Berberine and Cisplatin Exhibit Synergistic Anticancer Effects on Osteosarcoma MG-63 Cells by Inhibiting the MAPK Pathway
Abstract
:1. Introduction
2. Results
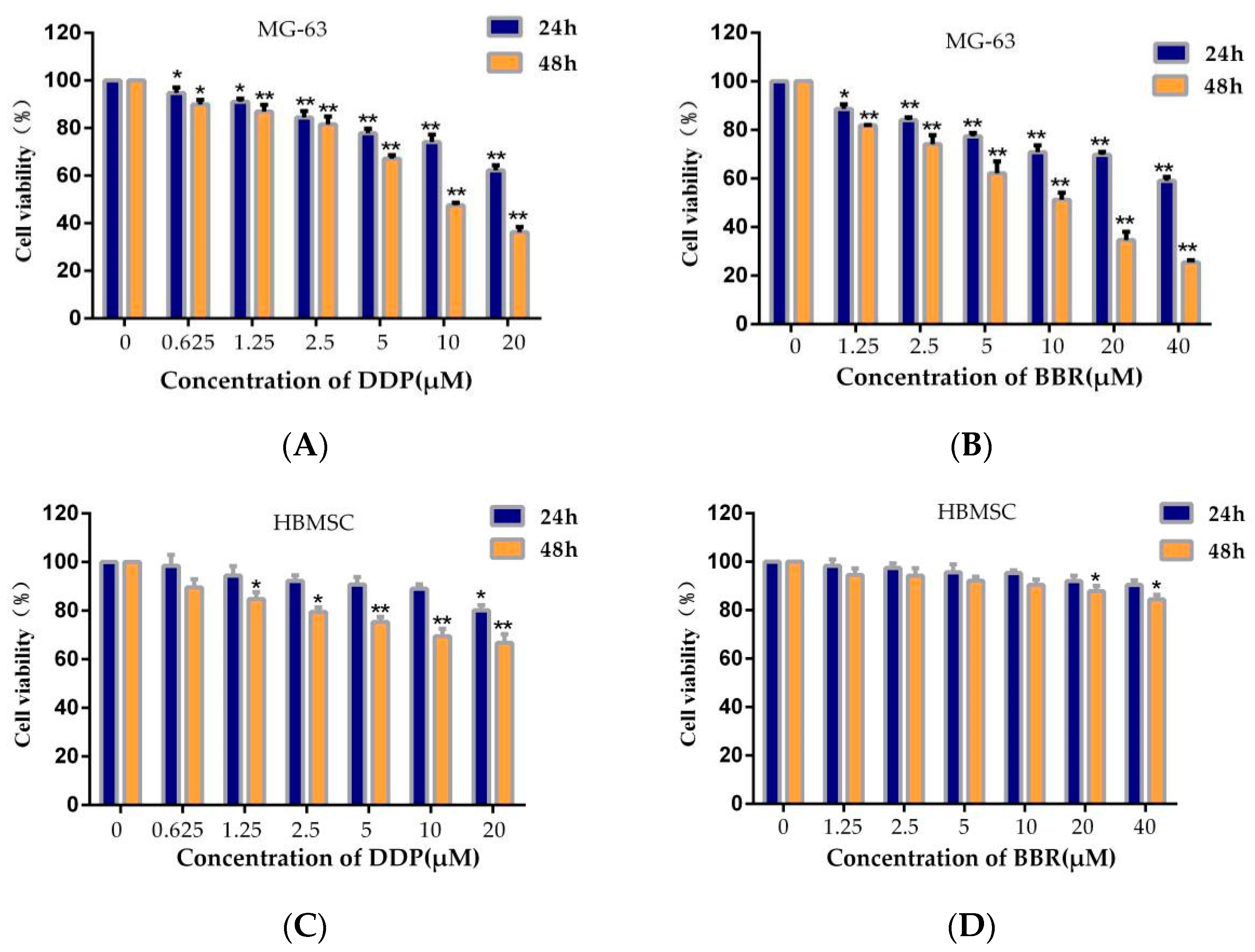
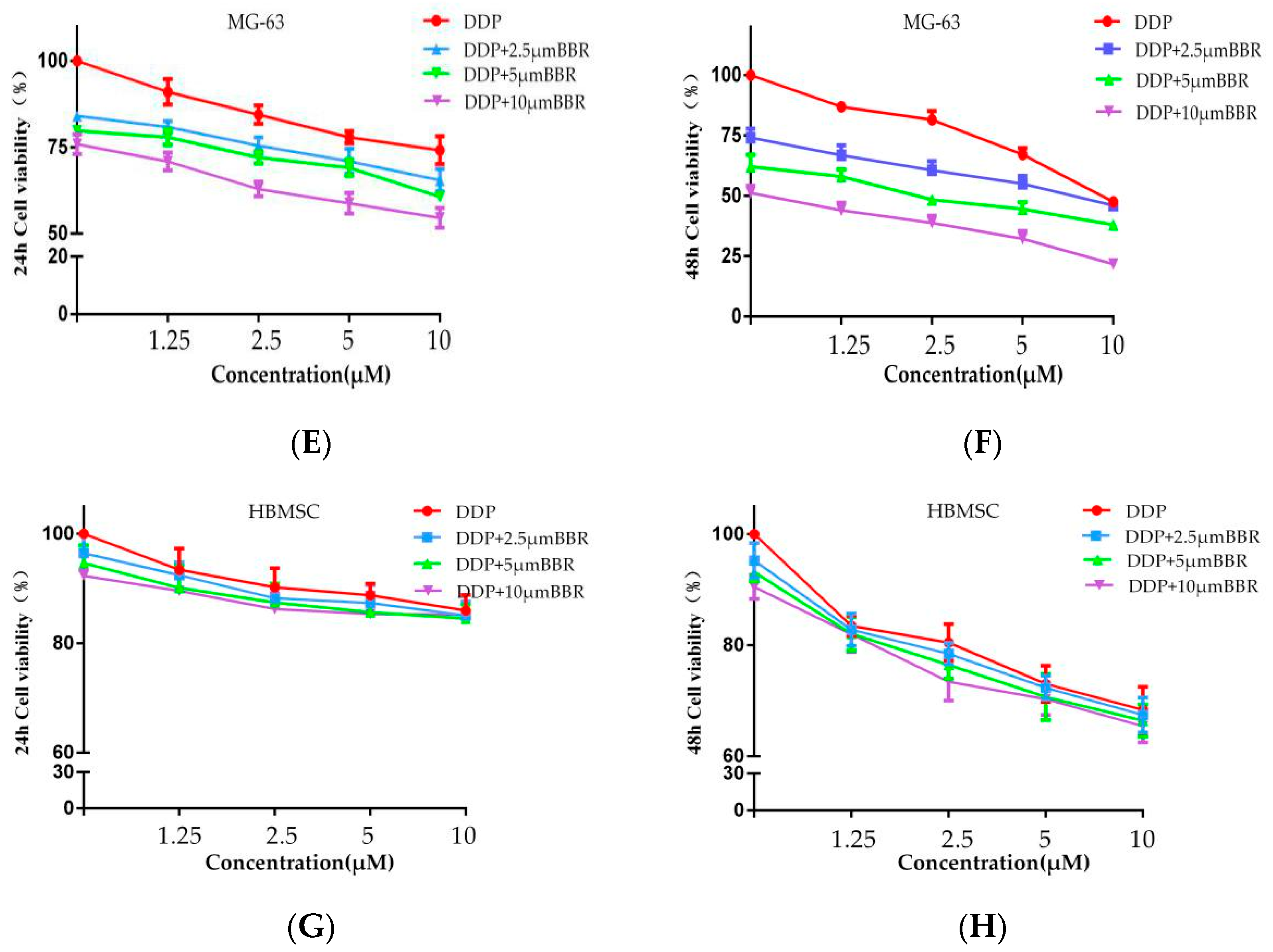
2.1. Effects of BBR Alone, DDP Alone, and Their Combination on the Viability of MG-63 Cells
2.2. Cotreatment with BBR and DDP Synergistically Inhibited the Migration and Invasion of MG-63 Cells
2.3. Cotreatment with BBR and DDP Synergistically Inhibited the Cloning Ability of MG-63 Cells
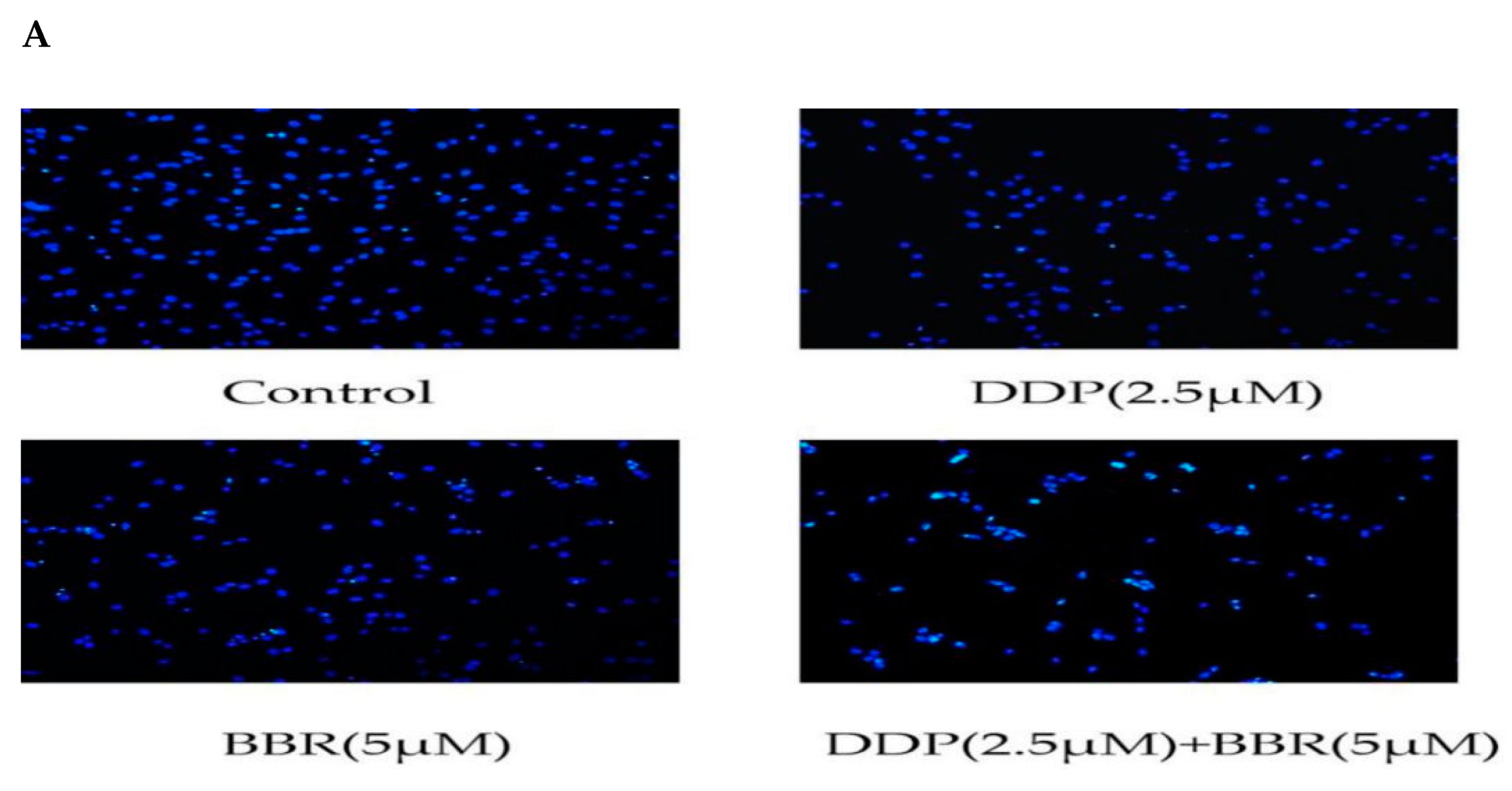
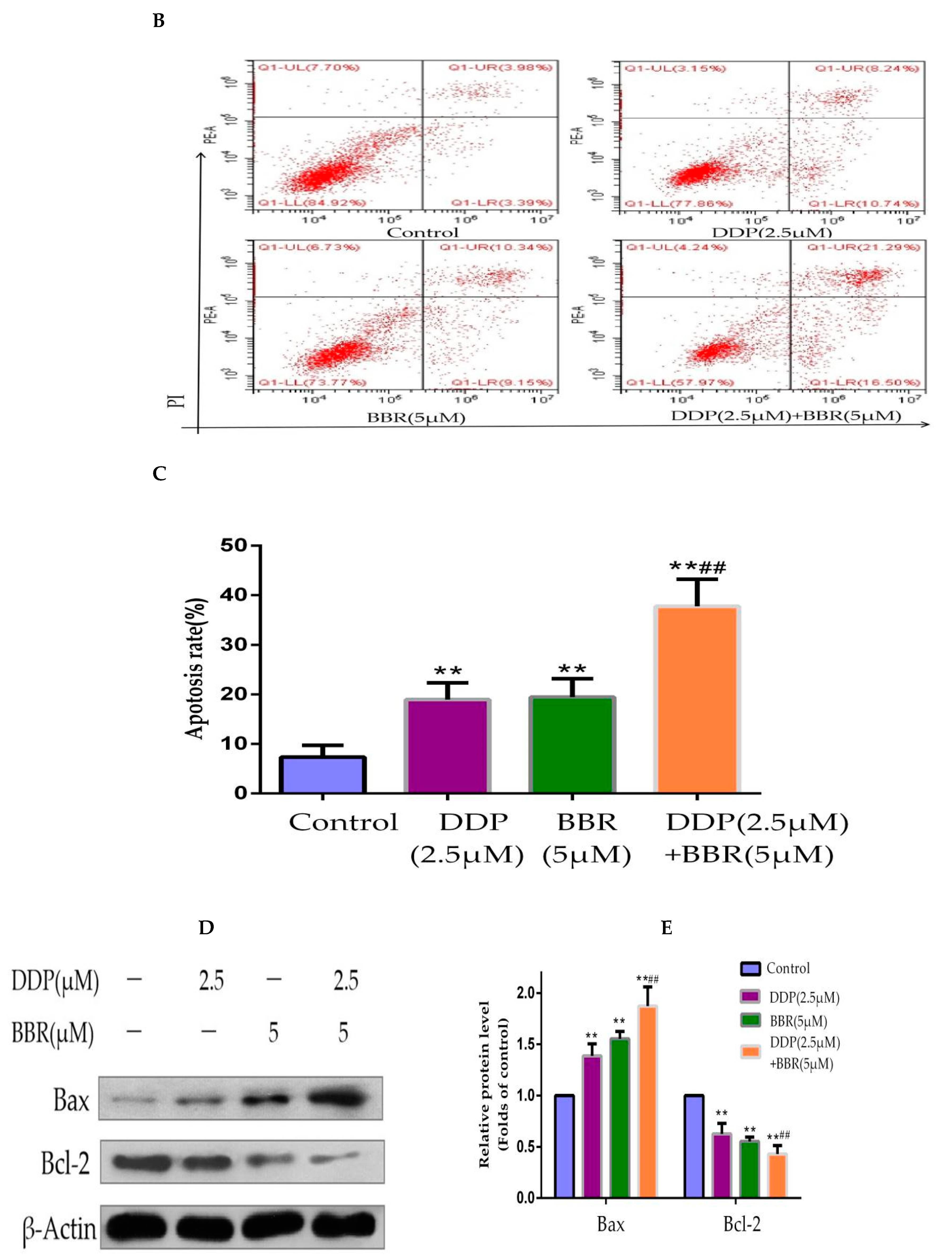
2.4. Cotreatment with BBR and DDP Synergistically Induced the Apoptosis of MG-63 Cells
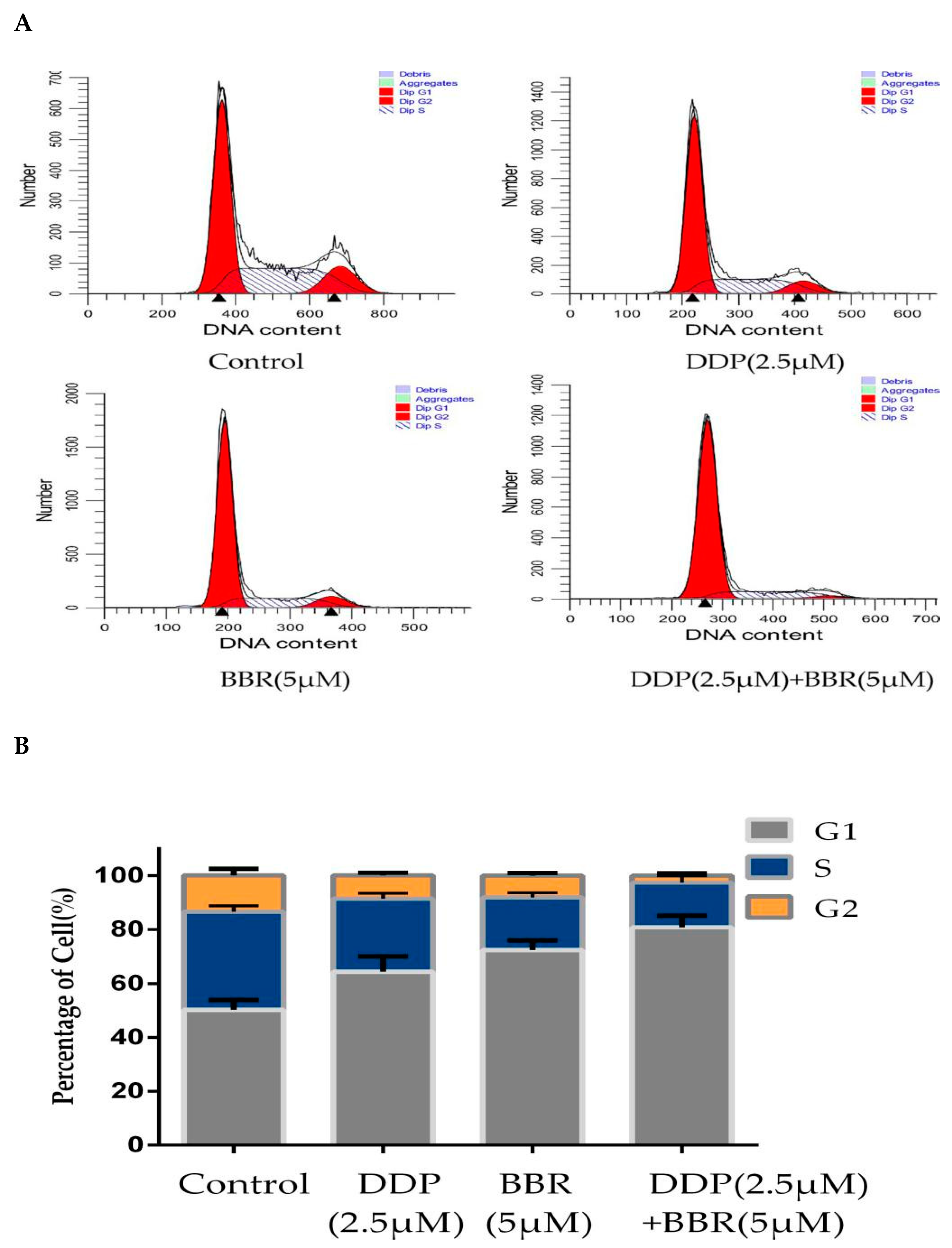
2.5. Cotreatment with BBR and DDP Synergistically Arrested the Cell Cycle of MG-63 Cells
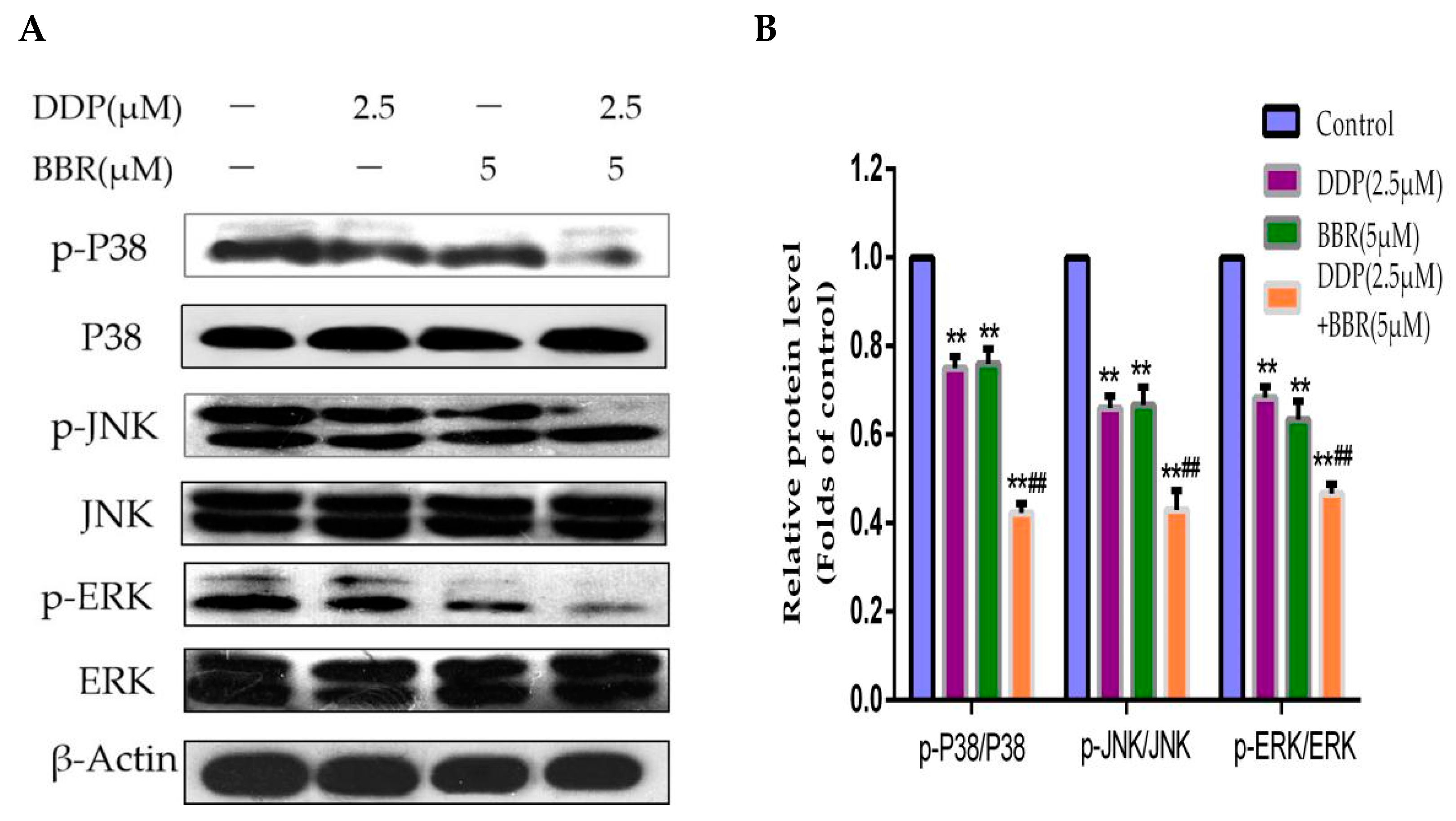
2.6. Mitogen-Activated Protein Kinase (MAPK) Pathway Participates in the Synergistic Effects of the Combined Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Drugs and Antibodies
4.3. Cell Viability Assay
4.4. Combination Index
4.5. Wound-Healing Assay
4.6. Transwell Assay
4.7. Cell Colony-Formation Assay
4.8. Hoechst 33258 Assay
4.9. Flow Cytometry Analysis
4.10. Western Blotting Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Biermann, J.S.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Butrynski, J.E.; Frassica, F.J. Bone cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 688–723. [Google Scholar] [CrossRef]
- Raymond, A.K.; Jaffe, N. Osteosarcoma multidisciplinary approach to the management from the pathologist’s perspective. Cancer Treat. Res. 2009, 152, 63–84. [Google Scholar] [CrossRef]
- Longhi, A.; Errani, C.; De Paolis, M.; Mercuri, M.; Bacci, G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat. Rev. 2006, 32, 423–436. [Google Scholar] [CrossRef]
- Meazza, C.; Scanagatta, P. Metastatic osteosarcoma: A challenging multidisciplinary treatment. Expert Rev. Anticancer Ther. 2016, 16, 543–556. [Google Scholar] [CrossRef]
- Hughes, D.P. Strategies for the targeted delivery of therapeutics for osteosarcoma. Expert Opin. Drug Deliv. 2009, 6, 1311–1321. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.A.; Peixoto, A.; Neves, M.; Gaiteiro, C.; Reis, C.A. Mechanisms of cisplatin resistance and targeting of cancer stem cells: Adding glycosylation to the equation. Drug Resist. Update 2016, 24, 34–54. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Ji, T.; Guo, W. Anti-angiogenesis target therapy for advanced osteosarcoma. Oncol. Rep. 2017, 15, 7409–7414. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, H.; Takeno, A.; Endo, S.; Makari, Y.; Kawada, J.; Taniguchi, H.; Tamura, S.; Sugimoto, N.; Kimura, Y.; Tamura, T. Randomized, open-label phase II study comparing Capecitabine-cisplatin every 3 weeks with S-1-cisplatin every 5 weeks in chemotherapy-naive patients with HER2-negative advanced gastric Cancer: OGSG1105, HERBIS-4A trial. Oncologist 2018, 12, 1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, R.; Li, J.; Bai, Y.; Liu, T.; Jiao, S.; Dai, G.; Xu, J.; Liu, Y.; Fan, N. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer 2016, 19, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.; Pearson, A.; English, M. Cisplatin dose rate as a risk factor for nephrotoxicity in children. Br. J. Cancer. 1998, 77, 1677–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reece, P.A.; Stafford, I.; Russell, J.; Khan, M.; Gill, P.G. Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: Relationship between peak ultrafilterable platinum plasma levels and nephrotoxicity. J. Clin. Oncol. 1987, 5, 304–309. [Google Scholar] [CrossRef]
- Stewart, D.J.; Dulberg, C.S.; Mikhae, N.Z. Association of cisplatin nephrotoxicity with patient characteristics and cisplatin administration methods. Cancer Chemother. Pharmacol. 1997, 40, 293. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef] [Green Version]
- Rackova, L.; Majekova, M.; Kost’alova, D.; Stefek, M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural Aspects. Bioorg. Med. Chem. 2004, 12, 4709–4715. [Google Scholar] [CrossRef]
- Menees, S.; Saad, R.; Chey, W.D. Agents that act luminally to treat diarrhoea and constipation. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Zhang, H.; Song, D.Q. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Mahajan, S. Berberine and its derivatives: A patent review (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.C.; Angers, S. Gli proteins in development and disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 513–537. [Google Scholar] [CrossRef] [Green Version]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol. Cancer Ther. 2006, 5, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cao, H.; Zhang, B.; Xu, X.; Ruan, H.; Yi, T. Berberine potently attenuates intestinal polyps growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signalling. J. Cell Mol. Med. 2013, 17, 1484–1493. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. p53 Cooperates berberineinduced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37. [Google Scholar] [CrossRef]
- James, M.A.; Fu, H.; Liu, Y.; Chen, D.R.; You, M. Dietary administration of berberine or Phellodendron amurense extract inhibits cell cycle progression and lung tumorigenesis. Mol. Carcinog. 2011, 50, 1–7. [Google Scholar] [CrossRef]
- Chappell, W.H.; Abrams, S.L.; Franklin, R.A.; LaHair, M.M.; Montalto, G.; Cervello, M. Ectopic NGAL expression can alter sensitivity of breast cancer cells to EGFR, Bcl-2, CaM-K inhibitors and the plant natural product berberine. Cell Cycle 2012, 11, 4447–4461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbarzadeh, K.P.; Rahmat, A.; Ismail, P.; Ling, K.H. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur. J. Pharmacol. 2014, 740, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z. Berberine induced apoptosis of human osteosarcoma cells by inhibiting phosphoinositide 3 kinase/protein kinase B (PI3K/Akt) signal pathway activation. Iran. J. Public Health 2016, 45, 578–585. [Google Scholar]
- Zhu, Y.; Ma, N.; Li, H. Berberine induces apoptosis and DNA damage in MG63 human osteosarcoma cells. Mol. Med. Rep. 2014, 10, 1734–1738. [Google Scholar] [CrossRef] [Green Version]
- Yue, P.; Fan, Z.; Yawei, Z. Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer. J. Cancer 2017, 8, 1679–1689. [Google Scholar] [CrossRef] [Green Version]
- Ping, J.; Chunjie, Z. Berberine exhibits antitumor effects in human ovarian cancer cells. Anticancer Agents Med. Chem. 2015, 15, 511–516. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, Z.; Li, Y. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol. Rep. 2016, 36, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Pasello, M.; Ferrari, S. Emerging drugs for high-grade osteosarcoma. Expert Opin. Emerg. Drugs 2010, 15, 615–634. [Google Scholar] [CrossRef]
- Sorenson, C.M.; Eastman, A. Influence of cis-diamminedichloroplatinum(II) on DNA synthesis and cell cycle progression in excision repair proficient and deficient chinese hamster ovary cells. Cancer Res. 1988, 48, 6703–6707. [Google Scholar] [CrossRef] [PubMed]
- Weijl, N.I.; Cleton, F.J.; Osanto, S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat. Rev. 1997, 23, 209–240. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, J.; Ai, G. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.Q.; Liu, J.; Ren, B.Y.; Zhang, L.; Owusu, L.; Liu, L.K.; Zhang, J. Anti-tumor and chemosensitization effects of Cryptotanshinone extracted from Salvia miltiorrhiza Bge. on ovarian cancer cells in vitro. J. Ethnopharmacol. 2017, 205, 33–40. [Google Scholar] [CrossRef]
- Youn, M.J.; So, H.S.; Cho, H.J. Berberine, a natural product, combined with cisplatin enhanced apoptosis through a mitochondria/caspasemediated pathway in HeLa cells. Biol. Pharm. Bull. 2008, 31, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libra, M.; Scalisi, A.; Vella, N.; Clementi, S.; Sorio, R. Uterine cervical carcinoma: Role of matrix metalloproteinases (Review). Int. J. Oncol. 2009, 34, 897–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, F.; Jiang, S. Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/Bax signaling pathways. Oncol. Lett. 2018, 15, 7409–7414. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [Green Version]
- Nussinov, R.; Tsai, C.J.; Jang, H.; Korcsmaros, T.; Csermely, P. Oncogenic KRAS signaling and YAP1/beta-catenin: Similar cell cycle control in tumor initiation. Semin. Cell Dev. Biol. 2016, 58, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Cao, S. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Guangzhi, M.; Yunfu, D. Artesunate exhibits synergistic anti-cancer effects with cisplatin on lung cancer A549 cells by inhibiting MAPK pathway. Gene 2020, 766, 145134. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| 24 h | 48 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NO. | BBR (μM) | DDP (μM) | FA * | CI | NO. | BBR (μM) | DDP (μM) | FA * | CI |
| 1 | 2.5 | 1.25 | 0.191 | 0.856 | 1 | 2.5 | 1.25 | 0.331 | 0.893 |
| 2 | 2.5 | 2.5 | 0.245 | 0.625 | 2 | 2.5 | 2.5 | 0.394 | 0.829 |
| 3 | 2.5 | 5 | 0.290 | 0.628 | 3 | 2.5 | 5 | 0.450 | 0.941 |
| 4 | 2.5 | 10 | 0.346 | 0.729 | 4 | 2.5 | 10 | 0.540 | 1.052 |
| 5 | 5 | 1.25 | 0.221 | 1.132 | 5 | 5 | 1.25 | 0.491 | 0.928 |
| 6 | 5 | 2.5 | 0.279 | 0.769 | 6 | 5 | 2.5 | 0.515 | 0.678 |
| 7 | 5 | 5 | 0.309 | 0.778 | 7 | 5 | 5 | 0.559 | 0.759 |
| 8 | 5 | 10 | 0.393 | 0.685 | 8 | 5 | 10 | 0.619 | 0.859 |
| 9 | 10 | 1.25 | 0.291 | 1.126 | 9 | 10 | 1.25 | 0.518 | 0.790 |
| 10 | 10 | 2.5 | 0.371 | 0.668 | 10 | 10 | 2.5 | 0.613 | 0.666 |
| 11 | 10 | 5 | 0.412 | 0.609 | 11 | 10 | 5 | 0.677 | 0.581 |
| 12 | 10 | 10 | 0.454 | 0.645 | 12 | 10 | 10 | 0.793 | 0.632 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Zhang, C.; Wang, Y.; Zhang, P.; Zhang, J.; Hong, T. Berberine and Cisplatin Exhibit Synergistic Anticancer Effects on Osteosarcoma MG-63 Cells by Inhibiting the MAPK Pathway. Molecules 2021, 26, 1666. https://doi.org/10.3390/molecules26061666
Gao X, Zhang C, Wang Y, Zhang P, Zhang J, Hong T. Berberine and Cisplatin Exhibit Synergistic Anticancer Effects on Osteosarcoma MG-63 Cells by Inhibiting the MAPK Pathway. Molecules. 2021; 26(6):1666. https://doi.org/10.3390/molecules26061666
Chicago/Turabian StyleGao, Xianxian, Chen Zhang, Yanjie Wang, Ping Zhang, Jingyu Zhang, and Tie Hong. 2021. "Berberine and Cisplatin Exhibit Synergistic Anticancer Effects on Osteosarcoma MG-63 Cells by Inhibiting the MAPK Pathway" Molecules 26, no. 6: 1666. https://doi.org/10.3390/molecules26061666





