Comparison of Coated and Immobilized Chiral Stationary Phases Based on Amylose tris-[(S)-α-Methylbenzylcarbamate] for the HPLC Enantiomer Separation of α-Lipoic Acid and Its Reduced Form
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enantioseparation under Normal-Phase Conditions
- (i)
- at 25 °C only ALA was baseline resolved (Rs > 1.5) on both CSPs, whereas the discrimination of the enantiomers of DHALA was very poor (i.e., the maximum value of α was 1.06);
- (ii)
- the chiral separation ability of the CSPs toward ALA decreased using ethanol as an alcoholic modifier (for example, α was 1.11 with ethanol and 1.29 with IPA on the Chirapak AS-H CSP).
2.2. Temperature-Variable HPLC
- (i)
- All the analyzed enantioseparations were characterized by the terms ΔΔH° and ΔΔS° of same negative sign. According to these results, the enantiorecognition process was favored by the enthalpic contribution and disfavored by the entropic term.
- (ii)
- The isoelution temperature values were, with a unique exception (entry 7, Table 2), higher than the explored range of temperature. Thus, when increasing the temperature within the enthalpy-controlled domain (|TISOΔΔS°| < |ΔΔH°|), no change in enantiomer elution order was observed. In the analysis of DHALA on the Chiralpak IH-3 with n-hexane/IPA/TFA 80:20:0.1 (v/v/v) (entry 7), a poor enantioseparation (α = 1.04) was achieved at 5 °C and above TISO (i.e., 25 °C); the two enantiomers coeluted and never split.
- (iii)
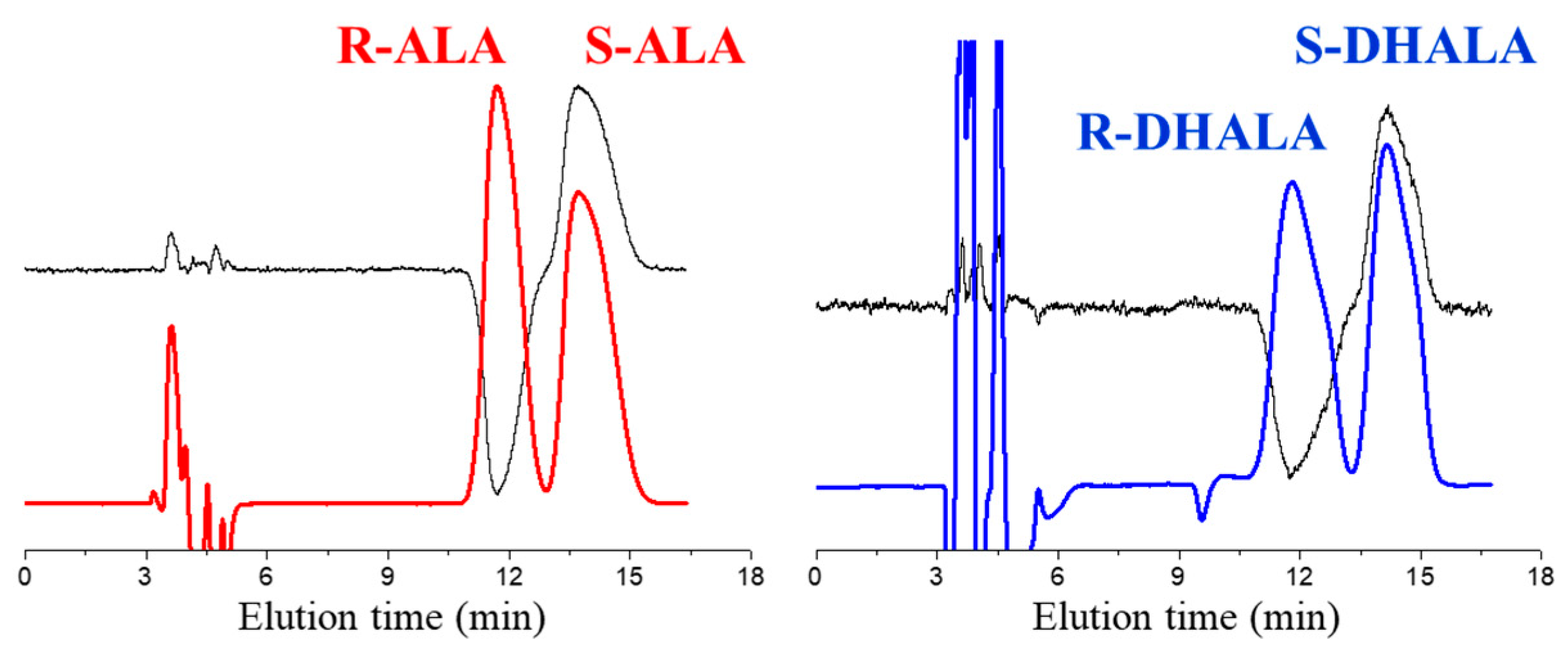
- The enantioseparation factors of both acids progressively increased as the column temperature decreased and moved away from the TISO value. Examples of enantioseparations of ALA and DHALA on the Chiralpak IH-3 optimized at the temperature of 5 °C are shown in Figure 2.
- (iv)
- The resolution factor values for DHALA obtained with non-standard mobile phases on the immobilized Chiralpak IH-3 (entries 8–10) were higher than those observed using the coated Chiralpak AS-H CSP in standard elution mode (entry 6).
- (v)
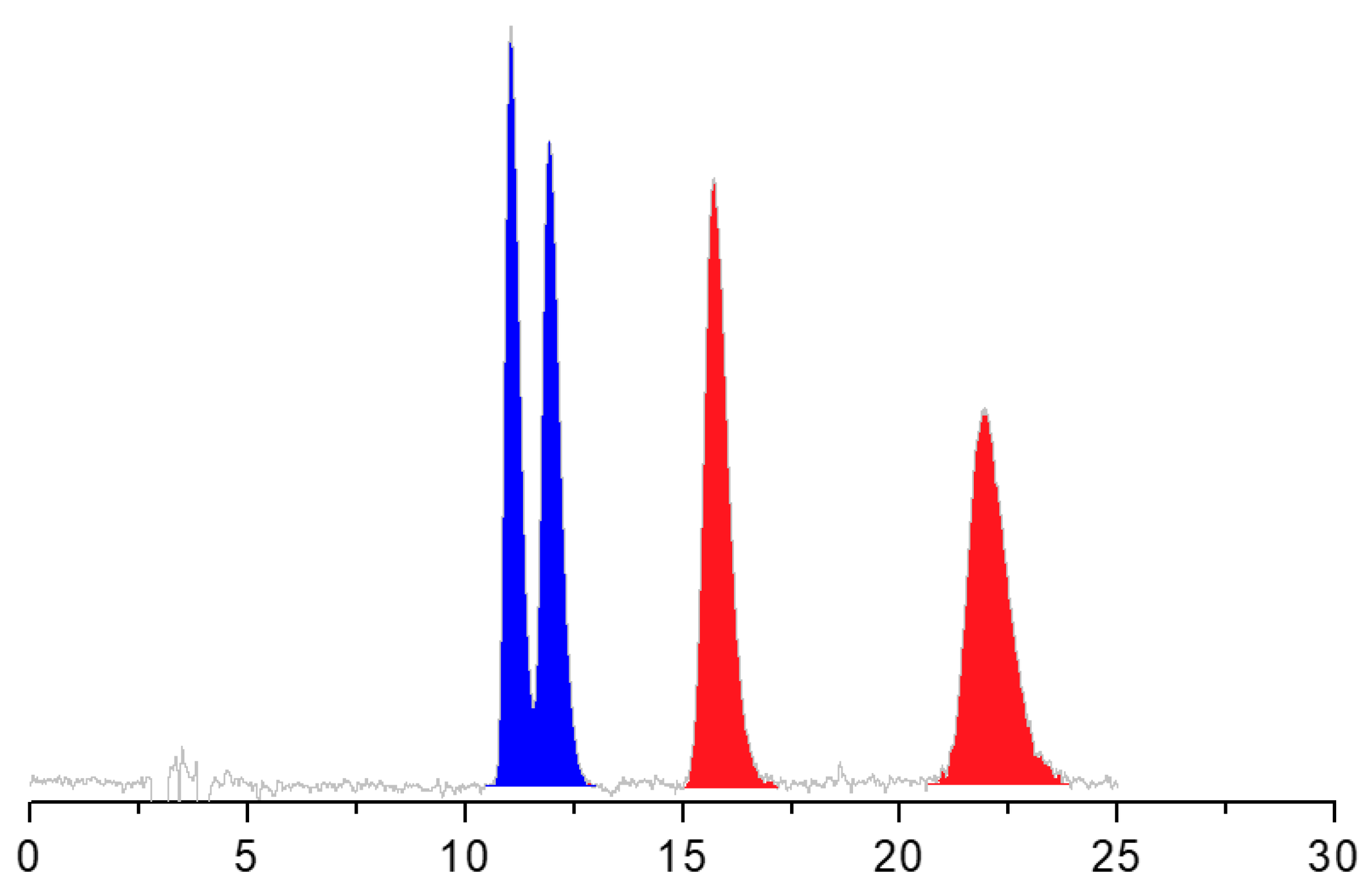
- To conclude this section, it is also interesting to mention that the enantiomers of ALA were separated from those of DHALA only on the Chiralpak AS-H CSP. The simultaneous chemo- and enantioseparation is shown in Figure 3.
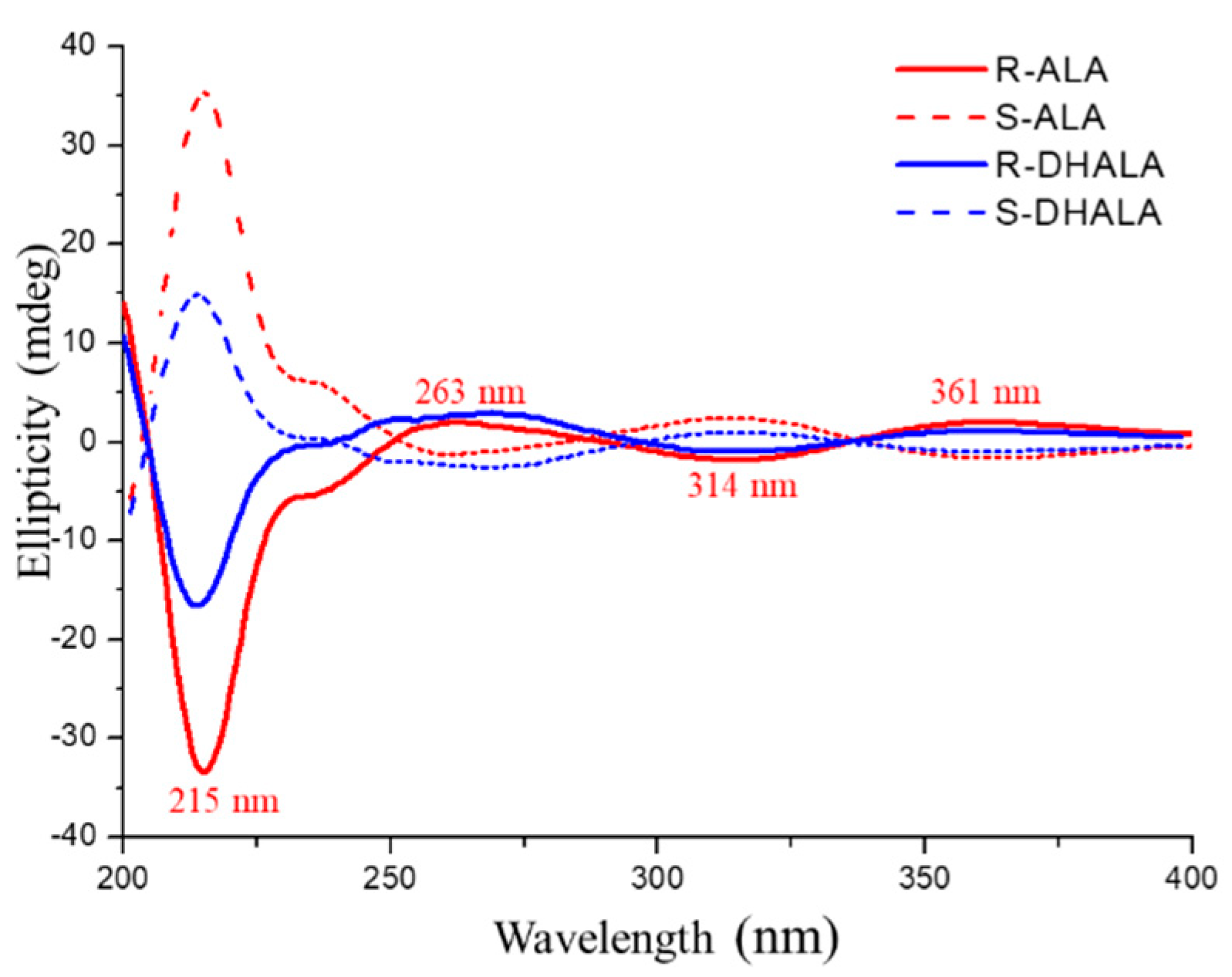
2.3. Absolute Configuration and Enantiomer Elution Order Determination
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Instruments and Chromatographic Conditions
3.3. Simulation of the Chiroptical Properties of (R)-DHALA Enantiomer Employed for the Assignment of the Absolute Configuration
- single point at the BLYP level of theory, employing the TZ2P Large Core basis set;
- ethanol as the solvent;
- 50 singlet excitations; diagonalization method: Davidson; velocity representation; scaling factor 1.3; peak width 20.0.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2011, 48, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Kemp, M.; Go, Y.M.; Jones, D.P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic. Biol. Med. 2008, 44, 921–937. [Google Scholar] [CrossRef] [Green Version]
- Vigna, L.; Solimena, G.; Bamonti, F.; Arcaro, M.; Fenoglio, C.; Oldoni, E.; Dellanoce, C.; Rossi, P.; Gori, F.; Figliola, R.; et al. Effects of 1-month R-α-lipoic acid supplementation on humans oxidative status: A pilot study. Prog. Nutr. 2017, 19, 14–25. [Google Scholar]
- Swaran, J.S. Flora Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar]
- Singh, U.; Jialal, I. Alpha-lipoic acid supplementation and diabetes. Nutr. Rev. 2008, 66, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Yılmaz, Y.B.; Antika, G.; Tumer, T.B.; Mahomoodally, M.F.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [Green Version]
- Carlson, D.A.; Smith, A.R.; Fischer, S.J.; Young, K.L.; Packer, L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern. Med. Rev. 2007, 12, 343–351. [Google Scholar]
- Niebch, G.; Bűchele, B.; Blome, J.; Grieb, S.; Brandt, G.; Kampa, P.; Raffael, H.H.; Locher, M.; Borbe, H.O.; Nubert, I.; et al. Enantioselective high-performance liquid chromatography assay of (+)R- and (−)S-α-Lipoic acid in human plasma. Chirality 1997, 9, 32–36. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ito, R.; Saito, K. Enantiomeric determination of α-lipoic acid in urine by LC/MS/MS. J. Pharm. Biomed. Anal. 2019, 166, 435–439. [Google Scholar] [CrossRef]
- Uchida, R.; Okamoto, H.; Ikuta, N.; Terao, K.; Hirota, T. Enantioselective Pharmacokinetics of α-Lipoic Acid in Rats. Int. J. Mol. Sci. 2015, 16, 22781–22794. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, R.; Carradori, S.; Guglielmic, P.; Pierini, M.; Casulli, A.; Cirilli, R. Enantiomers of triclabendazole sulfoxide: Analytical andsemipreparative HPLC separation, absolute configuration assignment, and transformation into sodium salt. J. Pharm. Biomed. Anal. 2017, 140, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Costi, R.; Di Santo, R.; Artico, M.; Roux, A.; Gallinella, B.; Zanitti, L.; La Torre, F. Enantioselective liquid chromatography of C3-chiral 2,3-dihydro-1,2,5-benzothiadiazepin-4(5H)-one and thione 1,1-dioxides on polyacrylamide- and polysaccharide-based chiral stationary phases. J. Chromatogr. A 2003, 99, 17–28. [Google Scholar] [CrossRef]
- Cirilli, R.; Ferretti, R.; La Torre, F.; Secci, D.; Bolasco, A.; Carradori, S.; Pierini, M. High-performance liquid chromatographic separation of enantiomers and diastereomers of 2-methylcyclohexanone thiosemicarbazone, and determination of absolute configuration and configurational stability. J. Chromatogr. A 2007, 1172, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.A.; Shah, P.A.; Shrivastav, P.S. Analytical separation of four stereoisomers of luliconazole using supercritical fluid chromatography: Thermodynamic aspects and simulation study with chiral stationary phase. J. Chromatogr. A 2020, 1625, 461299. [Google Scholar] [CrossRef]
- Aycock, D.F. Solvent Applications of 2-methyltetrahydrofuran in organometallic and biphasic reactions. Org. Process. Res. Dev. 2007, 11, 156–159. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A biomass-derived solvent with broad application in organic chemistry. Chem. Sus. Chem. 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Asnin, L.D.; Stepanova, M.V. Van’t Hoff analysis in chiral chromatography. J. Sep. Sci. 2018, 41, 1319–1337. [Google Scholar] [CrossRef]
- Panella, C.; Ferretti, R.; Casulli, A.; Cirilli, R. Temperature and eluent composition effects on enantiomer separation of carvedilol by high-performance liquid chromatography on immobilized amylose-based chiral stationary phases. J. Pharm. Anal. 2019, 9, 324–331. [Google Scholar] [CrossRef]
- Gasparrini, F.; Marini, F.; Misiti, D.; Pierini, M.; Villani, C. Temperature dependent elution order of enantiomers on a two-armed receptor HPLC chiral stationary phase. Enantiomer 1999, 4, 325–332. [Google Scholar]
- Schurig, V. Gas-chromatographic separation of enantiomers on optically-active metal-complex free stationary phases. Angew. Chem. Int. Ed. Engl. 1984, 23, 747–765. [Google Scholar] [CrossRef]
- Pirkle, W.H. Unusual effect of temperature on the retention of enantiomers on a chiral column. J. Chromatogr. 1991, 558, 1–6. [Google Scholar] [CrossRef]
- Díaz Merino, M.E.; Lancioni, C.; Padró, J.M.; Castells, C.B. Study of enantioseparation of β-blockers using amylose tris(3-chloro-5-methylphenylcarbamate) as chiral stationary phase under polar-organic, reversed-phase and hydrophilic interaction liquid chromatography conditions. J. Chromatogr. A 2020, 1634, 461685. [Google Scholar] [CrossRef] [PubMed]
- Zehnpfennig, B.; Wiriyasermkul, P.; Carlson, D.A.; Quick, M. Interaction of α-lipoic acid with the human Na+/multivitamin transporter (hSMVT)*. J. Biol. Chem. 2015, 290, 16372–16382. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Tuong, T.M.L.; Tian, J.M.; Pescitelli, G.; Gao, J.M. Ganorbifates A and B fromGanoderma orbiforme, determined by DFT calculations of NMR data and ECD spectra. Chem. Commun. 2020, 56, 10195–10198. [Google Scholar] [CrossRef] [PubMed]
- Górecki, M.; Zullo, V.; Iuliano, A.; Pescitelli, G. On the absolute stereochemistry of tolterodine: A circular dichroism study. Pharmaceuticals 2019, 12, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knežević, A.; Novak, J.; Pescitelli, G.; Vinković, V. Determination of the absolute configuration of (S)-N-(1-Aryl-allyl)-3,5-dinitrobenzamides and their elution order on brush-type chiral stationary phases. Eur. J. Org. Chem. 2018, 2018, 3982–3991. [Google Scholar] [CrossRef]

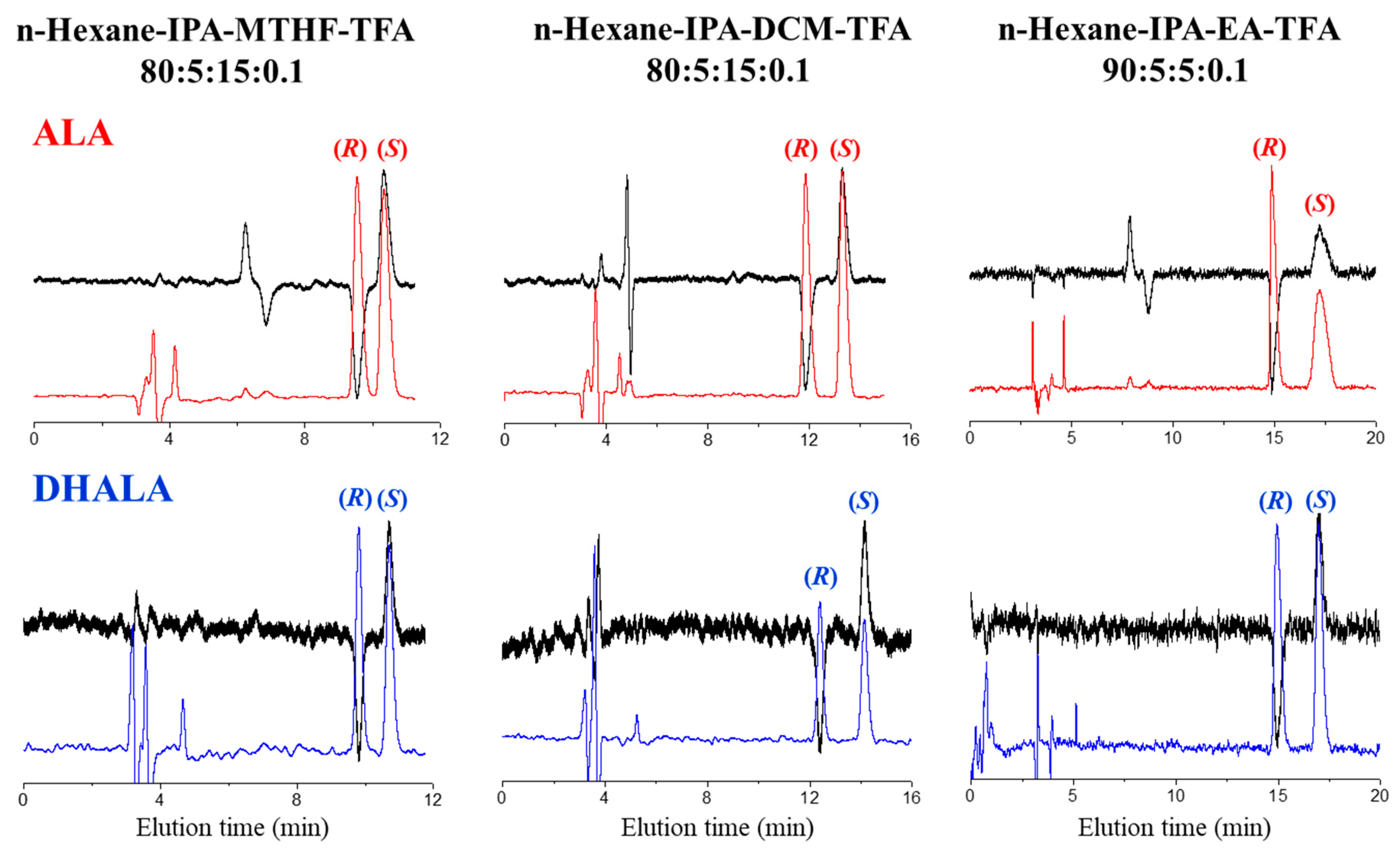

| Compound | CSP | Mobile Phase | k1 (AC)-(CD) 1 | α | Rs |
|---|---|---|---|---|---|
| ALA | AS-H | n-Hexane-IPA-TFA 80:20:0.1 | 2.06 (R)-(-) | 1.29 | 3.14 |
| IH-3 | n-Hexane-IPA-TFA 80:20:0.1 | 1.52 (R)-(-) | 1.16 | 2.77 | |
| AS-H | n-Hexane-EtOH-TFA 85:15:0.1 | 1.18 (R)-(-) | 1.11 | 1.23 | |
| IH-3 | n-Hexane-EtOH-TFA 85:15:0.1 | 1.19 (R)-(-) | 1.08 | 1.19 | |
| IH-3 | n-Hexane-IPA-DCM-TFA 80:5:15:0.1 | 2.23 (R)-(-) | 1.13 | 1.89 | |
| IH-3 | n-Hexane-IPA-EA-TFA 90:5:5:0.1 | 2.69 (R)-(-) | 1.09 | 1.75 | |
| IH-3 | n-Hexane-IPA-THF-TFA 80:5:15:0.1 | 1.09 (R)-(-) | 1.07 | <1 | |
| IH-3 | n-Hexane-IPA-MTHF-TFA 80:5:15:0.1 | 1.54 (R)-(-) | 1.10 | 1.12 | |
| DHALA | AS-H | n-Hexane-IPA-TFA 85:15:0.1 | 1.43 (R)-(-) | 1.06 | <1 |
| IH-3 | n-Hexane-IPA-TFA 85:15:0.1 | 1.18 (R)-(-) | 1.00 | - | |
| AS-H | n-Hexane-EtOH-TFA 85:15:0.1 | 0.89 (R)-(-) | 1.00 | - | |
| IH-3 | n-Hexane-EtOH-TFA 85:15:0.1 | 1.22 (R)-(-) | 1.00 | - | |
| IH-3 | n-Hexane-IPA-DCM-TFA 80:5:15:0.1 | 2.32 (R)-(-) | 1.14 | 2.88 | |
| IH-3 | n-Hexane-IPA-EA-TFA 90:5:5:0.1 | 2.79 (R)-(-) | 1.11 | 2.85 | |
| IH-3 | n-Hexane-IPA-THF-TFA 80:5:15:0.1 | 1.11 (R)-(-) | 1.07 | <1 | |
| IH-3 | n-Hexane-IPA-MTHF-TFA 80:5:15:0.1 | 1.67 (R)-(-) | 1.10 | 1.89 |
| Entry | Compound | CSP | Mobile Phase | α (5°C) | Rs (5°C) | ΔΔH° (kcal/mol) | ΔΔS° (e.u.) | TISO (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | ALA | AS-H | n-Hexane-IPA-TFA 80:20:0.1 | 1.49 | 4.62 | −1.23 | −3.61 | 68 |
| 2 | IH-3 | n-Hexane-IPA-TFA 80:20:0.1 | 1.22 | 3.16 | −0.36 | −0.95 | 108 | |
| 3 | IH-3 | n-Hexane-IPA-DCM-TFA 80:5:15:0.1 | 1.16 | 2.80 | −0.31 | −0.78 | 116 | |
| 4 | IH-3 | n-Hexane-IPA-EA-TFA 90:5:5:0.1 | 1.17 | 2.80 | −0.13 | −0.26 | 230 | |
| 5 | IH-3 | n-Hexane-IPA-MTHF-TFA 80:5:15:0.1 | 1.12 | 1.67 | −0.21 | −0.53 | 127 | |
| 6 | DHALA | AS-H | n-Hexane-IPA-TFA 80:20:0.1 | 1.11 | 1.24 | −0.40 | −1.24 | 52 |
| 7 | IH−3 | n-Hexane-IPA-TFA 80:20:0.1 | 1.04 | <1 | −0.30 | −1.00 | 25 | |
| 8 | IH-3 | n-Hexane-IPA-DCM-TFA 80:5:15:0.1 | 1.19 | 3.95 | −0.38 | −1.03 | 97 | |
| 9 | IH-3 | n-Hexane-IPA-EA-TFA 90:5:5:0.1 | 1.17 | 3.38 | −0.32 | −0.86 | 100 | |
| 10 | IH-3 | n-Hexane-IPA-MTHF-TFA 80:5:15:0.1 | 1.13 | 2.56 | −0.27 | −0.72 | 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosetti, A.; Villani, C.; Pierini, M.; Cirilli, R. Comparison of Coated and Immobilized Chiral Stationary Phases Based on Amylose tris-[(S)-α-Methylbenzylcarbamate] for the HPLC Enantiomer Separation of α-Lipoic Acid and Its Reduced Form. Molecules 2021, 26, 1747. https://doi.org/10.3390/molecules26061747
Rosetti A, Villani C, Pierini M, Cirilli R. Comparison of Coated and Immobilized Chiral Stationary Phases Based on Amylose tris-[(S)-α-Methylbenzylcarbamate] for the HPLC Enantiomer Separation of α-Lipoic Acid and Its Reduced Form. Molecules. 2021; 26(6):1747. https://doi.org/10.3390/molecules26061747
Chicago/Turabian StyleRosetti, Alessia, Claudio Villani, Marco Pierini, and Roberto Cirilli. 2021. "Comparison of Coated and Immobilized Chiral Stationary Phases Based on Amylose tris-[(S)-α-Methylbenzylcarbamate] for the HPLC Enantiomer Separation of α-Lipoic Acid and Its Reduced Form" Molecules 26, no. 6: 1747. https://doi.org/10.3390/molecules26061747
APA StyleRosetti, A., Villani, C., Pierini, M., & Cirilli, R. (2021). Comparison of Coated and Immobilized Chiral Stationary Phases Based on Amylose tris-[(S)-α-Methylbenzylcarbamate] for the HPLC Enantiomer Separation of α-Lipoic Acid and Its Reduced Form. Molecules, 26(6), 1747. https://doi.org/10.3390/molecules26061747







