Growth Restriction of Rhizoctonia solani via Breakage of Intracellular Organelles Using Crude Extracts of Gallnut and Clove
Abstract
:1. Introduction
2. Results
2.1. Inhibition of R. solani Plant-Derived Extracts
2.2. Indoor Toxicity Test of Plant-Derived Extracts against R. solani
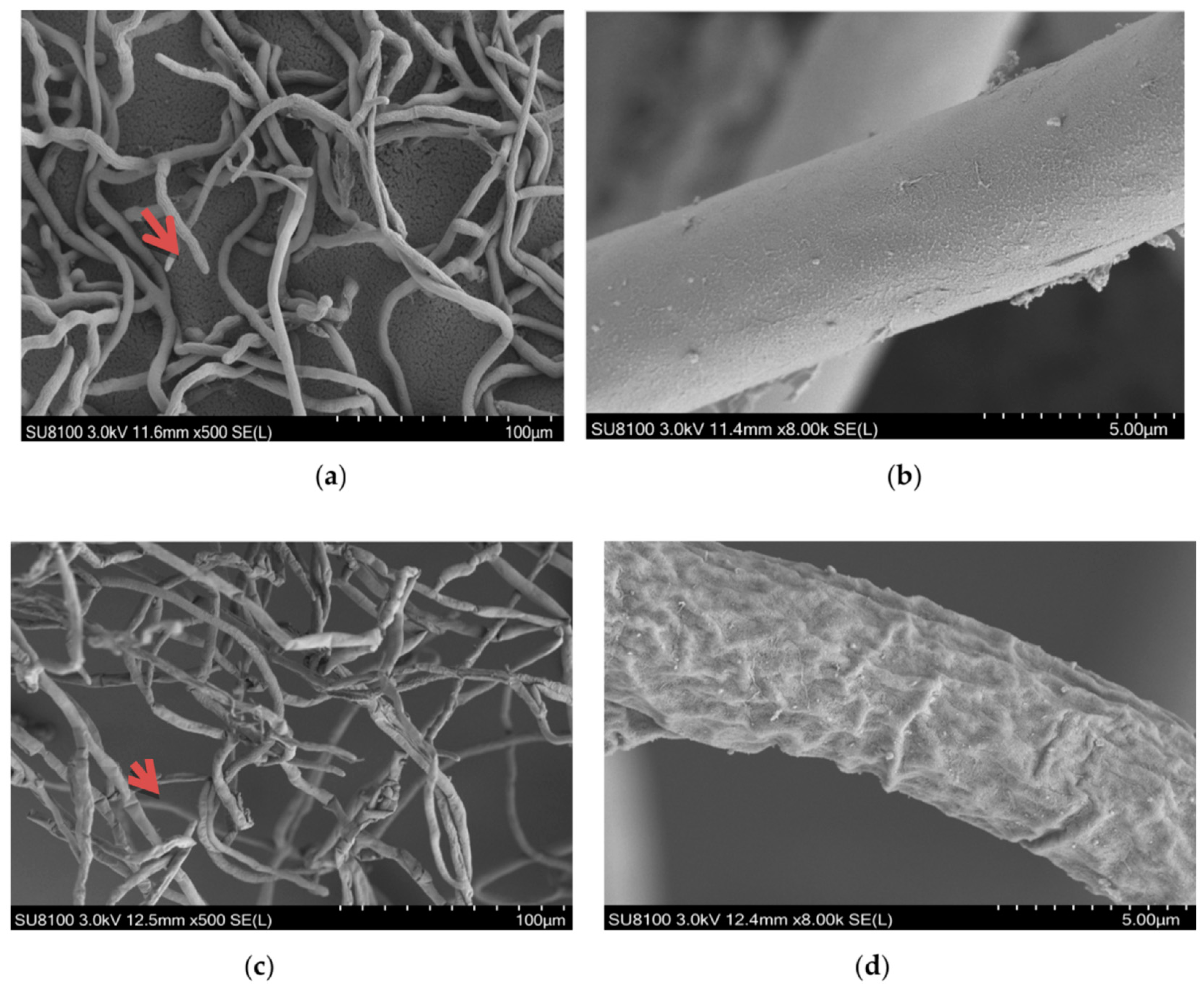
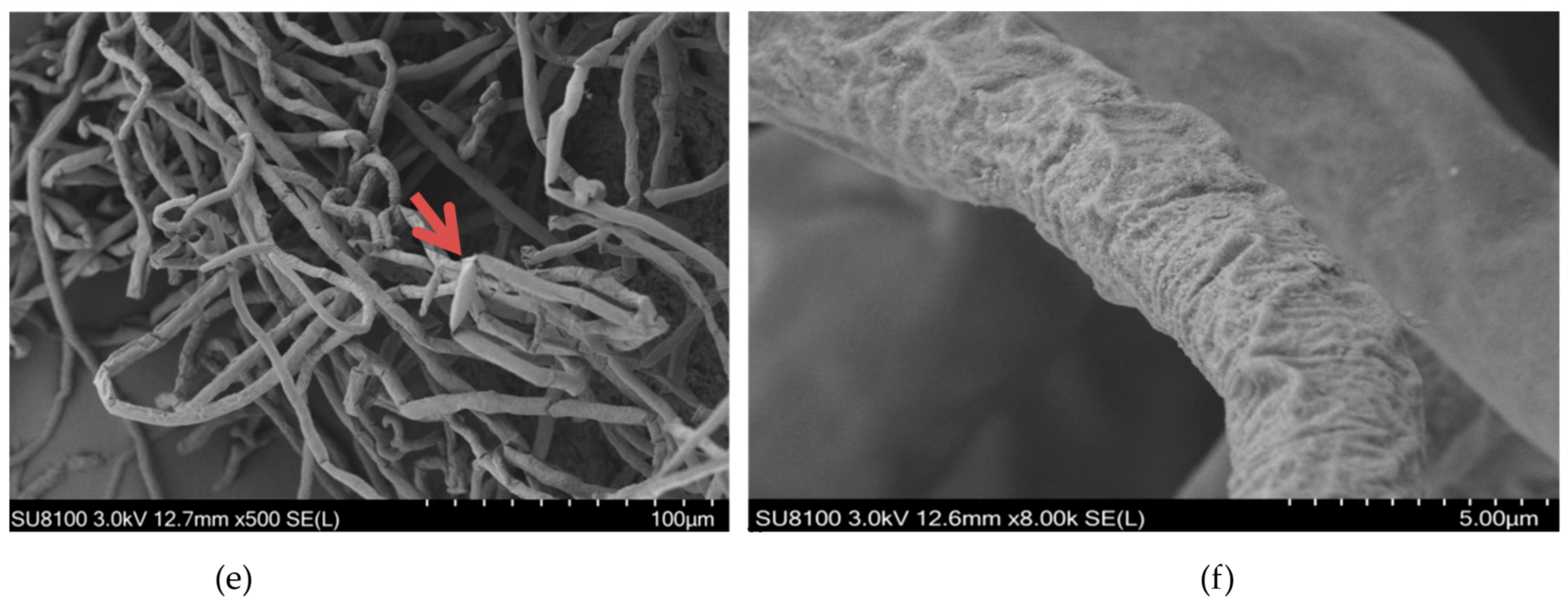
2.3. Effects of Ethanol Extracts from Clove and Gallnut on Mycelial Morphology of R. solani
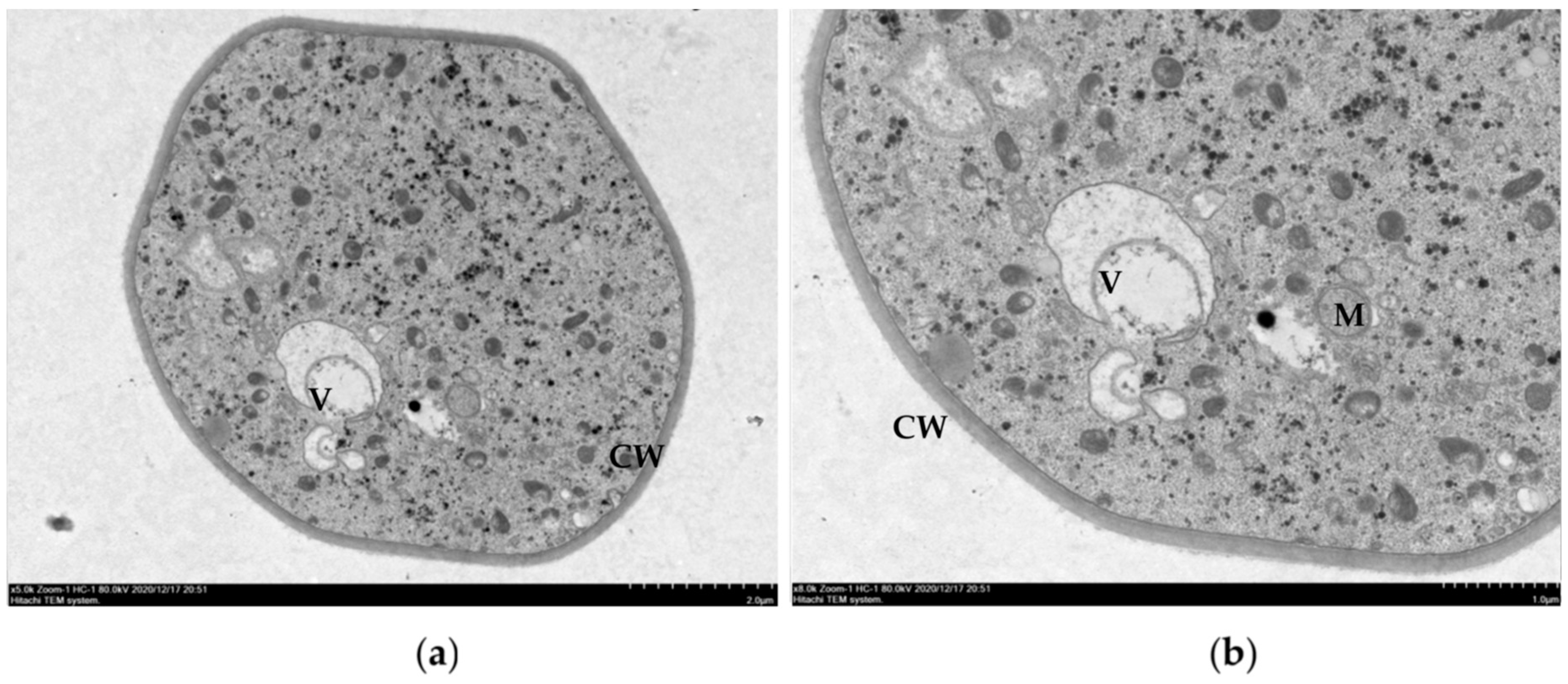
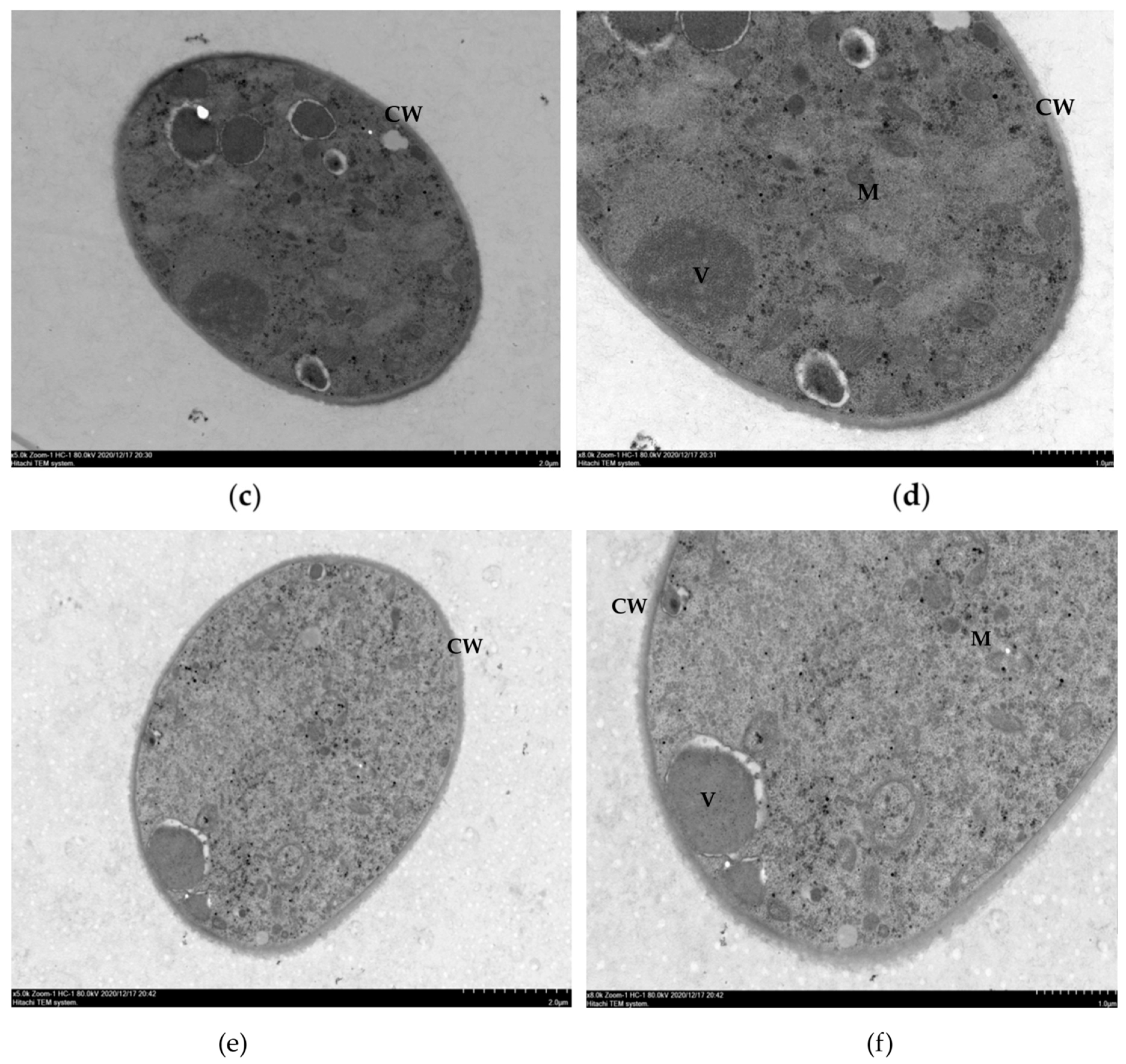
2.4. Effects of Ethanol Extracts from Clove and Gallnut on Ultrastructure of Mycelia of Rice Sheath Blight
2.5. Protective Effects of Clove and Gallnut Ethanol Extracts on Rice In Vivo
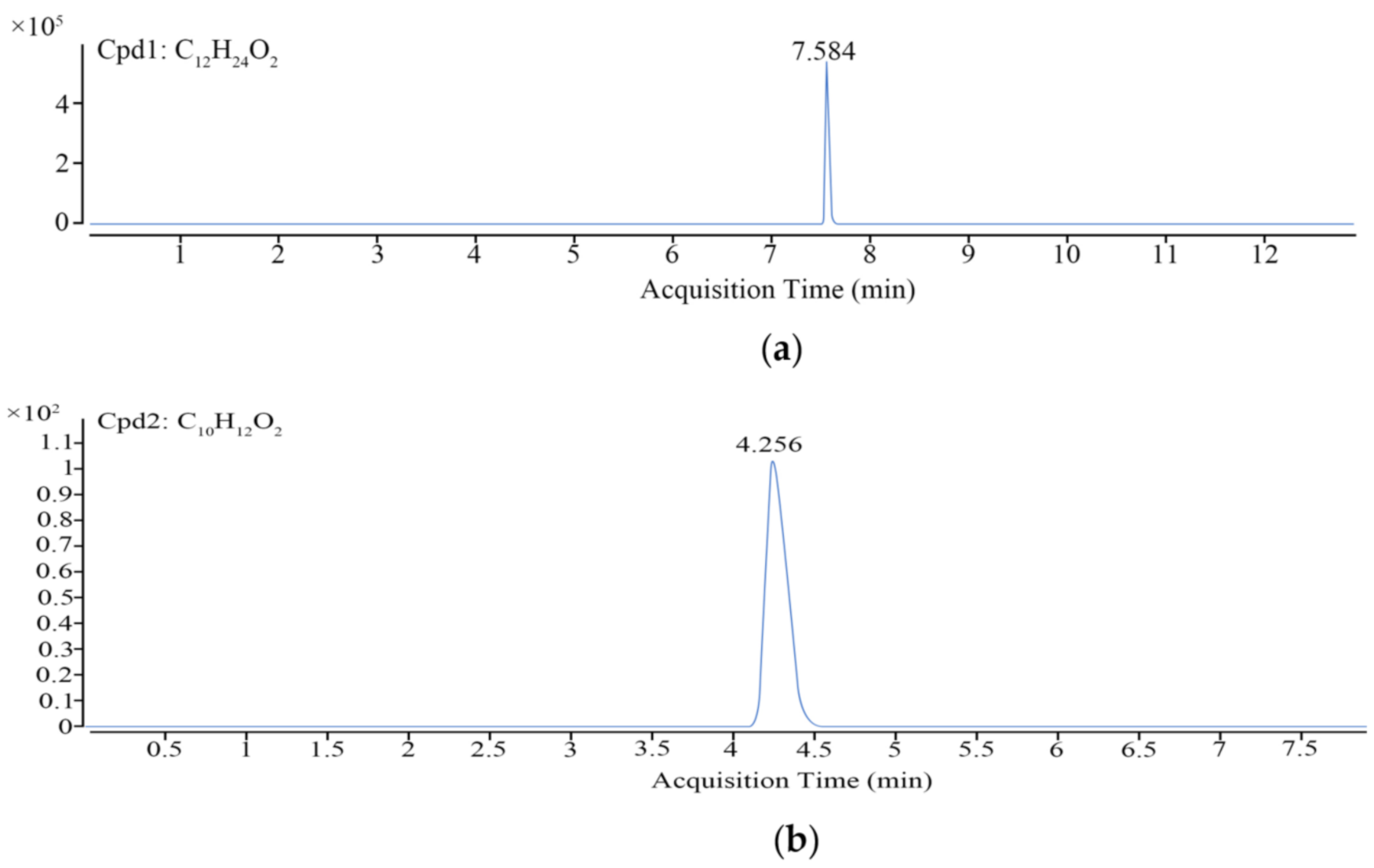
2.6. LC–MS and Data Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Isolation and Identification of Rice Sheath Blight Pathogen
4.1.2. Collection of Plant-Derived Materials
4.2. Methods
4.2.1. Collection and Preparation of Plant-Derived Extracts
4.2.2. In Vitro Evaluation of Rice Sheath Blight Inhibition Activity of Plant-Derived Extracts
4.2.3. Determination of Eugenol and Lauric Acid Content
4.2.4. Indoor Toxicity Determination of Plant-Derived Extracts
4.2.5. Scanning Electron Microscopy (SEM)
4.2.6. Transmission Electron Microscopy (TEM)
4.2.7. Protective Effect of Plant-Derived Extracts on Rice In Vivo
4.2.8. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wu, W.; Shah, F.; Shah, F.; Huang, J.L. Rice sheath blight evaluation as affected by fertilization rate and planting density. Australas Plant. Path. 2015, 44, 183–189. [Google Scholar] [CrossRef]
- Wu, W.; Liao, Y.C.; Shah, F.; Nie, L.X.; Peng, S.B.; Cui, K.H.; Huang, J.L. Plant growth suppression due to sheath blight and the associated yield reduction under double rice-cropping system in central China. Field Crops Res. 2013, 144, 268–280. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, H.Q.; Zhang, M.L.; Cao, X.; Zhou, E.X. The comnplete genomic sequence of a novel mycovirus from Rhizoctonia solani AG-1 1A strain B275. Arch. Virol. 2013, 158, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Srinivasachary, W.L.; Savary, S. Resistance to rice sheath blight (Rhizoctonia solani Kühn) [(teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk.] disease: Current status and perspectives. Euphytica 2011, 178, 1–22. [Google Scholar] [CrossRef]
- Singh, P.; Mazumdar, P.; Harikrishna, J.A.; Babu, S. Sheath blight of rice: A review and identification of priorities for future research. Planta 2019, 250, 1387–1407. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.H. Rice Diseases, 2nd ed.; Commonwealth Mycological Institute: Kew Surrey, UK, 1985; pp. 109–201. [Google Scholar]
- Basu, A.; Chowdhury, S.; Chaudhuri, R.T.; Kundu, S. Differential behaviour of sheath blight pathogen Rhizoctonia solani in tolerant and susceptible rice varieties before and during infection. Plant. Pathol. 2016, 65, 1333–1346. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Seo, M.J.; Kwon, H.J.; Rajkarnikar, A.; Kim, K.R.; Kim, S.O.; Suh, J.W. Genetic Localization and Heterologous Expression of Validamycin Biosynthetic Gene Cluster Isolated from Streptomyces hygroscopicus var. 1imoneus KCCM 11405(IFO 12704). Gene 2006, 376, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, W.X.; Xue, Y.G.; Chen, X.Y.; Wang, J.R. A Preliminary study on the Sensitivity of Rice Sheath Blight Fungi (Rhizoctonia solani) to Jinggangmycin in Zhengzhou County of Henan Province. Chin. J. Biol. Control. 1995, 11, 171–173, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Müller, J.; Boller, T.; Wiemken, A. Effect of Validamycin A a potent trehalase inhibitor, and phytohomones on trehalose metabolism in roots and nodules of soybean and cowpea. Planta 1995, 197, 362–368. [Google Scholar] [CrossRef]
- Wu, J.; Xi, Y.D.; Li, H.H.; Wang, L.X.; Peng, H.X. Monitoring of Resistance of Thenatephorus Cucumeris to Jinggangmysin in Sichuan. Southwest China J. Agric. Sci. 2015, 6, 2501–2504, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Chen, Y.; Yao, J.; Yang, X.; Zhang, A.F.; Gao, T.C. Sensitivity of Rhizoctonia solani causing rice sheath blight to fluxapyroxad in China. Eur. J. Plant. Pathol. 2014, 140, 419–428. [Google Scholar] [CrossRef]
- Ji, Y.R.; Yang, Q.L.; Dong, Y.; Ma, Z.J.; Liu, S.X.; Zhang, Z.H.; Guan, X.J. Research and Application on nematicidal activity of the Leguminosae plants. J. Anhui Agri. Sci. 2014, 42, 8884–8886. [Google Scholar] [CrossRef]
- Grange, M.; Ahmed, S. Handbook of Plants with Pest-Control Properties; Wiley-Interscience: New York, NY, USA, 1988; p. 65. [Google Scholar]
- Buckingham, J. Dictionary of Natural Products on DVD; Version 29.2; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019; pp. 102–167. [Google Scholar]
- Fogliani, B.; Bouraïma-Madjebi, S.; Medevielle, V.; Pineau, R. Screening of 50 Cunoniaceae species from New Caledonia for antimicrobial properties. N. Z. J. Bot. 2002, 40, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Ojala, T.; Rames, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Srinivasan, D.; Nathana, S.; Suresh, T.; Perumasamy, P.L. Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J. Ethnopharmacol. 2001, 74, 217–220. [Google Scholar] [CrossRef]
- Padaria, J.C.; Tarafdar, A.; Raipuria, R.; Lone, S.A.; Gahlot, P.; Shakil, N.A.; Kumar, J. Identification of phenazine-1-carboxylic acid gene (phc CD) from Bacillus pumilus MTCC7615 and its role in antagonism against Rhizoctonia solani. Basic Microbiol. 2016, 56, 999–1008. [Google Scholar] [CrossRef]
- Li, A.H.; Xu, X.P.; Dai, Z.Y.; Chen, Z.X.; Li, B.J.; Zhang, H.X.; Pan, X.B. Analysis of Resistance to Rice Sheath Blight for Transgenic line of rice. Chin. J. Rice Sci. 2003, 17, 302–306. [Google Scholar]
- Rajendra, P.; Ayub, K.; Wendy-Ann, I.; Wayne, G.; Duraisamy, S. Plant extracts, bioagents and new generation fungicides in the control of rice sheath blight in Guyana. Crop Prot. 2019, 119, 30–37. [Google Scholar] [CrossRef]
- Venkateswarlu, N.; Vijaya, T.; Suresh, B.D.; Chandra, M.K.; Pragathi, D.; Anitha, D.; Sreeramulu, A. In Vitro Inhibitory Effects of Medicinal Plants Extracts on Sclerotium Oryzae- a Fungi Causing Stem Rot Disease in Paddy. Int. J. Pharm. Biol. Sci. 2013, 3, 147–151. [Google Scholar]
- Lu, R.Y.; Wu, J.; Jiang, Y.Y. Study on the Antifungal Activity of Four Kinds of Traditional Chinese Medicine Extracts. Guangdong Chem. 2020, 22, 49–52, (In Chinese with English Abstract). [Google Scholar]
- Serrano, M.; Martínez-Romero, D.; Castillo, S.; Guillén, F.; Valero, D. The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov. Food Sci. Emerg. 2005, 1, 115–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Djakpoa, O.; Xie, Y.F.; Guo, Y.H.; Yu, H.; Cheng, Y.L.; Qian, H.; Shi, R.; Yao, W.R. Anti-quorum sensing of Galla chinensis and Coptis chinensis on bacteria. Food Sci. Technol. 2019, 101, 806–811. [Google Scholar] [CrossRef]
- Huang, X.L.; Liu, M.D.; Li, J.Y.; Zhou, X.D.; Cate, J.M.T. Chemical composition of Galla chinensis extract and the effect of its main component(s) on the prevention of enamel demineralization in vitro. Int. J. Oral Sci. 2012, 4, 146–151. [Google Scholar] [CrossRef]
- Wu, J.; Jahncke, M.L.; Eifert, J.D.; O’Keefe, S.F.; Welbaum, G.E. Pomegranate peel (Punica granatum L) extract and Chinese gall (Galla chinensis) extract inhibit Vibrio parahaemolyticus and Listeria monocytogenes on cooked shrimp and raw tuna. Food Control 2016, 59, 695–699. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Li, B.; Ji, B.; Zhang, G.Z.; Luo, Y.C. Identification and structure-activity relationship of gallotannins separated from Galla chinensis. LWT Food Sci. Technol. 2009, 42, 128–1295. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.P.; Yang, J.H.; Zhang, G.Z.; Chen, Y.; Luo, Y.C. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Li, R.Y.; Wu, X.M.; Yin, X.H.; Long, Y.H.; Li, M. Naturally produced citral can significantly inhibit normal physiology and induce cytotoxicity on Magnaporthe grisea. Pestic. Biochem. Physiol. 2014, 118, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.L.; Gou, J.Y.; Han, X.B.; Wu, Q.; Zhang, C.S. In vitro and in vivo activities of eugenol against tobacco black shank caused by Phytophthora nicotianae. Pestic. Biochem. Physiol. 2017, 142, 148–154. [Google Scholar] [CrossRef]
- Pasqua, D.R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Wright, A.V. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Zambonelli, A.D.; D’Aulerio, A.Z.; Severi, A.; Benvenuti, S.; Maggi, L.; Bianchi, A. Chemical Composition and Fungicidal Activity of Commercial Essential Oils of Thymus vulgaris L. J. Essent. Oil Res. 2004, 16, 69–74. [Google Scholar] [CrossRef]
- Mallavarapu, G.R.; Ramesh, S.; Chandrasekhara, R.S.; Rajeswara Rao, B.R.; Kaul, P.N.; Bhattacharya, A.K. In vestigation of the essential oil of cinnamon leaf grown at Bangalore and Hyderabad. Flavour. Frag. J. 1995, 10, 239–242. [Google Scholar] [CrossRef]
- Amrania, S.E.; Lalamib, A.E.O.; Zoubic, Y.E.; Moukhafia, K.; Bouslamtia, R.; Lairinia, S. Evaluation of antibacterial and antioxidant effects of cinnamon and clove essential oils from Madagascar. Mater. Today Proc. 2019, 13, 762–770. [Google Scholar] [CrossRef]
- Briozzo, J.; Núncez, L.; Chirife, J.; Herszage, L.; D’aquino, M. Antimicrohial activity of clove oil dispersed in a concentrated sugar solution. J. Appl. Microbiol. 1989, 66, 69–75. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, B.S.; Kim, M.K.; Choi, W.S.; Kim, H.T.; Cho, K.Y.; Lee, S.G.; Lee, H.S. Fungicidal activity of pipernonaline, a piperidine alkaloid derived from long pepper, Piper longum L., against phytopathogenic fungi. Crop Prot. 2001, 20, 523–528. [Google Scholar] [CrossRef]
- Khoa, N.D.; Thuy, P.T.H.; Thuy, T.T.T.; Collinge, D.B.; Jørgensen, H.J.L. Disease-reducing effect of Chromolaena odorata extract on sheath blight and other rice diseases. Phytopathology 2011, 101, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.B.; Guo, C.; Li, M.; Li, R.Y.; Wu, X.M.; Hu, A.L.; Hu, X.F.; Mo, F.X.; Wu, S. Physicochemical Properties and Bioactivity of a New Guar Gum-Based Film Incorporated with Citral to Brown Planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Molecules 2020, 25, 2044. [Google Scholar] [CrossRef]


| Plants Resources | Families | Extract Position | Colony Diameter (cm) | Inhibition Ratio (%) |
|---|---|---|---|---|
| Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f. ex Franch. et Sav | Apiaceae Lindl. | root | (6.07 ± 0.12) d | 4.56 |
| Angelica pubescens Maxim. f. biserrata Shan et Yuan | Apiaceae Lindl. | root | (3.42 ± 0.23) o | 38.93 |
| Angelica sinensis | Apiaceae Lindl. | root | (2.98 ± 0.2) q | 51.31 |
| Atractylodes macrocephala Koidz. | Asteraceae Bercht. & J. Presl | rhizome | (4.28 ± 0.37) n | 23.57 |
| Cinnamomum cassia Presl | Lauraceae Juss. | bark | (2.02 ± 0.03) r | 66.99 |
| Cnidium monnieri (L.) Cuss. | Apiaceae Lindl. | fruit | (4.58 ± 0.11) kl | 24.55 |
| Coptis chinensis Franch. | Ranunculaceae Juss. | total plant | (3.3 ± 0.17) p | 41.07 |
| Crataegus pinnatifida Bunge | Rosaceae Juss. | fruit | (4.35 ± 0.19) mn | 22.32 |
| Cynanchum otophyllum | Paeoniaceae Raf. | root | (6.45 ± 0.02) c | - |
| Eucommia ulmoides Oliver | Eucommiaceae Engl. | bark | (5.4 ± 0.02) g | 15.09 |
| Forsythia suspensa | Oleaceae | fruit | (4.62 ± 0.26) k | 17.5 |
| Glycyrrhiza uralensis Fisch. | Fabaceae Lindl. | rhizoma | (5.28 ± 0.015) h | 13.73 |
| Heartleaf Houttuynia Herb | Saururaceae Rich. ex T. Lestib. | leaves | (6.58 ± 0.21) b | - |
| Isatis tinctoria | Brassicaceae Burnett | rhizoma | (6.8 ± 0.16) a | - |
| Mentha haplocalyx Briq. | Labiatae | leaves | (4.42 ± 0.02) m | 21.07 |
| Morus alba L. | Moraceae Gaudich. | shoot | (5.02 ± 0.17) j | 10.36 |
| Reynoutria japonica Houtt. | Polygonaceae | rhizome | (5.62 ± 0.05) ef | 11.64 |
| Rheum palmatum L. | Polygonaceae | rhizoma | (4.5 ± 0.29) l | 29.25 |
| Rhizoma Pinelliae | Araceae Juss. | stem tuber | (5.22 ± 0.11) h | 6.79 |
| Rhus chinensis Mill. | Anacardiaceae R. Br. | Galls on leaves | (0) s | 100 |
| Salvia miltiorrhiza Bunge | Labiatae | root | (5.07 ± 0.53) i | 20.28 |
| Saposhnikovia divaricata (Trucz.) Schischk. | Apiaceae Lindl. | root | (5.63 ± 0.17) e | 7.24 |
| Syzygium aromaticum (L.) Merr. EtPerry | Myrtaceae Juss. | fruit | (0) s | 100 |
| Xanthium sibiricum Patrin ex Widder | Asteraceae Bercht. & J. Presl | fruit | (5.54 ± 0.05) f | 8.73 |
| Concentration (µg/mL) | The Ethanol Extracts from Clove | Concentration (µg/mL) | The Ethanol Extracts from Gallnut | ||
|---|---|---|---|---|---|
| Colony Diameter (cm) | Inhibition Ratio (%) | Colony Diameter (cm) | Inhibition Ratio (%) | ||
| 0 | (6.85 ± 0.1) a | - | 0 | (6.85 ± 0.1) a | - |
| 10 | (5.62 ± 0.2) b | 17.96 | 15 | (6.56 ± 0.07) b | 4.23 |
| 20 | (3.85 ± 0.2) c | 43.8 | 30 | (3.75 ± 0.1) c | 45.26 |
| 30 | (2.69 ± 0.12) d | 60.73 | 45 | (3.22 ± 0.11) d | 52.99 |
| 40 | (1.38 ± 0.11) e | 79.85 | 60 | (2.7 ± 0.05) e | 60.29 |
| 50 | (0.68 ± 0.15) f | 89.49 | 75 | (1.57 ± 0.03) f | 77.66 |
| Plants | Toxic Regression Equation (Y = A + B × X) | Correlation Coefficient | EC50 (µg/mL) | EC75 (µg/mL) |
|---|---|---|---|---|
| gallnut | Y = −0.6717 + 3.5590x | 0.9678 | 41.84 | 72.59 |
| clove | Y = 0.0250 + 3.9031x | 0.9593 | 21.68 | 36.34 |
| Ethanol Extract from Gallnut | Ethanol Extract from Clove | ||||
|---|---|---|---|---|---|
| Treatment | Disease Index | Disease Suppression (%) | Treatment | Disease Index | Disease Suppression (%) |
| 0 | 3.59 | 0 | 3.59 | ||
| 200 µg/mL | 2.92 | 18.66 e | 200 µg/mL | 3.26 | 9.19 e |
| 400 µg/mL | 2.75 | 23.40 d | 400 µg/mL | 2.96 | 17.55 d |
| 600 µg/mL | 2.47 | 31.20 c | 600 µg/mL | 2.83 | 21.17 c |
| 800 µg/mL | 2.32 | 35.38 b | 800 µg/mL | 2.56 | 28.69 b |
| 1000 µg/mL | 1.91 | 46.80 a | 1000 µg/mL | 2.23 | 37.88 a |
| Compounds | ESI | Parent (m/z) | Daughter (m/z) | Cone voltage/V | Colision energy/eV |
|---|---|---|---|---|---|
| eugenol | - | 163 | 148 | 29 | 13 |
| 121 | 24 | ||||
| lauric acid | - | 259 | 245 | 75 | 30 |
| 199 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hu, X.; Yang, C.; Wu, X.; Li, R.; Li, M. Growth Restriction of Rhizoctonia solani via Breakage of Intracellular Organelles Using Crude Extracts of Gallnut and Clove. Molecules 2021, 26, 1667. https://doi.org/10.3390/molecules26061667
Wang J, Hu X, Yang C, Wu X, Li R, Li M. Growth Restriction of Rhizoctonia solani via Breakage of Intracellular Organelles Using Crude Extracts of Gallnut and Clove. Molecules. 2021; 26(6):1667. https://doi.org/10.3390/molecules26061667
Chicago/Turabian StyleWang, Jian, Xianfeng Hu, Chenglong Yang, Xiaomao Wu, Rongyu Li, and Ming Li. 2021. "Growth Restriction of Rhizoctonia solani via Breakage of Intracellular Organelles Using Crude Extracts of Gallnut and Clove" Molecules 26, no. 6: 1667. https://doi.org/10.3390/molecules26061667






