Abstract
Phytophthora is a genus of microorganisms that cause devastating dieback and root-rot diseases in thousands of plant hosts worldwide. The economic impact of Phytophthora diseases on crops and native ecosystems is estimated to be billions of dollars per annum. These invasive pathogens are extremely difficult to control using existing chemical means, and the effectiveness of the few treatments available is being jeopardized by increasing rates of resistance. There is an urgent need to identify new chemical treatments that are effective against Phytophthora diseases. Natural products have long been regarded as “Nature’s medicine chest”, providing invaluable leads for developing front-line drugs and agrochemical agents. Here, we have screened a natural product-inspired library of 328 chemicals against two key Phytophthora species: Phytophthora cinnamomi and Phytophthora agathidicida. The library was initially screened for inhibition of zoospore germination. From these screens, we identified twenty-one hits that inhibited germination of one or both species. These hits were further tested in mycelial growth inhibition studies to determine their half-maximal inhibitory concentrations (IC50s). Four compounds had IC50 values of approximately 10 µM or less, and our best hit had IC50s of approximately 3 µM against both Phytophthora species tested. Overall, these hits may serve as promising leads for the development of new anti-Phytophthora agrochemicals
1. Introduction
Phytophthora (from the Greek for “plant-destroyers”) is a genus of plant pathogens in the class Oomycota. Oomycetes, or water molds, are eukaryotic microorganisms that superficially resemble fungi but are phylogenetically closer to algae [1]. Dieback and root rot diseases caused by Phytophthora species are notoriously difficult to control or eradicate. Phytophthora lack many common fungicide targets, such as the ergosterol biosynthesis pathway and chitin-based cell walls [2,3]. Compared to true fungi, Phytophthora also have a more complex lifecycle that includes not only fungus-like mycelia but also short-lived, motile zoospores and long-lived oospores and/or chlamydospores.
Globally, Phytophthora diseases cost the horticultural industry billions of dollars each year. One disease alone, potato late blight (caused by P. infestans), causes annual revenue losses of hundreds of millions of dollars in the USA through treatment costs and crop loss [4]. The effects of Phytophthora on natural ecosystems are equally devastating; sudden oak death caused by P. ramorum and root rot caused by P. cinnamomi are just two examples of Phytophthora diseases causing widespread destruction in the forests of the USA and Australia, respectively [5,6]. Given the widespread economic and ecological damage these organisms cause, there is a pressing need to develop new preventative and/or curative options for Phytophthora diseases.
Natural products have long been regarded as “Nature’s medicine chest”, providing invaluable scaffolds for developing front-line drugs and agrochemical agents. The chemical structures of natural products have evolved over several millennia for specific biochemical purposes, and their molecular frameworks can be considered “privileged scaffolds” [7]. Examples of natural products with promising anti-oomycete activity include the glucosinolates (and their hydrolysis products, isothiocyanates) present in the Brassicaceae plant family, which recent studies have shown are effective against different lifecycle stages of several Phytophthora species [8,9,10]. Similarly, metabolites from fungal extracts have also been shown to have anti-Phytophthora activities [11]. In this study, we have screened a natural product-inspired library of 328 chemicals against two key Phytophthora species: Phytophthora cinnamomi and Phytophthora agathidicida.
Phytophthora cinnamomi is a broad host range pathogen that infects ~5000 species of plants, devasting natural ecosystems, agriculture, forestry and horticulture worldwide [6]. Phytophthora agathidicida is the causative agent of kauri dieback, a disease afflicting the kauri (Agathis australis) forests of northern New Zealand. Kauri are one of the largest and longest-living tree species in the world, and these trees are both culturally and ecologically significant to New Zealand [12].
At present, one of the most effective treatments for both Phytophthora species is potassium phosphite [12,13,14,15], which functions both as an antimicrobial and as an inducer of host defence mechanisms [16,17,18]. While phosphite is relatively effective and has low environmental toxicity, it does not eradicate the pathogens, meaning ongoing treatment is required [14]. Further, there is evidence of resistance developing in some Phytophthora species [19,20,21]. Given the above limitations, the scarcity of alternative control options and the environmental concerns surrounding the use of existing fungicides [22], there is an urgent need to discover and develop new anti-Phytophthora treatments. Here, we describe the results of screening 328 nature-inspired products, intermediates and organocatalysts for activity against P. agathidicida and P. cinnamomi.
2. Results and Discussion
In the present study, we used a microtiter plate-based assay to screen a library of 328 natural product-inspired compounds (Supplementary Table S1) for anti-Phytophthora activity. Our initial screen focused on the inhibition of zoospore germination. This initial screen revealed twenty-one compounds (Figure 1) that prevented zoospore germination for at least one of the Phytophthora species tested.
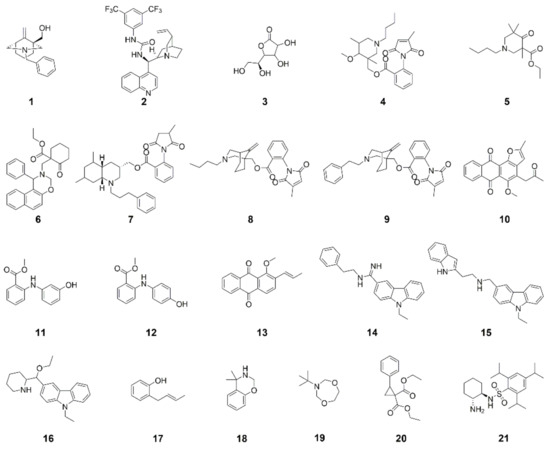
Figure 1.
Hits from the initial screening of a library of 328 compounds for inhibition of P. agathidicida and/or P. cinnamomi zoospore germination.
Of these twenty-one hits, fifteen inhibited both species (2–5, 8, 10–15, 17, 19–21), three inhibited P. cinnamomi only (6, 7, 18) and three inhibited P. agathidicida only (1, 9, 16) only.
The twenty-one compounds from the initial screen were next assayed for inhibition of mycelial growth at a range of concentrations to determine their IC50s. For compounds that were active against both species, IC50 values for mycelial inhibition were generally very similar between the species (Table 1).

Table 1.
Half-maximal mycelial inhibition concentrations (IC50s) of compounds active against P. cinnamomi and P. agathidicida.
Four of the compounds (2, 15, 20 and 21) showed particularly strong mycelial growth inhibition, with IC50 values of ~10 µM or less for at least one of the species tested. Overall, the most potent compound was 20, with IC50 values of ~3 µM for both species. These results compare favourably with existing oomycides such as copper sulfate and fosetyl-aluminium (P. cinnamomi IC50 values of ~30 and 150 µM, respectively) [23,24]. The compounds also show stronger inhibition than several other natural products with anti-Phytophthora activity. Examples targeting P. cinnamomi include a plant-derived triterpenoid (76% growth inhibition at 200 µM) [25]; Phlomis purpurea root extract (85.5% inhibition at 10 mg/mL) [26]; flufuran and derivative compounds (10–100% inhibition at ~1.4 mM) [27], Magnolia vovidessi extracts (~30–70% inhibition at 2 mg/mL) [28] and pomegranate extract (~40% inhibition at 10 mg/mL) [29].
Though there were no striking structural similarities between our top four compounds, there were a number of recurring moieties amongst the other hits. These promising scaffolds for further lead development are discussed below.
The structures of 4, 7, 8 and 9 all contain an N-substituted anthranilate ester and are based on the diterpene alkaloid methyllycaconitine (MLA), which is produced by plants of the Delphinium and Aconitum genera [30,31,32]. MLA is known to be a nicotinic acetylcholine receptor antagonist in mammals and insects, and structure-activity studies of MLA have demonstrated the importance of the N-substituted anthranilate ester moiety and N-ethyl tertiary amine for its activity [33,34]. Therefore, several research groups have explored the incorporation of these functional groups into simpler, MLA-related compounds, such as 4, 7, 8 and 9, for use as pesticides or pharmacological tools [30,35,36,37,38,39,40]. For 4, 7, 8 and 9, the N-substitution of the anthranilate ester is a methylsuccinimide or methylmaleimide group. Nicotinic acetylcholine receptors are absent in oomycetes [41], so the anti-Phytophthora activity of 4, 7, 8 and 9 observed in this work is not likely to be due to nAChR antagonism. Other maleimide-containing compounds have been reported to have antifungal and/or anti-Phytophthora activities [42,43]; however, the mode-of-action of these compounds is currently unknown. Interestingly, 7 and 9 displayed species-specific activities with 7 being active against only P. cinnamomi and 9 against only P. agathidicida, while 4 and 8 were active against both species. The only structural difference between 8 and 9 is in the N-substitution of the tertiary amine: 4 and 8 contain an N-butyl group, but 7 and 9 contain an N-propylphenyl and N-ethylphenyl group, respectively. Therefore, this is a possible moiety of interest in future structure-activity studies.
As with 4, 7, 8 and 9, compounds 11 and 12 also contain an N-substituted anthranilate ester. Both 11 and 12 are intermediates in the synthesis of inhibitors of Mycobacterium tuberculosis (Mtb) anthranilate phosphoribosyltransferase (AnPRT), which catalyzes a key step in the tryptophan biosynthesis pathway. Tryptophan biosynthesis is essential for Mtb pathogenesis and, therefore, is a target for the development of anti-Mtb drugs [44,45,46,47,48]. Oomycetes, including Phytophthora, also use an AnPRT for tryptophan biosynthesis [49]. In the present study, ten additional compounds structurally similar to 11 and 12 were tested in mycelial IC50 assays against P. cinnamomi and P. agathidicida to look for evidence of structure-activity relationships. Activity varied among these compounds and was often substantially lower than 11 and 12 (Supplementary Table S2), but four of the compounds were, to a degree, active against both species. Substitution of both phenyl groups at the position ortho to the amine with a methyl ester and substitution of the hydroxy group resulted in decreased activity.
Multiple hits contain an anthraquinone scaffold (i.e., 10 and 13). Anthraquinones are anthracene (three fused benzene rings)-derived structures, but the central ring contains two carbonyl groups, thus providing the quinone substructure. In nature, anthraquinones and their derivatives are produced as secondary metabolites by plants, lichens, insects and higher filamentous fungi. Naturally occurring anthraquinones extracted from plants have long been used in foods, cosmetics and traditional Chinese medicine [50]. Anthraquinones are also increasingly being explored as privileged scaffolds for pharmaceutical development due to their antimicrobial, anti-cancer and anti-thrombotic activities [51,52,53]. Various anthraquinones produced by plants have also been shown to have anti-Phytophthora activities [54,55,56,57]. However, this study is the first to report the anti-Phytophthora activities of 10 and 13.
The structures of hits 14, 15 and 16 all share a carbazole scaffold. Carbazoles are tricyclic aromatic compounds containing two benzene rings fused to either side of a pyrrole, and the majority of carbazole alkaloids have been isolated from plants of the Murraya, Glycosmis and Clausena genera and Rutaceae family [58]. These compounds are reported to have antimicrobial, insecticidal and antiprotozoal activity [59,60]. For example, a series of carbazole ellipticinium derivatives have notable inhibitory activity against P. infestans [61,62]. In the present study, a further fourteen compounds with structural similarity to 15 were tested in the mycelial IC50 assays against P. cinnamomi and P. agathidicida. Most were found to have activity, albeit lower than 15, against both species, suggesting a weak structure-activity relationship in the mechanism of action. Alterations to the identity (e.g., containing pyridine or piperidine), branching and position of the pendant groups of the ethylcarbazole resulted in decreased activity (Supplementary Table S3).
Overall, the hits identified herein represent promising leads and/or chemical scaffolds for further development of oomycides. Of particular interest are the six compounds that were active against only one of the Phytophthora species tested (1, 6, 7, 9, 16, 18). This specificity suggests targeted inhibition of one or more proteins, which is desirable for the development of species-specific antimicrobials. While promising, any off-target activity would need to be confirmed via phytotoxicity testing, antimicrobial assays and/or community sequencing prior to the implementation of potential oomycides in the field. Selective toxicity will be important for the control of P. agathidicida in particular, given the ecological significance of kauri trees and their surrounding biota. Future work could include medicinal chemistry studies to further explore the structure-activity relationships of these hits to improve potency and solubility (which relates to systemic movement in the plant) and could also include explorations into the mode of action of these compounds.
3. Materials and Methods
3.1. Compound Library
A set of 328 compounds with diverse chemical structures were selected from a larger 3500 compound library synthesized by the Brimble Lab at the University of Auckland, New Zealand. The full list of compounds screened can be found in Supplementary Table S1. The compounds in this set were produced during extensive synthetic work towards a range of diverse bioactive natural products and related scaffolds [31,32,39,40,47,60,63,64,65,66,67,68,69,70]. Additional information on individual compounds is available upon request. For anti-Phytophthora screening, compounds were resuspended in DMSO at a concentration of 10 mM and diluted to 100 µM with sterile Milli-Q water for use in assays.
3.2. Phytophthora Isolates and Culture Conditions
P. agathidicida isolate NZFS 3770 and P. cinnamomi isolate NZFS 3910 (both provided by Scion, Rotorua, New Zealand) were routinely cultured at 22 °C in darkness on clarified 20% V8 juice agar (cV8-agar) as described previously [71].
For zoospore production, 10 agar plugs (6 mm diameter) were removed from the edge of actively growing mycelia and then transferred to a petri dish containing 15 mL of a 1:10 dilution of 20% cV8 broth (P. cinnamomi) or carrot broth (P. agathidicida). These were grown for ~30 h at 25 °C. Broth was then removed and replaced with 15 mL of Chen-Zentmyer salt solution (for P. cinnamomi) or 5% (w/v) sterile soil extract (for P. agathidicida). Dishes were incubated at room temperature for 45 min. Then, the solutions were removed and replaced with fresh salt solution/soil extract. This was repeated after another 45 min, and the dishes were then incubated overnight at room temperature under light. The following morning, zoospore release was induced by removing the liquid and washing each dish three times with sterile Milli-Q water that had been cooled to 4 °C. Each wash was for 20 min, with the first two at room temperature and the final wash at 4 °C. Wash volumes were 15 mL per dish for the first two washes and 10 mL for the final wash. Following the final wash, dishes were returned to room temperature for 30–90 min until sufficient number of zoospores had been released. Zoospore densities were approximately 1500 per mL.
3.3. Inhibition Assays
Initial screening of the 328 compounds against zoospore germination was done in 96-well plates, with each well containing 100 µL of 4% cV8 broth amended with one of the compounds to a concentration of 200 µM. Each well then received 100 µL of zoospore suspension at a density of ~1500 zoospores per mL, resulting in a final volume of 200 µL 2% cV8 with 100 µM test compound and ~150 zoospores per well. Control wells were as above but were amended with 1% DMSO instead of the test compound. Plates were incubated for 24 h at 25 °C in darkness and qualitatively assessed for zoospore germination and mycelial growth using a Nikon SMZ-745T dissecting microscope.
Compounds that prevented zoospore germination relative to the negative control wells were then assayed for inhibition of mycelial growth at a range of concentrations to determine IC50 values. The mycelial growth IC50 assays were carried out in 24-well plates, with each well containing 1 mL cornmeal agar amended with the test compound at one of six concentrations (concentration ranges varied among compounds based on preliminary assay results (data not shown)). Negative control wells contained 1 mL cornmeal agar amended with DMSO at a final concentration of 1%. Plugs (~2 mm) were removed from the edge of actively growing mycelial mats and added to the centre of each well, and plates were then incubated for ~24 h at 25 °C in the dark. Mycelial mat diameters were measured (two perpendicular measurements were averaged for each well) and growth inhibition was calculated by subtracting the treatment mat diameter from the negative control mat diameter. To calculate the IC50 values, compound concentrations were log-transformed, and non-linear regression with curve fitting (by least squares) was carried out using GraphPad Prism version 6.0.
Supplementary Materials
The following are available online, Table S1. Plate 1–4; Tables S2 and S3. Half-maximal mycelial inhibition concentrations (IC50s).
Author Contributions
Conceptualization, M.L.G. and M.A.B.; resources, D.P.F. and M.A.B., investigation, S.A.L.; writing—original draft preparation, M.L.G., S.A.L. and H.F.R.; writing—review and editing, S.A.L., H.F.R., D.P.F., M.A.B. and M.L.G.; visualization, S.A.L. and H.F.R.; supervision, M.L.G.; project administration, M.L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Baldauf, S.L.; Roger, A.J.; Wenk-Siefert, I.; Doolittle, W.F. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 2000, 290, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, S.; Furzer, O.; Jones, J.D.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabieres, F.; et al. The top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.P.; Hewitt, H.G. Plant pathology and plant pathogens. In Fungicides in Crop Protection, 2nd ed.; CABI Publishing: Wallingford, UK, 2014; pp. 11–20. [Google Scholar]
- Guenthner, J.F.; Michael, K.C.; Nolte, P. The economic impact of potato late blight on US growers. Potato Res. 2001, 44, 121–125. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Garbelotto, M.; Goss, E.M.; Heungens, K.; Prospero, S. Emergence of the sudden oak death pathogen Phytophthora ramorum. Trends Microbiol. 2012, 20, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Brimble, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin. Chem. Biol. 2019, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baysal-Gurel, F.; Liyanapathiranage, P.; Mullican, J. Biofumigation: Opportunities and challenges for control of soilborne diseases in nursery production. Plant Health Prog. 2018, 19, 332–337. [Google Scholar] [CrossRef]
- Serrano-Perez, P.; Palo, C.; Rodriguez-Molina, M.D. Efficacy of Brassica carinata pellets to inhibit mycelial growth and chlamydospores germination of Phytophthora nicotianae at different temperature regimes. Sci. Hortic. 2017, 216, 126–133. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D.; Velasco, P. Natural control of plant pathogens through glucosinolates: An effective strategy against fungi and oomycetes. Phytochem. Rev. 2020, 19, 1045–1059. [Google Scholar] [CrossRef]
- Bae, S.J.; Mohanta, T.K.; Chung, J.Y.; Ryu, M.; Park, G.; Shim, S.; Hong, S.B.; Seo, H.; Bae, D.W.; Bae, I.; et al. Trichoderma metabolites as biological control agents against Phytophthora pathogens. Biol. Control 2016, 92, 128–138. [Google Scholar] [CrossRef]
- Bradshaw, R.E.; Bellgard, S.E.; Black, A.; Burns, B.R.; Gerth, M.L.; McDougal, R.L.; Scott, P.M.; Waipara, N.W.; Weir, B.S.; Williams, N.M.; et al. Phytophthora agathidicida: Research progress, cultural perspectives and knowledge gaps in the control and management of kauri dieback in New Zealand. Plant Pathol. 2020, 69, 3–16. [Google Scholar] [CrossRef]
- Horner, I.J.; Hough, E.G.; Horner, M.B. Forest efficacy trials on phosphite for control of kauri dieback. N. Z. Plant Prot. 2015, 68, 7–12. [Google Scholar] [CrossRef]
- Hardy, G.E.S.; Barrett, S.; Shearer, B.L. The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australas Plant Pathol. 2001, 30, 133–139. [Google Scholar] [CrossRef]
- Barrett, S.; Rathbone, D. Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecol. 2018, 43, 360–374. [Google Scholar] [CrossRef]
- Eshraghi, L.; Anderson, J.P.; Aryamanesh, N.; McComb, J.A.; Shearer, B.; Hardy, G.E.S. Suppression of the auxin response pathway enhances susceptibility to Phytophthora cinnamomi while phosphite-mediated resistance stimulates the auxin signalling pathway. BMC Plant Biol. 2014, 14, 68. [Google Scholar] [CrossRef]
- Vinas, M.; Mendez, J.C.; Jimenez, V.M. Effect of foliar applications of phosphites on growth, nutritional status and defense responses in tomato plants. Sci. Hortic. 2020, 265. [Google Scholar] [CrossRef]
- Dann, E.; McLeod, A. Phosphonic acid: A long-standing and versatile crop protectant. Pest. Manag. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, M.P.; Shearer, B.L.; Colquhoun, I.J.; O’Brien, P.A.; Hardy, G.E.S. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathol. 2008, 57, 928–936. [Google Scholar] [CrossRef]
- Hunter, S.; Williams, N.; McDougal, R.; Scott, P.; Garbelotto, M. Evidence for rapid adaptive evolution of tolerance to chemical treatments in Phytophthora species and its practical implications. PLoS ONE 2018, 13, e0208961. [Google Scholar] [CrossRef]
- Hao, W.; Förster, H.; Adaskaveg, J.E. Resistance to potassium phosphite in Phytophthora species causing Citrus Brown Rot and integrated practices for management of resistant isolates. Plant Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wightwick, A.M.; Walters, R.; Allinson, G.; Reichman, S.M.; Menzies, N.W. Environmental risks of fungicides used in horticultural production systems. Fungicides 2010, 1, 273–304. [Google Scholar]
- Lawrence, S.A.; Armstrong, C.B.; Patrick, W.M.; Gerth, M.L. High-throughput chemical screening identifies compounds that inhibit different stages of the Phytophthora agathidicida and Phytophthora cinnamomi life cycles. Front Microbiol. 2017. [Google Scholar] [CrossRef]
- Fenn, M.E.; Coffey, M.D. Studies on the in vitro and in vivo antifungal activity of fosetyl-al and phosphorus-acid. Phytopathology 1984, 74, 606–611. [Google Scholar] [CrossRef]
- Mateus, M.C.; Neves, D.; Dacunha, B.; Laczko, E.; Maia, C.; Teixeira, R.; Cravador, A. Structure, anti-Phytophthora and anti-tumor activities of a nortriterpenoid from the rhizome of Phlomis purpurea (Lamiaceae). Phytochemistry 2016, 131, 158–164. [Google Scholar] [CrossRef]
- Neves, D.; Caetano, P.; Oliveira, J.; Maia, C.; Horta, M.; Sousa, N.; Salgado, M.; Dionisio, L.; Magan, N.; Cravador, A. Anti-Phytophthora cinnamomi activity of Phlomis purpurea plant and root extracts. Eur. J. Plant Pathol. 2014, 138, 835–846. [Google Scholar] [CrossRef]
- Evidente, A.; Cristinzio, G.; Punzo, B.; Andolfi, A.; Testa, A.; Melck, D. Flufuran, an antifungal 3,5-disubstituted furan produced by Aspergillus flavus. Chem. Biodivers. 2009, 6, 328–334. [Google Scholar] [CrossRef]
- Ramirez-Reyes, T.; Monribot-Villanueva, J.L.; Jimenez-Martinez, O.D.; Aguilar-Colorado, A.S.; Bonilla-Landa, I.; Flores-Estevez, N.; Luna-Rodriguez, M.; Guerrero-Analco, J.A. Sesquiterpene lactones and phenols from polyfollicles of Magnolia vovidessi and their antimicrobial activity. Nat. Prod. Commun. 2018, 13, 521–525. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Sakr, S.H.; De Feo, V.; Camele, I. Study of bio-pharmaceutical and antimicrobial properties of pomegranate (Punica granatum L.) leathery exocarp extract. Plants 2021, 10, 153. [Google Scholar] [CrossRef]
- Baillie, L.C.; Bearder, J.R.; Li, W.-S.; Sherringham, J.A.; Whiting, D.A. Studies into the synthesis of a sub-unit of the neurotoxic alkaloid methyllycaconitine. J. Chem. Soc. Perkin Trans. I 1998, 24, 4047–4055. [Google Scholar] [CrossRef]
- Dickson, E.; Pilkington, L.I.; Brimble, M.A.; Barker, D. Enantioselective synthesis of BE ring analogues of methyllycaconitine. Tetrahedron 2016, 72, 400–414. [Google Scholar] [CrossRef]
- Goodall, K.J.; Brimble, M.A.; Barker, D. 1H and 13C NMR spectra of methylmaleimido- and methylsuccinimidoanthranilate esters of 1-hydroxymethyl-6-methoxy-3-azabicyclo [3.3.1]nonanes. Magn. Reson. Chem. 2007, 45, 695–699. [Google Scholar] [CrossRef]
- Hardick, D.J.; Blagbrough, I.S.; Cooper, G.; Potter, B.V.L.; Critchley, T.; Wonnacott, S. Nudicauline and elatine as potent norditerpenoid ligands at rat neuronal α-bungarotoxin binding sites: Importance of the 2-(methylsuccinimido)benzoyl moiety for neuronal nicotinic acetylcholine receptor binding. J. Med. Chem. 1996, 39, 4860–4866. [Google Scholar] [CrossRef] [PubMed]
- Manners, G.D.; Panter, K.E.; Pelletier, S.W. Structure-activity relationships of norditerpenoid alkaloids occurring in toxic larkspur (delphinium) species. J. Nat. Prod. 1995, 58, 863–869. [Google Scholar] [CrossRef]
- Bergmeier, S.C.; Lapinsky, D.J.; Free, R.B.; McKay, D.B. Ring E analogs of methyllycaconitine (MLA) as novel nicotinic antagonists. Bioorganic Med. Chem. Lett. 1999, 9, 2263–2266. [Google Scholar] [CrossRef]
- Bryant, D.L.; Free, R.B.; Thomasy, S.M.; Lapinsky, D.J.; Ismail, K.A.; McKay, S.B.; Bergmeier, S.C.; McKay, D.B. Structure-activity studies with ring E analogues of methyllycaconitine on bovine adrenal α3β4* nicotinic receptors. Neurosci. Res. 2002, 42, 57–63. [Google Scholar] [CrossRef]
- Doisy, X.; Blagbrough, I.S.; Wonnacott, S.; Potter, B.V.L. Design, synthesis, and biological evaluation of substituted benzoate analogues of the selective nicotinic acetylcholine receptor antagonist, methyllycaconitine. Pharm. Pharmacol. Commun. 1998, 4, 313–317. [Google Scholar]
- Kraus, G.A.; Dneprovskaia, E. A direct connection of a tricyclic analog of methyllycaconitine with 2-methylsuccinimidobenzoic acid. Tetrahedron Lett. 1998, 39, 2451–2454. [Google Scholar] [CrossRef]
- Barker, D.; McLeod, M.D.; Brimble, M.A.; Savage, G.P. Application of olefin metathesis to the synthesis of ABE ring analogues of methyllycaconitine. Tetrahedron Lett. 2002, 43, 6019–6022. [Google Scholar] [CrossRef]
- Barker, D.; Brimble, M.A.; McLeod, M.; Savage, G.P.; Wong, D.J. Synthesis of ABE tricyclic analogues of methyllycaconitine using a Wacker oxidation-aldol strategy to append the B ring to the AE fragment. J. Chem. Soc. Perkin Trans. I 2002, 7, 924–931. [Google Scholar] [CrossRef]
- Zheng, L.; Mackrill, J.J. Calcium Signaling in Oomycetes: An Evolutionary Perspective. Front. Physiol. 2016, 7, 123. [Google Scholar] [CrossRef]
- Putri, S.P.; Kinoshita, H.; Ihara, F.; Igarashi, Y.; Nihira, T. Farinomalein, a maleimide-bearing compound from the entomopathogenic fungus Paecilomyces farinosus. J. Nat. Prod. 2009, 72, 1544–1546. [Google Scholar] [CrossRef]
- Song, X.; Liu, C.; Chen, P.; Zhang, H.; Sun, R. Natural product-based pesticide discovery: Design, synthesis and bioactivity studies of N-amino-maleimide derivatives. Molecules 2018, 23, 1521. [Google Scholar] [CrossRef]
- Castell, A.; Short, F.L.; Evans, G.L.; Cookson, T.V.M.; Bulloch, E.M.M.; Joseph, D.D.A.; Lee, C.E.; Parker, E.J.; Baker, E.N.; Lott, J.S. The substrate capture mechanism of Mycobacterium tuberculosis anthranilate phosphoribosyltransferase provides a mode for inhibition. Biochemistry 2013, 52, 1776–1787. [Google Scholar] [CrossRef]
- Cookson, T.V.M.; Castell, A.; Bulloch, E.M.M.; Evans, G.L.; Short, F.L.; Baker, E.N.; Lott, J.S.; Parker, E.J. Alternative substrates reveal catalytic cycle and key binding events in the reaction catalysed by anthranilate phosphoribosyltransferase from Mycobacterium tuberculosis. Biochem. J. 2014, 461, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.L.; Gamage, S.A.; Bulloch, E.M.M.; Baker, E.N.; Denny, W.A.; Lott, J.S. Repurposing the chemical scaffold of the anti-arthritic drug Lobenzarit to target tryptophan biosynthesis in Mycobacterium tuberculosis. ChemBioChem 2014, 15, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.L.; Furkert, D.P.; Abermil, N.; Kundu, P.; de Lange, K.M.; Parker, E.J.; Brimble, M.A.; Baker, E.N.; Lott, J.S. Anthranilate phosphoribosyltransferase: Binding determinants for 5 ‘-phospho-alpha-D-ribosyl-1 ‘-pyrophosphate (PRPP) and the implications for inhibitor design. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Parish, T.; Stoker, N.G.; Bancroft, G.J. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 2001, 69, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Jiroutová, K.; Horák, A.; Bowler, C.; Oborník, M. Tryptophan biosynthesis in stramenopiles: Eukaryotic winners in the diatom complex chloroplast. J. Mol. Evol. 2007, 65, 496–511. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.G. Health functions and structure-activity relationships of natural anthraquinones from plants. Food Funct. 2018, 9, 6064–6081. [Google Scholar] [CrossRef] [PubMed]
- Baqi, Y. Anthraquinones as a privileged scaffold in drug discovery targeting nucleotide-binding proteins. Drug Discov. Today 2016, 21, 1571–1577. [Google Scholar] [CrossRef]
- Diaz-Muñoz, G.; Miranda, I.L.; Sartori, S.K.; de Rezende, D.C.; Diaz, M.A.N. Anthraquinones: An overview. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 313–338. [Google Scholar]
- Siddamurthi, S.; Gutti, G.; Jana, S.; Kumar, A.; Singh, S.K. Anthraquinone: A promising scaffold for the discovery and development of therapeutic agents in cancer therapy. Future Med. Chem. 2020, 12, 1037–1069. [Google Scholar] [PubMed]
- Jang, S.J.; Kuk, Y.I. Effects of different fractions of Rheum palmatum root extract and anthraquinone compounds on fungicidal, insecticidal, and herbicidal activities. J. Plant Dis. Prot. 2018, 125, 451–460. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, C.-H.; Kim, H.-G.; Lee, H.-S. Anthraquinones isolated from Cassia tora (Leguminosae) seed show an antifungal property against phytopathogenic fungi. J. Agric. Food Chem. 2004, 52, 6096–6100. [Google Scholar] [CrossRef]
- Tala, M.F.; Ansary, M.W.R.; Talontsi, F.M.; Kowa, T.K.; Tofazzal Islam, M.; Tane, P. Anthraquinones and flavanols isolated from the vegetable herb Rumex abyssinicus inhibit motility of Phytophthora capsici zoospores. South Afr. J. Bot. 2018, 115, 1–4. [Google Scholar] [CrossRef]
- Wijnsma, R.; van Weerden, I.N.; Verpoorte, R.; Harkes, P.A.A.; Lugt, C.B.; Scheffer, J.J.C.; Baerheim Svendsen, A. Anthraquinones in Cinchona ledgeriana bark infected with Phytophthora cinnamomi. Planta Med. 1986, 52, 211–212. [Google Scholar] [CrossRef]
- Knölker, H.-J.; Reddy, K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4427. [Google Scholar] [CrossRef]
- Greger, H. Phytocarbazoles: Alkaloids with great structural diversity and pronounced biological activities. Phytochem. Rev. 2017, 16, 1095–1153. [Google Scholar] [CrossRef]
- Rennison, D.; Gueret, S.M.; Laita, O.; Bland, R.J.; Sutherland, I.A.; Boddy, I.K.; Brimble, M.A. Substituted carbazoles—A new class of anthelmintic agent. Austral. J. Chem. 2016, 69, 1268–1276. [Google Scholar] [CrossRef]
- Mackrill, J.J.; Kehoe, R.A.; Zheng, L.; McKee, M.L.; O’Sullivan, E.C.; Doyle Prestwich, B.M.; McCarthy, F.O. Inhibitory properties of aldehydes and related compounds against Phytophthora infestans—Identification of a new lead. Pathogens 2020, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.L.; Zheng, L.; O’sullivan, E.C.; Kehoe, R.A.; Doyle Prestwich, B.M.; Mackrill, J.J.; McCarthy, F.O. Synthesis and evaluation of novel ellipticines and derivatives as inhibitors of Phytophthora infestans. Pathogens 2020, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Boddy, I.K.; Boniface, P.J.; Cambie, R.C.; Craw, P.A.; Huang, Z.D.; Larsen, D.S.; Mcdonald, H.; Rutledge, P.S.; Woodgate, P.D. Experiments directed towards the synthesis of anthracyclinones. VIII: Functionalization of hydroxyanthraquinones by reductive Claisen rearrangements. Aust. J. Chem. 1984, 37, 1511–1529. [Google Scholar] [CrossRef]
- Balsells, J.; Mejorado, L.; Phillips, M.; Ortega, F.; Aguirre, G.; Somanathan, R.; Walsh, P.J. Synthesis of chiral sulfonamide/Schiff base ligands. Tetrahedron Asymmetry 1998, 9, 4135–4142. [Google Scholar] [CrossRef]
- Li, B.J.; Jiang, L.; Liu, M.; Chen, Y.C.; Ding, L.S.; Wu, Y. Asymmetric Michael addition of arylthiols to alpha,beta-unsaturated carbonyl compounds catalyzed by bifunctional organocatalysts. Synlett 2005, 4, 603–606. [Google Scholar]
- Chan, Y.; Guthmann, H.; Brimble, M.A.; Barker, D. Diastereoselective synthesis of substituted 4-piperidones and 4-piperidols using a double mannich reaction. Synlett 2008, 2008, 2601–2604. [Google Scholar] [CrossRef]
- Chan, Y.; Balle, J.; Kevin Sparrow, J.; Boyd, P.D.W.; Brimble, M.A.; Barker, D. A double Mannich approach to the synthesis of substituted piperidones—application to the synthesis of substituted E-ring analogues of methyllycaconitine. Tetrahedron 2010, 66, 7179–7191. [Google Scholar] [CrossRef]
- Guéret, S.M.; Furkert, D.P.; Brimble, M.A. Synthesis of a functionalized 7,6-bicyclic spiroimine ring fragment of the spirolides. Org. Lett. 2010, 12, 5226–5229. [Google Scholar] [CrossRef]
- Sparrow, K.; Barker, D.; Brimble, M.A. An efficient synthesis of 3-alkyl-1,5,3-dioxazepanes and their use as electrophiles in double-Mannich reactions. Tetrahedron 2012, 68, 1017–1028. [Google Scholar] [CrossRef]
- Pieroni, M.; Annunziato, G.; Azzali, E.; Dessanti, P.; Mercurio, C.; Meroni, G.; Trifiro, P.; Vianello, P.; Villa, M.; Beato, C.; et al. Further insights into the SAR of alpha-substituted cyclopropylamine derivatives as inhibitors of histone demethylase KDM1A. Eur. J. Med. Chem. 2015, 92, 377–386. [Google Scholar] [CrossRef]
- Lawrence, S.A.; Burgess, E.J.; Pairama, C.; Black, A.; Patrick, W.M.; Mitchell, I.; Perry, N.B.; Gerth, M.L. Mātauranga-guided screening of New Zealand native plants reveals flavonoids from kānuka (Kunzea robusta) with anti-Phytophthora activity. J. R. Soc. N. Z. 2019, 49, 137–154. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).