmospheric Plasma Etching of Polymers: A Palette of Applications in Cleaning/Ashing, Pattern Formation, Nanotexturing and Superhydrophobic Surface Fabrication. Microelectron. Eng. 2018, 194, 109–115. [Google Scholar] [CrossRef]
- Siegel, J.; Řezníčková, A.; Chaloupka, A.; Slepička, P.; Švorčík, V. Ablation and Water Etching of Plasma-Treated Polymers. Radiat. Eff. Defects Solids 2008, 163, 779–788. [Google Scholar] [CrossRef]
- Fricke, K.; Steffen, H.; von Woedtke, T.; Schröder, K.; Weltmann, K.-D. High Rate Etching of Polymers by Means of an Atmospheric Pressure Plasma Jet. Plasma Process. Polym. 2011, 8, 51–58. [Google Scholar] [CrossRef]
- Spence, T.B.; Pellegrino, J.; Ricci, J.L.; Coelho, P.G. The Influence of Atmospheric Pressure Plasma Surface-Modified Polymers PVDF, ECTFE, and PEEK on Primary Mesenchymal Stem Cell Response. In Proceedings of the 72nd annual Technical Conference of the Society of Plastics Engineers, Las Vegas, NV, USA, 28–30 April 2014. [Google Scholar]
- Gonzalez-Simon, A.; Eniola-Adefeso, L. Host Response to Biomaterials. In Engineering Biomaterials for Regenerative Medicine; Springer: New York, NY, USA, 2012; pp. 143–159. ISBN 978-1-4614-1079-9. [Google Scholar]
- Tagaya, M. In Situ QCM-D Study of Nano-Bio Interfaces with Enhanced Biocompatibility. Polym. J. 2015, 47, 599–608. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wang, D.; Namihira, T.; Akiyama, H. Non-Thermal Plasma Technic for Air Pollution Control. Air Pollut.—Compr. Perspect. 2012. [Google Scholar] [CrossRef] [Green Version]
- Morent, R.; Geyter, N.D.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Aiyelabegan, H.T.; Sadroddiny, E. Fundamentals of Protein and Cell Interactions in Biomaterials. Biomed. Pharmacother. 2017, 88, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone Biomaterials and Interactions with Stem Cells. Bone Res. 2017, 5, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Biomaterials That Promote Cell-Cell Interactions Enhance the Paracrine Function of MSCs. Biomaterials 2017, 140, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.; Cillero Pastor, B.; van Rijt, S.; Habibovic, P. Understanding Interactions between Biomaterials and Biological Systems Using Proteomics. Biomaterials 2018, 167, 191–204. [Google Scholar] [CrossRef]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef] [Green Version]
- Khandwekar, A.P.; Patil, D.P.; Shouche, Y.S.; Doble, M. The Biocompatibility of Sulfobetaine Engineered Polymethylmethacrylate by Surface Entrapment Technique. J. Mater. Sci. Mater. Med. 2010, 21, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hipler, U.-C. Evaluation of Biocompatibility and Cytotoxicity Using Keratinocyte and Fibroblast Cultures. Skin Pharmacol. Physiol. 2009, 22, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu Sasmazel, H.; Aday Aydin, S.; Manolache, S.; Gumusderelioglu, M. Influence of Water/O2 Plasma Treatment on Cellular Responses of PCL and PET Surfaces. Biomed. Mater. Eng. 2011, 21, 191. [Google Scholar] [CrossRef]
- Gozutok, M.; Baitukha, A.; Arefi-Khonsari, F.; Sasmazel, H.T. Novel Thin Films Deposited on Electrospun PCL Scaffolds by Atmospheric Pressure Plasma Jet for L929 Fibroblast Cell Cultivation. J. Phys. Appl. Phys. 2016, 49, 474002. [Google Scholar] [CrossRef]
- Surucu, S.; Sasmazel, H.T. DBD Atmospheric Plasma-Modified, Electrospun, Layer-by-Layer Polymeric Scaffolds for L929 Fibroblast Cell Cultivation. J. Biomater. Sci. Polym. Ed. 2016, 27, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, O.; Turkoglu Sasmazel, H. Dielectric Barrier Discharge and Jet Type Plasma Surface Modifications of Hybrid Polymeric Poly (ε-Caprolactone)/Chitosan Scaffolds. J. Biomater. Appl. 2018, 32, 1300–1313. [Google Scholar] [CrossRef]
- Han, I.; Kwon, B.-J.; Vagaska, B.; Kim, B.-J.; Kang, J.K.; Lee, M.H.; Kim, H.H.; Park, J.-C.; Wang, K.-K.; Kim, Y.-R.; et al. Nitrogen Grafting onto Polycarprolactone by a Simple Surface Modification with Atmospheric Pressure Glow Discharge (Ar-APGD) and Promoted Neonatal Human Fibroblast Growth. Macromol. Res. 2011, 19, 1134. [Google Scholar] [CrossRef]
- Navaneetha Pandiyaraj, K.; Arun Kumar, A.; RamKumar, M.C.; Deshmukh, R.R.; Bendavid, A.; Su, P.-G.; Uday Kumar, S.; Gopinath, P. Effect of Cold Atmospheric Pressure Plasma Gas Composition on the Surface and Cyto-Compatible Properties of Low Density Polyethylene (LDPE) Films. Curr. Appl. Phys. 2016, 16, 784–792. [Google Scholar] [CrossRef]
- Salapare, H., III; Guittard, F.; Noblin, X.; Taffin de Givenchy, E.; Celestini, F.; Ramos, H. Stability of the Hydrophilic and Superhydrophobic Properties of Oxygen Plasma-Treated Poly(Tetrafluoroethylene) Surfaces. J. Colloid Interface Sci. 2013, 396, 287–292. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic Materials for Biomedical Applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Kasálková, N.S.; Slepička, P.; Kolská, Z.; Švorčík, V. Wettability and Other Surface Properties of Modified Polymers. Wetting Wettability 2015, 12, 323–356. [Google Scholar]
- Šrámková, P.; Zahoranová, A.; Kelar, J.; Kelar Tučeková, Z.; Stupavská, M.; Krumpolec, R.; Jurmanová, J.; Kováčik, D.; Černák, M. Cold Atmospheric Pressure Plasma: Simple and Efficient Strategy for Preparation of Poly(2-Oxazoline)-Based Coatings Designed for Biomedical Applications. Sci. Rep. 2020, 10, 9478. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kwon, J.-S.; Om, J.; Kim, Y.-H.; Choi, E.-H.; Kim, K.-M.; Kim, K.-N. Cell Immobilization on Polymer by Air Atmospheric Pressure Plasma Jet Treatment. Jpn. J. Appl. Phys. 2014, 53, 086202. [Google Scholar] [CrossRef]
- Basar, A.O.; Sadhu, V.; Turkoglu Sasmazel, H. Preparation of Electrospun PCL-Based Scaffolds by Mono/Multi-Functionalized GO. Biomed. Mater. Bristol Engl. 2019, 14, 045012. [Google Scholar] [CrossRef] [PubMed]
- Causa, F.; Netti, P.; Ambrosio, L.; Ciapetti, G.; Baldini, N.; Pagani, S.; Martini, D.; Giunti, A. Poly-??-Caprolactone/Hydroxyapatite Composites for Bone Regeneration: In Vitro Characterization and Human Osteoblast Response. J. Biomed. Mater. Res. A 2006, 76, 151–162. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Nair, L.; Laurencin, C. Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. Adv. Biochem. Eng. Biotechnol. 2006, 102, 47–90. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.; Lee, H.; Kim, G. A Surface-Modified Poly(ɛ-Caprolactone) Scaffold Comprising Variable Nanosized Surface-Roughness Using a Plasma Treatment. Tissue Eng. Part C Methods 2014, 20, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Tiaw, K.S.; Goh, S.W.; Hong, M.; Wang, Z.; Lan, B.; Teoh, S.H. Laser Surface Modification of Poly(Epsilon-Caprolactone) (PCL) Membrane for Tissue Engineering Applications. Biomaterials 2005, 26, 763–769. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the Biomimetic Behavior of Scaffolds for Regenerative Medicine through Surface Modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Trizio, I.; Intranuovo, F.; Gristina, R.; Dilecce, G.; Favia, P. He/O2 Atmospheric Pressure Plasma Jet Treatments of PCL Scaffolds for Tissue Engineering and Regenerative Medicine. Plasma Process. Polym. 2015, 12, 1451–1458. [Google Scholar] [CrossRef]
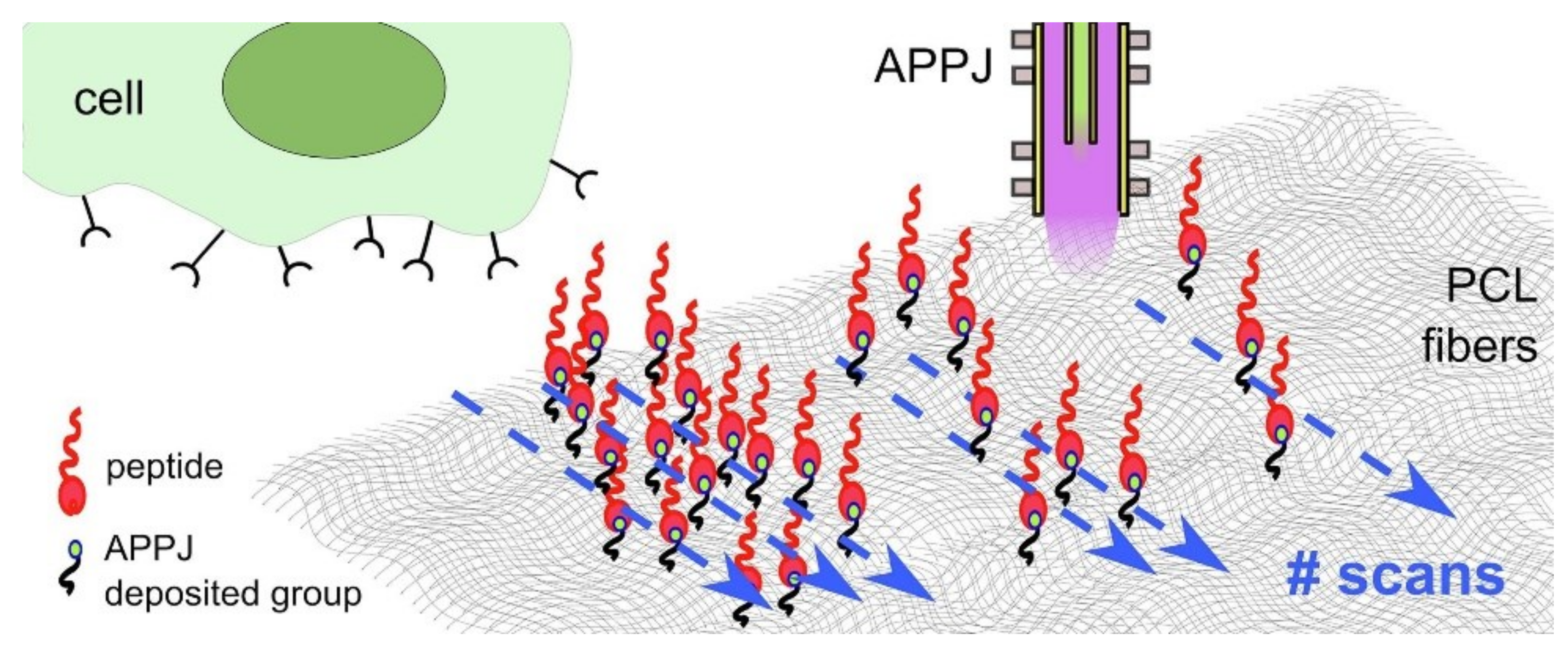
- Maffei, A.; Michieli, N.; Brun, P.; Zamuner, A.; Zaggia, A.; Roso, M.; Kalinic, B.; Verga Falzacappa, E.; Scopece, P.; Gross, S.; et al. An Atmospheric Pressure Plasma Jet to Tune the Bioactive Peptide Coupling to Polycaprolactone Electrospun Layers. Appl. Surf. Sci. 2020, 507, 144713. [Google Scholar] [CrossRef]
- Kung, F.-C.; Kuo, Y.-L.; Gunduz, O.; Lin, C.-C. Dual RGD-Immobilized Poly(L-Lactic Acid) by Atmospheric Pressure Plasma Jet for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2019, 178, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.M.; Yang, Y.; Marton, D.; Carnes, D.L.; Ong, J.L.; Sylvia, V.L.; Dean, D.D.; Reis, R.L.; Agrawal, C.M. Plasma Surface Modification of Poly(D,L-Lactic Acid) as a Tool to Enhance Protein Adsorption and the Attachment of Different Cell Types. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, M.; Teraoka, F.; Fujimoto, S.; Hamada, Y.; Kibayashi, H.; Takahashi, J. Improvement of Cell Adhesion on Poly(L-Lactide) by Atmospheric Plasma Treatment. J. Biomed. Mater. Res. A 2006, 77, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, F.; Nakagawa, M.; Hara, M. Surface Modification of Poly(L-Lactide) by Atmospheric Pressure Plasma Treatment and Cell Response. Dent. Mater. J. 2006, 25, 560–565. [Google Scholar] [CrossRef] [Green Version]
- Sastri, V.R. Plastics in Medical Devices: Properties, Requirements, and Applications, 2nd ed.; William Andrew: New York, NY, USA, 2013. [Google Scholar]
- Ho, B.; Roberts, T.; Lucas, S. An Overview on Biodegradation of Polystyrene and Modified Polystyrene: The Microbial Approach. Crit. Rev. Biotechnol. 2017, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ayan, H.; Yildirim, E.D.; Pappas, D.D.; Sun, W. Development of a Cold Atmospheric Pressure Microplasma Jet for Freeform Cell Printing. Appl. Phys. Lett. 2011, 99, 111502. [Google Scholar] [CrossRef]
- Perni, S.; Kong, M.G.; Prokopovich, P. Cold Atmospheric Pressure Gas Plasma Enhances the Wear Performance of Ultra-High Molecular Weight Polyethylene. Acta Biomater. 2012, 8, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of Surface Wettability and Topography on the Adhesion of Osteosarcoma Cells on Plasma-Modified Polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef]
- D’Sa, R.A.; Raj, J.; Dickinson, P.J.; McCabe, F.; Meenan, B.J. Human Fetal Osteoblast Response on Poly(Methyl Methacrylate)/Polystyrene Demixed Thin Film Blends: Surface Chemistry Vs Topography Effects. ACS Appl. Mater. Interfaces 2016, 8, 14920–14931. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.G.; Niklason, L.E. A Short Discourse on Vascular Tissue Engineering. Npj Regen. Med. 2017, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Challenges with the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertram, U.; Steiner, D.; Poppitz, B.; Dippold, D.; Köhn, K.; Beier, J.P.; Detsch, R.; Boccaccini, A.R.; Schubert, D.W.; Horch, R.E.; et al. Vascular Tissue Engineering: Effects of Integrating Collagen into a PCL Based Nanofiber Material. BioMed Res. Int. 2017, 2017, 9616939. [Google Scholar] [CrossRef]
- Ravi, S.; Chaikof, E.L. Biomaterials for Vascular Tissue Engineering. Regen. Med. 2010, 5, 107. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; He, W.; Yong, T.; Ramakrishna, S. Grafting of Gelatin on Electrospun Poly(Caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Eng. 2005, 11, 1149–1158. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Stankevich, K.; Gudima, A.; Kibler, E.; Zhukov, Y.; Bolbasov, E.; Malashicheva, A.; Zhuravlev, M.; Riabov, V.; Liu, T.; et al. Atmospheric Pressure Plasma Assisted Immobilization of Hyaluronic Acid on Tissue Engineering PLA-Based Scaffolds and Its Effect on Primary Human Macrophages. Mater. Des. 2017, 127, 261–271. [Google Scholar] [CrossRef]
- De, S.; Sharma, R.; Trigwell, S.; Laska, B.; Ali, N.; Mazumder, M.K.; Mehta, J.L. Plasma Treatment of Polyurethane Coating for Improving Endothelial Cell Growth and Adhesion. J. Biomater. Sci. Polym. Ed. 2005, 16, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Bilek, M.M.M.; Vandrovcová, M.; Shelemin, A.; Kuzminova, A.; Kylián, O.; Biederman, H.; Bačáková, L.; Weiss, A.S. Plasma Treatment in Air at Atmospheric Pressure That Enables Reagent-Free Covalent Immobilization of Biomolecules on Polytetrafluoroethylene (PTFE). Appl. Surf. Sci. 2020, 518, 146128. [Google Scholar] [CrossRef]
- Park, B.J.; Seo, H.J.; Kim, J.; Kim, H.-L.; Kim, J.K.; Choi, J.B.; Han, I.; Hyun, S.O.; Chung, K.-H.; Park, J.-C. Cellular Responses of Vascular Endothelial Cells on Surface Modified Polyurethane Films Grafted Electrospun PLGA Fiber with Microwave-Induced Plasma at Atmospheric Pressure. Surf. Coat. Technol. 2010, 205, S222–S226. [Google Scholar] [CrossRef]
- Lee, H.-U.; Jeong, Y.-S.; Jeong, S.-Y.; Park, S.-Y.; Bae, J.-S.; Kim, H.-G.; Cho, C.-R. Role of Reactive Gas in Atmospheric Plasma for Cell Attachment and Proliferation on Biocompatible Poly Ɛ-Caprolactone Film. Appl. Surf. Sci. 2008, 254, 5700–5705. [Google Scholar] [CrossRef]
- Alemi, P.S.; Atyabi, S.A.; Sharifi, F.; Mohamadali, M.; Irani, S.; Bakhshi, H.; Atyabi, S.M. Synergistic Effect of Pressure Cold Atmospheric Plasma and Carboxymethyl Chitosan to Mesenchymal Stem Cell Differentiation on PCL/CMC Nanofibers for Cartilage Tissue Engineering. Polym. Adv. Technol. 2019, 30, 1356–1364. [Google Scholar] [CrossRef]
- Fabbri, M.; Gigli, M.; Costa, M.; Govoni, M.; Seri, P.; Lotti, N.; Giordano, E.; Munari, A.; Gamberini, R.; Rimini, B.; et al. The Effect of Plasma Surface Modification on the Biodegradation Rate and Biocompatibility of a Poly(Butylene Succinate)-Based Copolymer. Polym. Degrad. Stab. 2015, 121, 271–279. [Google Scholar] [CrossRef]
- Reznickova, A.; Novotna, Z.; Kolska, Z.; Kasalkova, N.S.; Rimpelova, S.; Svorcik, V. Enhanced Adherence of Mouse Fibroblast and Vascular Cells to Plasma Modified Polyethylene. Mater. Sci. Eng. C 2015, 52, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-Y.; Chang, C.-H.; Yang, Y.-W.; Liao, G.-C.; Liu, C.-T.; Wu, J.-S. Enhancement of Cell Growth on Honeycomb-Structured Polylactide Surface Using Atmospheric-Pressure Plasma Jet Modification. Appl. Surf. Sci. 2017, 394, 534–542. [Google Scholar] [CrossRef]
- Kuzminova, A.; Vandrovcová, M.; Shelemin, A.; Kylián, O.; Choukourov, A.; Hanuš, J.; Bačáková, L.; Slavínská, D.; Biederman, H. Treatment of Poly(Ethylene Terephthalate) Foils by Atmospheric Pressure Air Dielectric Barrier Discharge and Its Influence on Cell Growth. Appl. Surf. Sci. 2015, 357, 689–695. [Google Scholar] [CrossRef]




| Substrate | Plasma Type | Plasma Gas | Grafted or Deposited Layer | Cell Type | Observation | Reference |
|---|---|---|---|---|---|---|
| PCL | APPJ | Ar | PCL homopolymer PCL/PEG copolymer | L929 | Enhancement in cell adhesion, proliferation and growth for deposited PCL/PEG scaffold | [94] |
| PCL/Chitosan/PCL | DBD | Ar/O2 Ar/N2 | - | L929 | Increased cell viability, attachment and proliferation for Ar/O2 treatment. | [95] |
| PCL/Chitosan/PCL | DBD Plasma jet | Ar/O2 Ar/N2 Dry air | - | MRC5 | Enhancement of initial cell attachment and seven-day cell viability, proliferation, and growth for both treatments in comparison with the untreated scaffold. | [96] |
| LDPE | DBD | Ar /O2 | - | NIH 3T3 | Improvement in adhesion and cytocompatibility of cells. | [98] |
| PLLA | Linear corona discharge | N2 | - | MEF | Higher field/body area ratio Reduced percentage of apoptotic nuclei elongated with dendritic morphology | [17] |
| PTFE and POx copolymer | DCSBD | Ar/air | - | 3T3 | Increased cells adhesion number | [102] |
| PS | APPJ | Air | - | L929 | Increase in the number of the attached fibroblast Wider spreading phenotype Vinculin protein and PTK2 increased | [103] |
| Substrate | Plasma Type | Plasma Gas | Grafted or Deposited Layer | Cell Type | Observation | Reference |
|---|---|---|---|---|---|---|
| PCL | DBD | O2 | - | 7F2 | improves the initial attachment, proliferation, and migration | [18] |
| PCL | APPJ | He/O2 He | - | Saos-2 | Clusterization of cells Presence of actin stress fibers | [113] |
| PCL | APPJ | Ar | APTES precursor | Human osteoblast | Improvement in cell viability after HVP functionalization and increase in cell number | [114] |
| PLLA | APPJ | Air CO2 C3F8 | - | MC3T3-E1 | Samples treated in air or CO2 gas were significantly superior in number and growth of adhering cells comparing to C3F8 treated substrate | [117,118] |
| PLA | APPJ | Ar | - | MG-63 | cell proliferation and ALP activity significantly enhanced | [115] |
| PE | APPJ (microplasma) | He/O2 | - | 7F2 | The cells attached and survived on the treated substrate | [121] |
| UHMWPE | APPJ | He/O2 | - | MG-63 | No significant difference between treated and untreated substrate | [122] |
| PS | APPJ | He/O2 | PDMS precursor | MG-63 | Enhancement of cell adhesion | [123] |
| PMMA/PS | DBD | Air | - | hFOB | Enhancement of cellular response | [124] |
| Substrate | Plasma Type | Plasma Gas | Grafted or Deposited Layer | Cell Type | Observation | Reference |
|---|---|---|---|---|---|---|
| PCL | RFGD | Air | - | HCAEC | Introduction of -COOH group, that enhance gelatin grafting, and therefore increase cell adhesion, proliferation and maintain the expression of characteristic markers. | [130] |
| PLLA | Self-sustained barrier discharge | Air | - | HUVEC | Enhanced biocompatibility | [131] |
| PU | APP | He | - | HCAEC | Enhancement of HCAEC growth and adhesion under laminar flow. | [132] |
| PTFE | DBD | Air | - | EC | Good adhesion and growth of ECs Better spreading of ECs than the untreated substrate | [133] |
| PU PU/PLGA | MW induced | Ar | PU | HUVEC | PU/PLGA plasma treated substrate significantly increased the attachment of HUVEC a slightly enhanced the proliferation of the cells | [134] |
| Substrate | Plasma Type | Plasma Gas | Cell Type | Observation | Reference |
|---|---|---|---|---|---|
| PCL | RF-plasma | Ar/O2 Ar/N2 Ar/H2 | HPEC | Ar/N2 and Ar/O2 plasma-treated substrate improved adhesion properties and showed better cell proliferation and growth | [135] |
| PVDF ECTFE PEEK | APP | Air | MSC | Increased viability, cellular activity, and attachment on atmospheric pressure plasma treated surfaces. Spherically shaped MSC | [81] |
| PCL/Chitosan PCL/CMC | CAP | He | MSC | Improved cell attachment Induce chondrocyte cell formation. | [136] |
| PBS | DBD | Air | H9c2 | Absence of cytotoxic products Support cell adhesion and proliferation | [137] |
| LDPE HDPE UHMWPE | Glow discharge plasma | Ar | L929 VSMC | Adhesion and proliferation of L929 cells were enhanced on all the plasma-treated samples. High viability values of VSMC in plasma treated substrate. | [138] |
| Flat PLA Honey-comb PLA | DBD-APPJ system | N2/O2 pretreatment N2/NH3 plasma treatment | NIH-3T3 Neuro-2A | Improve cell attachment and proliferation under all surface conditions. | [139] |
| PET | DBD | Air | Saos-2 HUVEC | Positive influence on the growth of both cell types | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkoglu Sasmazel, H.; Alazzawi, M.; Kadim Abid Alsahib, N. Atmospheric Pressure Plasma Surface Treatment of Polymers and Influence on Cell Cultivation. Molecules 2021, 26, 1665. https://doi.org/10.3390/molecules26061665
Turkoglu Sasmazel H, Alazzawi M, Kadim Abid Alsahib N. Atmospheric Pressure Plasma Surface Treatment of Polymers and Influence on Cell Cultivation. Molecules. 2021; 26(6):1665. https://doi.org/10.3390/molecules26061665
Chicago/Turabian StyleTurkoglu Sasmazel, Hilal, Marwa Alazzawi, and Nabeel Kadim Abid Alsahib. 2021. "Atmospheric Pressure Plasma Surface Treatment of Polymers and Influence on Cell Cultivation" Molecules 26, no. 6: 1665. https://doi.org/10.3390/molecules26061665
APA StyleTurkoglu Sasmazel, H., Alazzawi, M., & Kadim Abid Alsahib, N. (2021). Atmospheric Pressure Plasma Surface Treatment of Polymers and Influence on Cell Cultivation. Molecules, 26(6), 1665. https://doi.org/10.3390/molecules26061665







