Searching for Potential Markers of Glomerulopathy in Urine by HS-SPME-GC×GC TOFMS
Abstract
:1. Introduction
2. Results
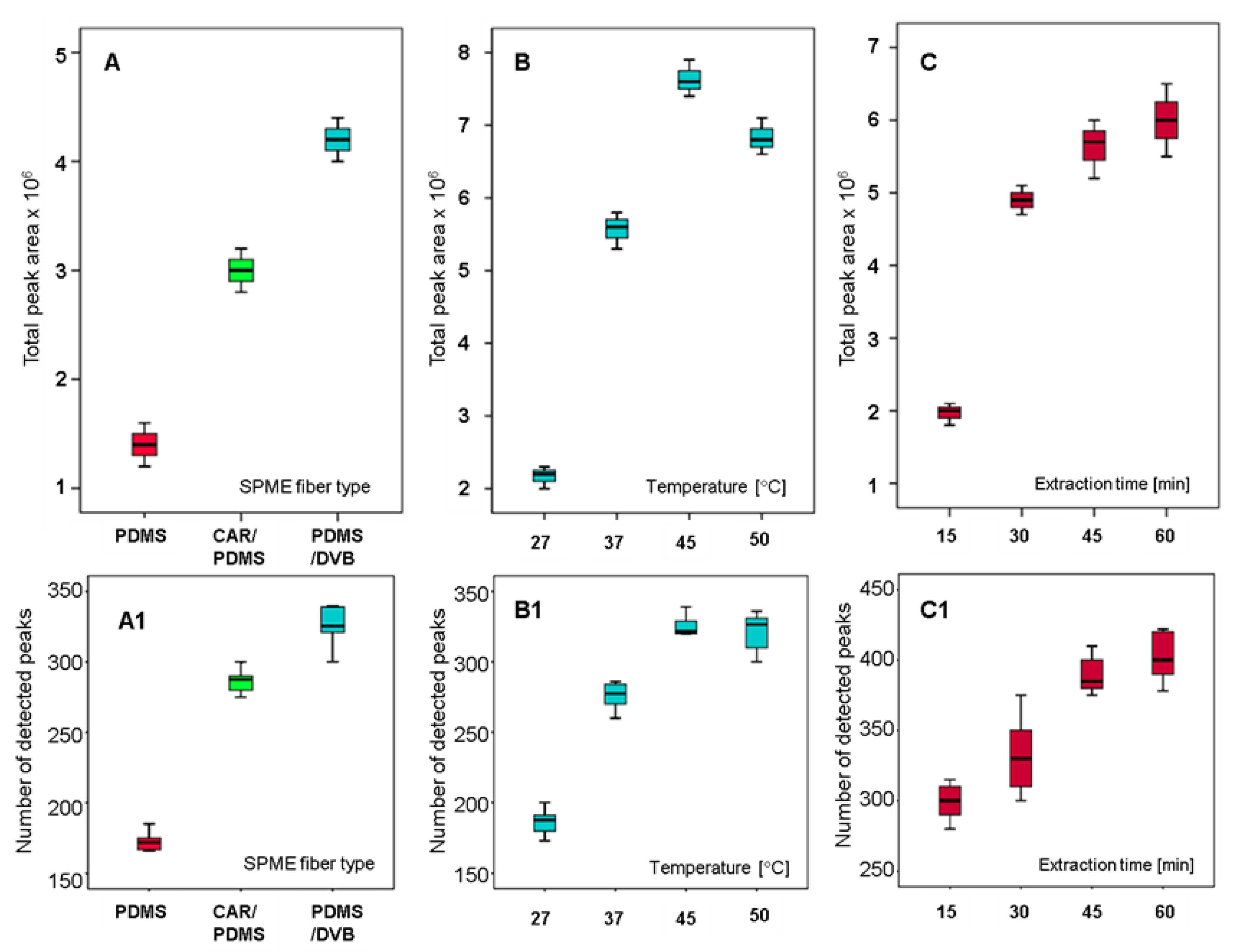
2.1. Fiber Selection
2.2. Extraction Temperature
2.3. Extraction Time
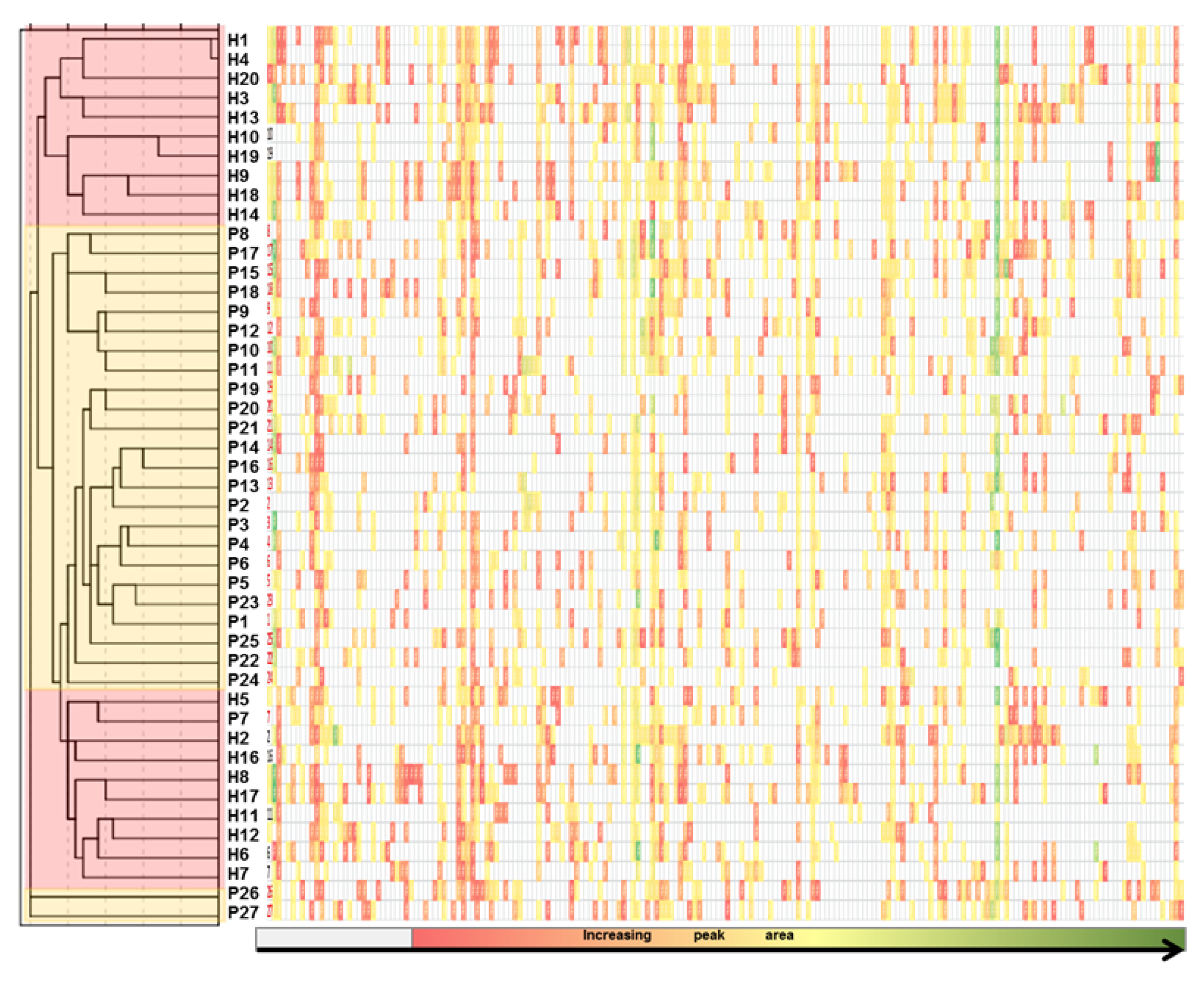
2.4. Identification
2.5. Statistical Analyses
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Apparatus
4.3. Data Processing
4.4. Sample Pre-Processing and Extraction
4.5. Human Subjects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef]
- Wagenstaller, M.; Buettner, A. Characterization of odorants in human urine using a combined chemo-analytical and human-sensory approach: A potential diagnostic strategy. Metabolomics 2013, 9, 9–20. [Google Scholar] [CrossRef]
- Zlatkis, A.; Brazell, R.S.; Poole, C.F. The role of organic volatile profiles in clinical diagnosis. Clin. Chem. 1981, 27/6, 789–797. [Google Scholar] [CrossRef]
- Mills, G.A.; Walker, V.; Mughal, H. Quantitative determination of trimethylamine in urine by solidphase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. B 1999, 723, 281–285. [Google Scholar] [CrossRef]
- Smith, S.; Burden, H.; Persad, R.; Whittington, K.; de Lacy Costello, B.; Ratcliffe, N.M.; Probert, C.S. A comparative study of the analysis of human urine headspace using gas chromatography–mass spectrometry. J. Breath Res. 2008, 2, 037022. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Silva, C.L.; Passos, M.; Camara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; del Nogal Sánchez, M.; Pozas, Á.P.C.; Pavón, J.L.P.; Cordero, B.M. Determination of ketones and ethyl acetate−a preliminary study for the discrimination of patients with lung cancer. Anal. Bioanal. Chem. 2017, 409, 5689–5696. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Carvalho, M.; Henrique, R.; Jeronimo, C.; Moreira, N.; de Lourdes Bastos, M.; Guedes de Pinho, P. Analysis of volatile human urinary metabolome by solid-phase microextraction in combination with gas chromatography–mass spectrometry for biomarker discovery: Application in a pilot study to discriminate patients with renal cell carcinoma. Eur. J. Cancer 2014, 50, 1993–2002. [Google Scholar] [CrossRef]
- Silva, A.I., Jr.; Pereira, H.M.G.; Casilli, A.; Conceic¸, F.C.; Aquino Neto, F.R. Analytical challenges in doping control: Comprehensive two-dimensional gas chromatography with time of flight mass spectrometry, a promising option. J. Chromatogr. A 2009, 1216, 2913–2922. [Google Scholar] [CrossRef]
- Mitrevski, B.S.; Brenna, J.T.; Zhang, Y.; Marriott, P.J. Application of comprehensive two-dimensional gas chromatography to sterols analysis. J. Chromatogr. A 2008, 1214, 134–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Auchu, H.J.T.R.J.; Brenna, J.T. Comprehensive two dimensional gas chromatography fast quadrupole mass spectrometry (GC×GC-qMS) for urinary steroid profiling. mass spectral characteristics with chemical ionization. Drug Test. Anal. 2011, 3, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.B.; Snow, N.H. Analysis of Salvinorin A in plants, water, and urine using solid-phase microextraction-comprehensive two-dimensional gas chromatography–time of flight mass spectrometry. J. Chromatogr. A 2012, 1226, 110–115. [Google Scholar] [CrossRef]
- Vasquez, N.P.; Crosnier de Bellaistre-Bonose, M.; Lévêque, N.; Thioulouse, E.; Doummar, D.; Billette de Villemeur, T.; Rodriguez, D.; Couderc, R.; Robin, S.; Courderot-Masuyer, C.; et al. Advances in the metabolic profiling of acidic compounds in children’s urines achieved by comprehensive two-dimensional gas chromatography. J. Chromatogr. B 2015, 1002, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Caldeira, M.; Carrola, J.; Santos, M.; Cruz, N.; Duarte, I.F. Exploring the human urine metabolomic potentialities by comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry. J. Chromatogr. A 2012, 1252, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; Benigni, A.; Remuzzi, A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J. Clin. Investig. 2006, 116, 288–296. [Google Scholar] [CrossRef]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lu, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1917–2014. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.; Rodrigez-Peria, J.M.A.B.; Ortiz, A.; Martinez-Salgado, C.; Hernandez, L.F.J. Etiopathology of chronic tubular, glomerular and renovascular nephropaties. Clinical implications. J. Transl. Med. 2011, 9, 13–39. [Google Scholar] [CrossRef]
- Couser, W.G. Pathogenesis of glomerular damage in glomerulonephritis. Nephrol. Dial. Transplant. 1998, 13, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Couser, W.G. Mechanisms of immune-deposit formation and the mediation of immune renal injury. Clin. Exp. Nephrol. 2005, 9, 183–191. [Google Scholar] [CrossRef]
- Couser, W.G. Complement inhibitors and glomerulonephritis: Are we there yet? J. Am. Soc. Nephrol. 2003, 14, 815–818. [Google Scholar] [CrossRef]


| ID | Compound | SSGroup | df | SSError | df | F | p |
|---|---|---|---|---|---|---|---|
| 1 | Cyclohexanone | 160.14 | 1 | 99.80 | 39 | 54.56 | 0.000 |
| 2 | 3-Ethylcyclopentanone | 60.33 | 1 | 121.36 | 39 | 16.90 | 0.000 |
| 3 | 3-Hexanone | 87.83 | 1 | 166.31 | 39 | 17.96 | 0.000 |
| 4 | 3-Heptanone | 102.78 | 1 | 145.92 | 39 | 23.95 | 0.000 |
| 5 | Methyl hexadecanoate | 68.88 | 1 | 134.76 | 39 | 17.38 | 0.000 |
| 6 | 9-Hexadecen-1-ol | 75.72 | 1 | 142.22 | 39 | 18.10 | 0.000 |
| 7 | 3-Methyl-2-pentanone | 73.83 | 1 | 209.25 | 39 | 12.00 | 0.001 |
| 8 | 6,10-Dimethyl-5,9-undecadien-2-one | 49.61 | 1 | 117.49 | 39 | 14.36 | 0.001 |
| 9 | 2-Pentanone | 44.38 | 1 | 144.91 | 39 | 10.41 | 0.003 |
| 10 | Acetophenone | 25.60 | 1 | 117.55 | 39 | 7.40 | 0.010 |
| 11 | 2-Methoxy-4-vinylphenol | 19.72 | 1 | 119.53 | 39 | 5.61 | 0.024 |
| 12 | 1-Decanol | 28.56 | 1 | 184.65 | 39 | 5.26 | 0.028 |
| 13 | N-Acetylpyrrole | 28.44 | 1 | 204.56 | 39 | 4.73 | 0.037 |
| 14 | 6-Methylhept-5-en-2-one | 22.96 | 1 | 198.97 | 39 | 3.92 | 0.056 |
| 15 | Dimethyl sulfone | 3.24 | 1 | 37.79 | 39 | 2.91 | 0.097 |
| 16 | 1-Tetradecanol | 14.96 | 1 | 190.06 | 39 | 2.68 | 0.111 |
| 17 | 4-Heptanone | 9.43 | 1 | 128.86 | 39 | 2.49 | 0.124 |
| 18 | Benzaldehyde | 1.66 | 1 | 23.90 | 39 | 2.36 | 0.134 |
| 19 | 2-Nonanone | 14.35 | 1 | 213.06 | 39 | 2.29 | 0.139 |
| 20 | 5-Methyl-3-hexanone | 13.07 | 1 | 210.41 | 39 | 2.11 | 0.155 |
| 21 | Dimethyl trisulfide | 10.23 | 1 | 190.57 | 39 | 1.83 | 0.186 |
| 22 | 2-Aminobenzaldehyde | 9.40 | 1 | 222.11 | 39 | 1.44 | 0.239 |
| 23 | 3-Methylcyclopentanone | 8.12 | 1 | 230.47 | 39 | 1.20 | 0.281 |
| 24 | Hexanal | 3.85 | 1 | 217.07 | 39 | 0.60 | 0.443 |
| 25 | 1-Octanol | 3.59 | 1 | 245.37 | 39 | 0.50 | 0.485 |
| 26 | Benzeneacetaldehyde | 3.43 | 1 | 234.68 | 39 | 0.50 | 0.486 |
| 27 | 2,5-Dimethylpyrazine | 2.85 | 1 | 198.49 | 39 | 0.49 | 0.490 |
| 28 | Nonanal | 1.50 | 1 | 155.73 | 39 | 0.33 | 0.571 |
| 29 | 9-Octadecen-1-ol | 1.47 | 1 | 183.26 | 39 | 0.27 | 0.605 |
| 30 | Indole | 0.35 | 1 | 49.34 | 39 | 0.24 | 0.626 |
| 31 | Theaspirane | 1.37 | 1 | 218.11 | 39 | 0.21 | 0.647 |
| 32 | Benzonitrile | 0.52 | 1 | 185.47 | 39 | 0.09 | 0.760 |
| 33 | 2-Heptanone | 0.27 | 1 | 157.55 | 39 | 0.06 | 0.811 |
| 34 | 4-Methylphenol | 0.27 | 1 | 170.99 | 39 | 0.05 | 0.818 |
| 35 | Phenol | 0.24 | 1 | 166.54 | 39 | 0.05 | 0.828 |
| 36 | Decanal | 0.09 | 1 | 73.76 | 39 | 0.04 | 0.837 |
| 37 | 1-Methyl-4-(1-methylethenyl)-benzene | 0.08 | 1 | 192.35 | 39 | 0.01 | 0.905 |
| 38 | N-Phenylformamide | 0.00 | 1 | 227.31 | 39 | 0.00 | 0.979 |
| 39 | Ethyl acetate | 0.00 | 1 | 328.20 | 39 | 0.00 | 0.986 |
| 40 | Octanal | 0.00 | 1 | 199.51 | 39 | 0.00 | 0.986 |
| Diseased Group | Healthy Group | ||||||
|---|---|---|---|---|---|---|---|
| ID | Compound | Mean | Min | Max | Mean | Min | Max |
| 1 | Cyclohexanone | 3.28 × 105 | 1.33 × 104 | 9.43 × 105 | 3.54 × 104 | 2.23 × 104 | 4.64 × 104 |
| 2 | 3-Ethylcyclopentanone | 1.10 × 105 | 5.14 × 103 | 4.53 × 105 | 3.41 × 104 | 9.57 × 103 | 6.58 × 104 |
| 3 | 3-Hexanone | 9.41 × 105 | 1.65 × 104 | 1.35 × 106 | 3.44 × 104 | 2.92 × 104 | 1.06 × 106 |
| 4 | 3-Heptanone | 2.84 × 106 | 6.06 × 104 | 2.57 × 107 | 1.75 × 105 | 7.46 × 104 | 3.90 × 106 |
| 5 | Methyl hexadecanoate | 7.63 × 104 | 7.64 × 103 | 3.64 × 105 | 2.19 × 104 | 6.99 × 103 | 8.38 × 104 |
| 6 | 9-Hexadecen-1-ol | 7.39 × 104 | 5.28 × 103 | 4.34 × 105 | 9.39 × 103 | 2.78 × 103 | 1.93 × 105 |
| 7 | 3-Methyl-2-pentanone | 2.69 × 106 | 2.90 × 104 | 2.81 × 107 | 1.81 × 105 | 8.95 × 103 | 1.19 × 107 |
| 8 | 6,10-Dimethyl-5,9-undecadien-2-one | 1.41 × 105 | 2.47 × 104 | 3.91 × 105 | 7.41 × 104 | 1.93 × 104 | 1.73 × 105 |
| 9 | 2-Pentanone | 1.94 × 107 | 6.39 × 104 | 7.62 × 107 | 3.18 × 106 | 1.46 × 106 | 1.05 × 108 |
| 10 | Acetophenone | 1.08 × 105 | 4.37 × 104 | 1.40 × 105 | 1.23 × 104 | 8.19 × 104 | 1.79 × 105 |
| 11 | 2-Methoxy-4-vinylphenol | 1.95 × 105 | 1.76 × 104 | 9.97 × 105 | 1.07 × 105 | 1.19 × 104 | 4.58 × 105 |
| 12 | 1-Decanol | 1.62 × 105 | 2.46 × 104 | 5.20 × 105 | 2.82 × 105 | 1.99 × 104 | 7.00 × 105 |
| 13 | N-Acetylpyrrole | 3.12 × 105 | 1.11 × 104 | 1.06 × 106 | 6.87 × 104 | 3.17 × 104 | 7.43 × 105 |
| 14 | 6-Methylhept-5-en-2-one | 1.84 × 105 | 9.19 × 103 | 9.31 × 105 | 2.00 × 105 | 2.30 × 104 | 7.26 × 105 |
| 15 | Dimethyl sulfone | 3.24 × 104 | 1.10 × 104 | 1.97 × 105 | 2.97 × 104 | 4.16 × 103 | 9.29 × 104 |
| 16 | 1-Tetradecanol | 2.93 × 105 | 1.33 × 104 | 1.23 × 106 | 2.11 × 105 | 3.10 × 104 | 4.69 × 105 |
| 17 | 4-Heptanone | 6.50 × 106 | 2.24 × 104 | 3.82 × 107 | 5.73 × 106 | 1.77 × 104 | 2.25 × 107 |
| 18 | Benzaldehyde | 6.23 × 104 | 6.18 × 103 | 1.89 × 105 | 1.01 × 105 | 1.26 × 104 | 3.58 × 105 |
| 19 | 2-Nonanone | 1.56 × 105 | 1.31 × 104 | 7.72 × 105 | 1.54 × 105 | 1.07 × 104 | 5.24 × 105 |
| 20 | 5-Methyl-3-hexanone | 1.89 × 106 | 5.78 × 104 | 1.48 × 107 | 3.22 × 106 | 1.92 × 104 | 1.90 × 107 |
| 21 | Dimethyl trisulfide | 6.17 × 104 | 5.14 × 103 | 1.88 × 105 | 4.51 × 104 | 5.76 × 103 | 7.46 × 104 |
| 22 | 2-Aminobenzaldehyde | 1.93 × 105 | 6.12 × 103 | 6.80 × 105 | 2.65 × 105 | 8.50 × 104 | 5.20 × 105 |
| 23 | 3-Methylcyclopentanone | 2.76 × 105 | 4.29 × 104 | 8.61 × 105 | 2.05 × 105 | 3.19 × 104 | 7.21 × 105 |
| 24 | Hexanal | 1.80 × 106 | 1.16 × 105 | 6.48 × 106 | 9.01 × 105 | 8.86 × 104 | 1.86 × 106 |
| 25 | 1-Octanol | 5.13 × 105 | 1.01 × 104 | 3.61 × 106 | 6.37 × 105 | 7.98 × 104 | 3.42 × 106 |
| 26 | Benzeneacetaldehyde | 1.43 × 105 | 2.67 × 104 | 1.25 × 106 | 1.47 × 105 | 5.34 × 104 | 5.60 × 105 |
| 27 | 2,5-Dimethylpyrazine | 1.19 × 105 | 1.06 × 104 | 5.67 × 105 | 7.50 × 104 | 1.82 × 104 | 2.36 × 105 |
| 28 | Nonanal | 2.26 × 105 | 2.04 × 104 | 1.09 × 106 | 1.28 × 105 | 7.82 × 104 | 1.77 × 105 |
| 29 | 9-Octadecen-1-ol | 4.98 × 105 | 3.25 × 104 | 1.79 × 106 | 4.60 × 105 | 3.20 × 104 | 9.43 × 105 |
| 30 | Indole | 1.20 × 105 | 2.19 × 104 | 3.47 × 105 | 9.08 × 104 | 1.45 × 104 | 2.00 × 105 |
| 31 | Theaspirane | 1.09 × 105 | 7.87 × 103 | 2.62 × 105 | 1.29 × 105 | 4.64× 104 | 3.72 × 105 |
| 32 | Benzonitrile | 4.53 × 104 | 6.57 × 103 | 6.30 × 104 | 1.67 × 104 | 8.76 × 103 | 3.10 × 104 |
| 33 | 2-Heptanone | 1.92 × 105 | 1.13 × 104 | 1.41 × 106 | 1.75 × 105 | 7.46 × 104 | 5.69 × 105 |
| 34 | 4-Methylphenol | 1.22 × 105 | 1.71 × 104 | 5.13 × 105 | 1.71 × 105 | 2.71 × 104 | 1.18 × 106 |
| 35 | Phenol | 3.52 × 104 | 9.95 × 103 | 4.67 × 105 | 2.90 × 104 | 2.08 × 104 | 1.11 × 105 |
| 36 | Decanal | 1.39 × 105 | 1.62 × 104 | 7.38 × 105 | 1.56 × 104 | 2.06 × 104 | 2.06 × 104 |
| 37 | 1-Methyl-4-(1-methylethenyl)-benzene | 1.13 × 105 | 1.12 × 104 | 1.41 × 105 | 1.41 × 105 | 1.90 × 104 | 6.09 × 105 |
| 38 | N-Phenylformamide | 1.17 × 105 | 1.59 × 104 | 3.32 × 105 | 1.54 × 105 | 3.02 × 104 | 3.35 × 105 |
| 39 | Ethyl acetate | 1.56 × 107 | 2.02 × 105 | 7.11 × 107 | 1.41 × 107 | 1.21 × 101 | 6.41 × 1057 |
| 40 | Octanal | 2.17 × 105 | 5.62 × 104 | 6.97 × 105 | 1.62 × 105 | 1.49 × 105 | 4.34 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ligor, T.; Zawadzka, J.; Strączyński, G.; González Paredes, R.M.; Wenda-Piesik, A.; Ratiu, I.A.; Muszytowski, M. Searching for Potential Markers of Glomerulopathy in Urine by HS-SPME-GC×GC TOFMS. Molecules 2021, 26, 1817. https://doi.org/10.3390/molecules26071817
Ligor T, Zawadzka J, Strączyński G, González Paredes RM, Wenda-Piesik A, Ratiu IA, Muszytowski M. Searching for Potential Markers of Glomerulopathy in Urine by HS-SPME-GC×GC TOFMS. Molecules. 2021; 26(7):1817. https://doi.org/10.3390/molecules26071817
Chicago/Turabian StyleLigor, Tomasz, Joanna Zawadzka, Grzegorz Strączyński, Rosa M. González Paredes, Anna Wenda-Piesik, Ileana Andreea Ratiu, and Marek Muszytowski. 2021. "Searching for Potential Markers of Glomerulopathy in Urine by HS-SPME-GC×GC TOFMS" Molecules 26, no. 7: 1817. https://doi.org/10.3390/molecules26071817
APA StyleLigor, T., Zawadzka, J., Strączyński, G., González Paredes, R. M., Wenda-Piesik, A., Ratiu, I. A., & Muszytowski, M. (2021). Searching for Potential Markers of Glomerulopathy in Urine by HS-SPME-GC×GC TOFMS. Molecules, 26(7), 1817. https://doi.org/10.3390/molecules26071817







