Approaches for Mitigating Microbial Biofilm-Related Drug Resistance: A Focus on Micro- and Nanotechnologies
Abstract
:1. Introduction
1.1. Biofilms and Their Role in Resistance
1.2. Biofilms in Healthcare-Associated Infections (HAIs)
1.3. Implications of Biofilm-Related HAIs and Possible Treatments
2. Characteristics of Biofilms
2.1. Physiological State
2.2. Extracellular Matrix (ECM)
3. Factors Contributing towards Drug Resistance
3.1. Cell Density
3.2. Quorum Sensing (QS)
3.3. Upregulation of Drug Efflux Pumps
3.4. Point Mutation and Overexpression of CDR1, ERG11
3.5. Presence of Persister Cells
3.6. Antimicrobial Tolerance
4. Potential Alternative Therapeutics for Biofilm Mitigation
4.1. Antimicrobial Photodynamic Therapy (aPDT)
4.2. Antimicrobial Lock Therapy (ALT)
4.3. Antimicrobial Peptides (AMPs)
4.4. Electrical Method
4.5. Antimicrobial Coatings
5. Nanotechnology and Microtechnology in Antimicrobial Resistance
5.1. Nanotechnology and Its Mechanisms to Mitigate Biofilms
5.2. Types of Nanomaterials
5.2.1. Quantum Dots (QDs)
5.2.2. Carbon-Based Nanoparticles
5.2.3. Carbon Nanotubes (CNTs)
5.2.4. Fullerenes
5.2.5. Graphene
5.2.6. Nanodiamonds
5.2.7. Dendrimers
5.2.8. Mesoporous Silica Particles
5.2.9. Chitosan-Based Nanoparticles
5.3. Microtechnology and Biofilms
5.3.1. Microparticles for Delivery of Antimicrobials
5.3.2. Novel Microtechnology Approaches with Antibiofilm Properties
5.3.3. Coatings with Microparticles
5.3.4. Micro Textured/Patterned Surfaces
5.3.5. Microdressing
5.3.6. Microspray
5.3.7. Microrods
5.3.8. Microswimmers
5.3.9. Microneedles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, K.F.; Goldberg, M.; Rosenthal, S.; Carlson, L.; Chen, J.; Chen, C.; Ramachandran, S. Global rise in human infectious disease outbreaks. J. R. Soc. Interface 2014, 11, 20140950. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nat. Cell Biol. 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Aparna, M.S.; Yadav, S. Biofilms: Microbes and disease. Braz. J. Infect. Dis. 2008, 12, 526–530. [Google Scholar] [CrossRef] [Green Version]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.; Ha, S. Current and recent advanced strategies for combating biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Srinivasan, A.; Ramasubramanian, A.K.; López-Ribot, J.L. From biology to drug development: New approaches to combat the threat of fungal biofilms. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef] [Green Version]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; De Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal biofilms and polymicrobial diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [Green Version]
- Briones, E.; Colino, C.I.; Lanao, J.M. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J. Control. Release 2008, 125, 210–227. [Google Scholar] [CrossRef]
- Centre for Disease Control (CDC). Healthcare-Associated Infections (HAI). 2020. Available online: https://www.cdc.gov/policy/polaris/healthtopics/hai.html (accessed on 12 April 2020).
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. New. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- Vergidis, P.; Patel, R. Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect. Dis. Clin. N. Am. 2012, 26, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Nowakowska, J.; Landmann, R.; Khanna, N. Foreign body infection models to study host-pathogen response and antimicrobial tolerance of bacterial biofilm. Antibiotics 2014, 3, 378–397. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deorukhkar, S.C.; Saini, S.; Mathew, S. Non-albicans Candida infection: An emerging threat. Interdiscip Perspect Infect Dis. 2014, 2014, 615958. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Septimus, E.J.; Moody, J. Prevention of device-related healthcare-associated infections. F1000Research 2016, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Keyt, H.; Faverio, P.; Restrepo, M.I. Prevention of ventilator-associated pneumonia in the intensive care unit: A review of the clinically relevant recent advancements. Indian J. Med. Res. 2014, 139, 814–821. [Google Scholar] [PubMed]
- Percival, S.L. Importance of biofilm formation in surgical infection. BJS 2017, 104, e85–e94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillie, G.S.; Douglas, L.J. Iron-limited biofilms of Candida albicans and their susceptibility to Amphotericin B. Antimicrob. Agents Chemother. 1998, 42, 2146–2149. [Google Scholar] [CrossRef] [Green Version]
- Dumitru, R.; Hornby, J.M.; Nickerson, K.W. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 2004, 48, 2350–2354. [Google Scholar] [CrossRef] [Green Version]
- Walters, M.C., III; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciproflaxin and tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Scorzoni, L.; De Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; De Melo, W.C.M.A.; De Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taraszkiewicz, A.; Fila, G.; Grinholc, M.; Nakonieczna, J. Innovative strategies to overcome biofilm resistance. BioMed Res. Int. 2012, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Cramton, S.E.; Gerke, C.; Schnell, N.F.; Nichols, W.W.; Götz, F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 1999, 67, 5427–5433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, N.; Kolodkins-Gal, I. The matrix reloaded: How sensing the extracellular matrix synchronizes bacterial communities. J. Bacteriol. 2015, 197, 2092–2103. [Google Scholar] [CrossRef] [Green Version]
- Al-Fattani, M.A.; Douglas, L.J. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006, 55, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, E.G.; Zarnowski, R.; Choy, H.L.; Zhao, M.; Sanchez, H.; Nett, J.E.; Andes, D.R. Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. mSphere 2019, 4, e00680-18. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 2007, 73, 4592–4601. [Google Scholar] [CrossRef] [Green Version]
- Beauvais, A.; Schmidt, C.; Guadagnini, S.; Roux, P.; Perret, E.; Henry, C.; Paris, S.; Mallet, A.; Prévost, M.-C.; Latgé, J.P. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell. Microbiol. 2007, 9, 1588–1600. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Gutierrez-Correa, M.; Jones, B.; Williams, C. Aspergillus biofilms: Clinical and industrial significance. FEMS Microbiol. Lett. 2011, 324, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, R.; Williams, C.; Lappin, D.F.; Millington, O.; Martins, M.; Ramage, G. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot. Cell 2013, 12, 420–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, M.; Uppuluri, P.; Thomas, D.P.; Cleary, I.A.; Henriques, M.; Lopez-Ribot, J.L.; Oliveira, R. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia 2009, 169, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghupathi, P.K.; Liu, W.; Sabbe, K.; Houf, K.; Burmølle, M.; Sørensen, S.J. Synergistic interactions within a multispecies biofilm enhance individual species protection against grazing by a pelagic protozoan. Front. Microbiol. 2018, 8, 2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perumal, P.; Mekala, S.; Chaffin, W.L. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob. Agents Chemother. 2007, 51, 2454–2463. [Google Scholar] [CrossRef] [Green Version]
- Kirby, A.E.; Garner, K.; Levin, B.R. The relative contributions of physical structure and cell density to the antibiotic susceptibility of bacteria in biofilms. Antimicrob. Agents Chemother. 2012, 56, 2967–2975. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.-H.; Wang, L.-H.; Xu, J.-L.; Zhang, H.-B.; Zhang, X.-F.; Zhang, L.-H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nat. Cell Biol. 2001, 411, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.B.; Chormey, D.S.; Büyükpınar, Ç.; Engin, G.O.; Bakirdere, S. Quorum sensing: Little talks for an effective bacterial coordination. TrAC Trends Anal. Chem. 2017, 91, 1–11. [Google Scholar] [CrossRef]
- Albuquerque, P.; Casadevall, A. Quorum sensing in fungi—A review. Med. Mycol. 2012, 50, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Genet. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Sturme, M.H.J.; Kleerebezem, M.; Nakayama, J.; Akkermas, A.D.L.; Vaugha, E.E.; de Vos, W.M. Cell to cell communication by autoinducing peptides in Gram-positive bacteria. Antonie Leeuwenhoek 2002, 81, 233–243. [Google Scholar] [CrossRef]
- Deep, A.; Chaudhary, U.; Gupta, V. Quorum sensing and bacterial pathogenicity: From molecules to disease. J. Lab. Physicians 2011, 3, 4–11. [Google Scholar] [CrossRef]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.Y.; Cao, Y.-B.; Xu, Z.; Ying, K.; Li, Y.; Xie, Y.; Zhu, Z.-Y.; Chen, W.-S.; Jiang, Y.-Y. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob. Agents Chemother. 2005, 49, 584–589. [Google Scholar] [CrossRef] [Green Version]
- Dou, Y.; Song, F.; Guo, F.; Zhou, Z.; Zhu, C.; Xiang, J.; Huan, J. Acinetobacter baumanii quorum-sensing signalling molecule induces the expression of drug-resistance genes. Mol. Med. Rep. 2017, 15, 4061–4068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramage, G.; Bachmann, S.; Patterson, T.F.; Wickes, B.L.; López-Ribot, J.L. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 2002, 49, 973–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.W.; Shin, J.H.; Kee, S.J.; Kim, S.H.; Shin, M.G.; Suh, S.P.; Ryang, D.W. Expression of CgCDR1, CgCDR2, and CgERG11 in Candida glabrata biofilms formed by bloodstream isolates. Med. Mycol. 2009, 47, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, E.; Silva, S.; Rodrigues, C.F.; Alves, C.T.; Azeredo, J.; Henriques, M. Effects of fluconazole on Candida glabrata biofilms and its relationship with ABC transporter gene expression. Biofouling 2014, 30, 447–457. [Google Scholar] [CrossRef]
- Pulcrano, G.; Panellis, D.; de Domenico, G.; Rossano, F.; Catania, M.R. Ambroxol influences voriconazole resistance of Candida parapsilosis biofilm. FEMS Yeast Res. 2012, 12, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arana, D.M.; Nombela, C.; Pla, J. Fluconazole at subinhibitory concentrations induces the oxidative- and nitrosative-responsive genes TRR1, GRE2 and YHB1, and enhances the resistance of Candida albicans to phagocytes. J. Antimicrob. Chemother. 2009, 65, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, R.; Mowat, E.; McCulloch, E.; Lappin, D.F.; Jones, B.; Lang, S.; Majithiya, J.B.; Warn, P.; Williams, C.; Ramage, G. Azole Resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob. Agents Chemother. 2011, 55, 2092–2097. [Google Scholar] [CrossRef] [Green Version]
- Higgins, C.F. ABC transporters physiology, structure and mechanisms—An overview. Res. Microbiol. 2001, 152, 205–210. [Google Scholar] [CrossRef]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H., Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.H.; Paulsen, I.T.; Skurray, R.A. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 1999, 31, 394–395. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Skurray, R.A.; Tam, R.; Saier, M.H., Jr.; Turner, R.J.; Weiner, J.H.; Goldberg, E.B.; Grinius, L.L. The SMR family: A novel family of multidrug efflux proteins involved with the efflux of lipophilic drug. Mol. Microbiol. 1996, 19, 1167–1175. [Google Scholar] [CrossRef]
- Nikaido, H.; Takatsuka, Y. Mechanisms of RND multidrug efflux pumps. Biochim. Biophys. Acta 2009, 1794, 769–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-T.; Huang, Y.-W.; Liou, R.-S.; Chang, Y.-C.; Yang, T.-C. MacABCsm, an ABC-type tripartite efflux pump of Stenotrophomonas maltophilia involved in drug resistance, oxidative and envelope stress tolerances and biofilm formation. J. Antimicrob. Chemother. 2014, 69, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, E.; Metlay, J.P.; Mao, X.; Han, X.; Fishman, N.O.; Bilker, W.B.; Tolomeo, P.; Wheeler, M.; Nachamkin, I. The prevalence of fluoroquinolone resistance mechanisms in colonizing Escherichia coli isolates recovered from hospitalized patients. Clin. Infect. Dis. 2010, 51, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Z.; Zhang, L.; Poole, K. Interplay between the MexA-MexB-OprM multidrug efflux system and the outer membrane barrier in the multiple antibiotic resistance of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2000, 45, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Looi, C.Y.; D’ Silva, E.C.; Seow, H.F.; Rosli, R.; Ng, K.P.; Chong, P.P. Increased expression and hotspot mutations of the multidrug efflux transporter, CDR1 in azole-resistant Candida albicans isolates from vaginitis patients. FEMS Microbiol. Lett. 2005, 249, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Berila, N.; Borecká, S.; Dzugasova, V.; Bojnansky, J.; Šubík, J. Mutations in the CgPDR1 and CgERG11 genes in azole-resistant Candida glabrata clinical isolates from Slovakia. Int. J. Antimicrob. Agents 2009, 33, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, S3–S13. [Google Scholar] [CrossRef]
- Fuentefria, A.M.; Pippi, B.; Lana, D.D.; Donato, K.; De Andrade, S. Antifungals discovery: An insight into new strategies to combat antifungal resistance. Lett. Appl. Microbiol. 2017, 66, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spettel, K.; Barousch, W.; Makristathis, A.; Zeller, I.; Nehr, M.; Selitsch, B.; Lackner, M.; Rath, P.-M.; Steinmann, J.; Willinger, B. Analysis of antifungal resistance genes in Candida albicans and Candida glabrata using next generation sequencing. PLoS ONE 2019, 14, e0210397. [Google Scholar] [CrossRef]
- Borecká-Melkusová, S.; Moran, G.P.; Sullivan, D.J.; Kucharíková, S.; Chorvát, D., Jr.; Bujdáková, H. The expression of genes involved in the ergosterol biosynthesis pathway in Candida albicans and Candida dubliniensis biofilms exposed to fluconazole. Mycoses 2009, 52, 118–128. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Gonçalves, B.; Rodrigues, M.E.; Silva, S.; Azeredo, J.; Henriques, M. The effectiveness of voriconazole in therapy of Candida glabrata’s biofilms oral infections and its influence on the matrix composition and gene expression. Mycopathologia 2017, 182, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossignol, T.; Ding, C.; Guida, A.; D’Enfert, C.; Higgins, D.G.; Butler, G. Correlation between biofilm formation and the hypoxic response in Candida parapsilosis. Eukaryot. Cell 2009, 8, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Nailis, H.; VandenBosch, D.; Deforce, D.; Nelis, H.J.; Coenye, T. Transcriptional response to fluconazole and Amphotericin B in Candida albicans biofilms. Res. Microbiol. 2010, 161, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Saran, N.; Saha, S. Survey of drug resistance associated gene mutations in Mycobacterium tuberculosis, ESKAPE and other bacterial species. Sci. Rep. 2020, 10, 8957. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Zhou, Y.; Zhang, Z.; Zhang, X. Survival of bactericidal antibiotic treatment by tolerant persister cells of Klebsiella pneumoniae. J. Med. Microbiol. 2018, 67, 273–281. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [Green Version]
- Miyaue, S.; Suzuki, E.; Komiyama, Y.; Kondo, Y.; Morikawa, M.; Maeda, S. Bacterial memory of persisters: Bacterial persister cells can retain their phenotype for days or weeks after withdrawal from colony–biofilm culture. Front. Microbiol. 2018, 9, 1396. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhaheri, R.S.; Douglas, L.J. Apoptosis in Candida biofilms exposed to Amphotericin B. J. Med. Microbiol. 2010, 59, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bink, A.; VandenBosch, D.; Coenye, T.; Nelis, H.; Cammue, B.P.A.; Thevissen, K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob. Agents Chemother. 2011, 55, 4033–4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuyts, J.; Van Dijck, P.; Holtappels, M. Fungal persister cells: The basis for recalcitrant infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.M.; Lancaster, A.K.; Scherz-Shouval, R.; Whitesell, L.; Lindquist, S. Fitness trade-offs restrict the evolution of resistance to Amphotericin B. PLoS Biol. 2013, 11, e1001692. [Google Scholar] [CrossRef]
- Cohen, N.R.; Lobritz, M.A.; Collins, J.J. Microbial persistence and the road to drug resistance. Cell Host Microbe. 2013, 13, 632–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delarze, E.; Sanglard, D. Defining the frontiers between antifungal resistance, tolerance and the concept of persistence. Drug Resist. Updat. 2015, 23, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Genet. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Held, K.; Gasper, J.; Morgan, S.; Siehnel, R.; Singh, P.; Manoil, C. Determinants of extreme β-lactam tolerance in the Burkholderia pseudomallei complex. Antimicrob. Agents Chemother. 2018, 62, e00068-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederberg, J.; Zinder, N. Concentration of biochemical mutants of bacteria with penicillin. J. Am. Chem. Soc. 1948, 70, 4267–4268. [Google Scholar] [CrossRef]
- Caza, M.; Kronstad, J.W. The cAMP/protein kinase A pathway regulates virulence and adaptation to host conditions in Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2019, 9, 212. [Google Scholar] [CrossRef]
- Akins, R.A. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 2005, 43, 285–318. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yu, B.; Sun, W.; Liang, C.; Ying, H.; Zhou, S.; Niu, H.; Wang, Y.; Liu, D.; Chen, Y. Calcineurin signaling pathway influences Aspergillus niger biofilm formation by affecting hydrophobicity and cell wall integrity. Biotechnol. Biofuels 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Nett, J.; Heitman, J.; Andes, D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 52, 1127–1132. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-J.; Wu, C.-Y.; Yu, S.-J.; Chen, Y.-L. Protein kinase A governs growth and virulence in Candida tropicalis. Virulence 2018, 9, 331–347. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Liu, D.; Zhao, N.; Cheng, H.; Ren, H.; Guo, T.; Niu, H.; Zhuang, W.; Wi, J.; et al. Involvement of glycolysis/gluconeogenesis and signaling regulatory pathways in Saccharomyces cerevisiae biofilms during fermentation. Front. Microbiol. 2015, 6, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Uppuluri, P.; Nett, J.; Rajendran, R.; Ramage, G.; Lopez-Ribot, J.L.; Andes, D.; Cowen, L.E. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011, 7, e1002257. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M.; Woolfson, A.D. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J. Photochem. Photobiol. B Biol. 2007, 86, 59–69. [Google Scholar] [CrossRef]
- De Melo, W.C.M.A.; Avci, P.; de Oliveira, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huang, Y.-Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert. Rev. Anti Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, L.P.; de Silva, F.C. Antimicrobial photodynamic therapy: A new therapeutic option to combat infections. J. Med. Microb. Diagn. 2014, 3, 158. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Zhang, J.; Chen, R.; Zhong, X.; Wu, X.; Zheng, L.; Zhao, J. In vitro Evaluation of photodynamic effects against biofilms of dermatophytes involved in onychomycosis. Front. Microbiol. 2019, 10, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junqueira, J.C.; Jorge, A.O.C.; Barbosa, J.O.; Rossoni, R.D.; Vilela, S.F.G.; Costa, A.C.B.P.; Primo, F.L.; Gonçalves, J.M.; Tedesco, A.C.; Suleiman, J.M.A.H. Photodynamic inactivation of biofilms formed by Candida spp., Trichosporon mucoides, and Kodamaea ohmeri by cationic nanoemulsion of zinc 2,9,16,23-tetrakis(phenylthio)-29H, 31H-phthalocyanine (ZnPc). Lasers Med. Sci. 2012, 27, 1205–1212. [Google Scholar] [CrossRef]
- Biel, M.A.; Sievert, C.; Usacheva, M.; Bs, M.T.; Balcom, J. Antimicrobial photodynamic therapy treatment of chronic recurrent sinusitis biofilms. Int. Forum Allergy Rhinol. 2011, 1, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Meire, M.A.; Coenye, T.; Nelis, H.J.; De Moor, R.J.G. Evaluation of Nd:YAG and Er:YAG irradiation, antibacterial photodynamic therapy and sodium hypochlorite treatment on Enterococcus faecalis biofilms. Int. Endod. J. 2012, 45, 482–491. [Google Scholar] [CrossRef]
- Walraven, C.J.; Lee, S.A. Antifungal lock therapy. Antimicrob. Agents Chemother. 2012, 57, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Justo, J.A.; Bookstaver, P.B. Antibiotic lock therapy: Review of technique and logistical challenges. Infect. Drug Resist. 2014, 7, 343–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schinabeck, M.K.; Long, L.A.; Hossain, M.A.; Chandra, J.; Mukherjee, P.K.; Mohamed, S.; Ghannoum, M.A. Rabbit model of Candida albicans biofilm infection: Liposomal Amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 2004, 48, 1727–1732. [Google Scholar] [CrossRef] [Green Version]
- Lazzell, A.L.; Chaturvedi, A.K.; Pierce, C.G.; Prasad, D.; Uppuluri, P.; Lopez-Ribot, J.L. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J. Antimicrob. Chemother. 2009, 64, 567–570. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Isham, N.; Jacobs, M.R. Antimicrobial activity of B-lock against bacterial and Candida spp. causing catheter-related bloodstream infections. Antimicrob. Agents Chemother. 2011, 55, 4430–4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delattin, N.; De Brucker, K.; De Cremer, K.; Cammue, B.P.; Thevissen, K. Antimicrobial peptides as a strategy to combat fungal biofilms. Curr. Top. Med. Chem. 2016, 17, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, E.; Lombardi, L.; Falanga, A.; Libralato, G.; Guida, M.; Carotenuto, R. Biofilms: Novel strategies based on antimicrobial peptides. Pharmaceutics 2019, 11, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raheem, N.; Straus, S.K. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.M.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [Green Version]
- De Alteriis, E.; Lombardi, L.; Falanga, A.; Napolano, M.; Galdiero, S.; Siciliano, A.; Carotenuto, R.; Guida, M.; Galdiero, E. Polymicrobial antibiofilm activity of the membranotropic peptide gH625 and its analogue. Microb. Pathog. 2018, 125, 189–195. [Google Scholar] [CrossRef]
- Burrows, L.L.; Stark, M.; Chan, C.; Glukhov, E.; Sinnadurai, S.; Deber, C.M. Activity of novel non-amphipathic cationic antimicrobial peptides against Candida species. J. Antimicrob. Chemother. 2006, 57, 899–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect. Immun. 2006, 74, 6118–6123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Ruigomez, M.; Badiola, J.; Schmidt-Malan, S.M.; Greenwood-Quaintance, K.; Karau, M.J.; Brinkman, C.L.; Mandrekar, J.N.; Patel, R. Direct electrical current reduces bacterial and yeast biofilm formation. Int. J. Bacteriol. 2016, 2016, 9727810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boda, S.K.; Bajpai, I.; Basu, B. Inhibitory effect of direct electric field and HA-ZnO composites on S. aureus biofilm formation. J. Biomed. Mater. Res B Appl. Biomater. 2016, 104, 1064–1075. [Google Scholar] [CrossRef]
- Sandvik, E.L.; McLeod, B.R.; Parker, A.E.; Stewart, P.S. Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid. PLoS ONE 2013, 8, e55118. [Google Scholar] [CrossRef]
- Zander, Z.K.; Becker, M.L. Antimicrobial and antifouling strategies for polymeric medical devices. ACS Macro Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef] [Green Version]
- De Prijck, K.; De Smet, N.; Rymarczyk-Machal, M.; Van Driessche, G.; Devreese, B.; Coenye, T.; Schacht, E.; Nelis, H.J. Candida albicans biofilm formation on peptide functionalized polydimethylsiloxane. Biofouling 2010, 26, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Centre for Disease Control and Prevention (CDC). Antibiotic/Antimicrobial Resistance. 2020. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 13 February 2021).
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Armstead, A.L.; Li, B. Nanomedicine as an emerging approach against intracellular pathogens. Int. J. Nanomed. 2011, 6, 3281–3293. [Google Scholar] [CrossRef] [Green Version]
- Kuan, C.-Y.; Wai, Y.-F.; Yuen, K.-H.; Liong, M.-T. Nanotech: Propensity in foods and bioactives. Crit. Rev. Food Sci. Nutr. 2012, 52, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S. Food nanotechnology—An overview. Nanotechnol. Sci. Appl. 2010, 3, 1–15. [Google Scholar]
- Neethirajan, S.; Jayas, D.S. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol. 2011, 4, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C.; Bainbridge, W.S. The new world of discovery, invention, and innovation: Convergence of knowledge, technology, and society. J. Nanopart Res. 2013, 15, 1946. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nanostrategies to fight multidrug resistant bacteria-“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, W.; Sharma, C. Inorganic nanoparticles for targeted drug delivery. In Biointegration of Medical Implant Materials: Science and Design, 1st ed.; Woodhead Publishing Limited: Cambridge, UK; New Delhi, India; Boca Raton, FL, USA, 2010; pp. 204–234. [Google Scholar]
- Martin-Serrano, Á.; Gómez, R.; Ortega, P.; De La Mata, F.J. Nanosystems as vehicles for the delivery of antimicrobial peptides (AMPs). Pharmaceutics 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, H.K.S.; Raizaday, A. Inorganic nanobiomaterials for medical imaging. In Nanobiomaterials in Medical Imaging; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2016; pp. 365–401. [Google Scholar]
- Keane, P.A.; Ruiz-Garcia, H.; Sadda, S.R. Advanced imaging technologies. Retina 2013, 133–150. [Google Scholar] [CrossRef]
- Davey, M.E. Tracking dynamic interactions during plaque formation. J. Bacteriol. 2008, 190, 7869–7870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, G.; Mitra, A.K.; Cholkar, K. Nanosystems for Diagnostic imaging, biodetectors, and biosensors. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2017; pp. 217–248. [Google Scholar]
- Morrow, J.B.; Arango, C.P.; Holbrook, R.D. Association of quantum dot nanoparticles with Pseudomonas aeruginosa Biofilm. J. Environ. Qual. 2010, 39, 1934–1941. [Google Scholar] [CrossRef]
- Li, X.; Yeh, Y.-C.; Giri, K.; Mout, R.; Landis, R.F.; Prakash, Y.S.; Rotello, V.M. Control of nanoparticle penetration into biofilms through surface design. Chem. Commun. 2015, 51, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, N.; Ahmad, R.; Bang, S.H.; Khang, G.; Min, J.; Hahn, Y.-B. Outstanding antibiofilm features of quanta-CuO film on glass surface. ACS Appl. Mater. Interfaces 2016, 8, 15128–15137. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, D.; He, Z.; Liu, J.; Xiao, F.-X.; Zhang, Y.; Wang, R.; Bhattacharyya, D.; Tan, T.T.Y. Graphene oxide quantum dots covalently functionalized PVDF membrane with significantly enhanced bactericidal and antibiofouling performances. Sci. Rep. 2016, 6, 20142. [Google Scholar] [CrossRef] [Green Version]
- Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Influence of zinc oxide quantum dots in the antibacterial activity and cytotoxicity of an experimental adhesive resin. J. Dent. 2018, 73, 57–60. [Google Scholar] [CrossRef]
- Garcia, I.M.; Souza, V.S.; Scholten, J.D.; Collares, F.M. Quantum dots of tantalum oxide with an imidazolium ionic liquid as antibacterial agent for adhesive resin. J. Adhes. Dent. 2020, 22, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.F.; Tamboli, M.S.; Patil, R.H.; Bhan, A.; Ambekar, J.D.; Kale, B.B. Bioinspired carbon quantum dots: An antibiofilm agents. J. Nanosci. Nanotechnol. 2019, 19, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Parker, S.; Chiniforush, N.; Bahador, A. Photoexcitation triggering via semiconductor graphene quantum dots by photochemical doping with Curcumin versus perio-pathogens mixed biofilms. Photodiagnosis Photodyn. Ther. 2019, 28, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kadiyala, U.; Qu, Z.; Elvati, P.; Altheim, C.; Kotov, N.A.; Violi, A.; Vanepps, J.S. Anti-biofilm activity of graphene quantum dots via self-assembly with bacterial amyloid proteins. ACS Nano 2019, 13, 4278–4289. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Dahiya, M. Carbon nanotubes: Types, methods of preparation and applications. Int. J. Pharm. Sci. Res. 2016, 1, 15–21. [Google Scholar]
- Tan, J.M.; Arulselvan, P.; Fakurazi, S.; Ithnin, H.; Hussein, M.Z. A review on characterizations and biocompatibility of functionalised carbon nanotubes in drug delivery design. J. Nanomater. 2014, 2014, 917024. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Chuong, P.-H. Carbon nanotubes: Applications in pharmacy and medicine. BioMed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Igielska-Kalwat, J. Prospects for the use of carbon nanotubes in medicine. J. Oncol. Med. Pract. 2018, 3, 116. [Google Scholar] [CrossRef]
- Jain, S.; Singh, S.R.; Pillai, S. Toxicity issues related to biomedical applications of carbon nanotubes. J. Nanomed. Nanotechnol. 2012, 3, 140. [Google Scholar] [CrossRef]
- Behra, N.; Sahu, G.; Sharma, R.; Sen, K.S.; Bohidar, S. Review on effect of various parameter on properties of carbon nano tube. Int. J. Sci. Technol. Eng. 2015, 2, 113–116. [Google Scholar]
- Hirschfeld, J.; Akinoglu, E.M.; Wirtz, D.C.; Hoerauf, A.; Bekeredjian-Ding, I.; Jepsen, S.; Haddouti, E.; Limmer, A.; Giersig, M. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomedicine 2017, 13, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, B.; Pandiyan, N.; Kasinathan, K.; Rajaiah, A.; Arumuga, M.; Subramanian, P.; Sonamuthu, J.; Samayanan, S.; Arumugam, V.R.; Marimuthu, K.; et al. Fabrication of heteroatom doped NFP-MWCNT and NFB-MWCNT nanocomposite from imidazolium ionic liquid functionalized MWCNT for antibiofilm and wound healing in Wistar rats: Synthesis, characterization, in vitro and in vivo studies. Mater. Sci. Eng. C 2020, 111, 110791. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Sahle-Demessie, E.; Sorial, G.A. Inhibition of biofilm growth on polymer-MWCNTs composites and metal surfaces. Sci. Total. Environ. 2018, 633, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Anju, V.T.; Paramanantham, P.; Sruthil Lal, S.B.; Sharan, A.; Alsaedi, M.H.; Dawoud, T.M.S.; Syed, A.; Siddhardha, B. Antimicrobial photodynamic activity of rose bengal conjugated multi walled carbon nanotubes against planktonic cells and biofilm of Escherichia coli. Photodiagnosis Photodyn. Ther. 2018, 24, 300–310. [Google Scholar] [CrossRef]
- Anju, V.T.; Paramanantham, P.; Sruthil Lal, S.B.; Sharan, A.; Syed, A.; Bahkali, N.A.; Alsaedi, M.H.; Kaviyarasu, K.; Busi, S. Antimicrobial photodynamic activity of toluidine blue-carbon nanotube conjugate against Pseudomonas aeruginosa and Staphylococcus aureus—Under-standing the mechanism of action. Photodiagnosis Photodyn. Ther. 2019, 27, 305–316. [Google Scholar] [CrossRef]
- Anju, V.T.; Paramanantham, P.; Siddhardha, B.; Sruthil Lal, S.B.; Sharan, A.; Alyousef, A.A.; Arshad, M.; Syed, A. Malachite green-conjugated multi-walled carbon nanotubes potentiate antimicrobial photodynamic inactivation of planktonic cells and biofilms of Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Nanomed. 2019, 14, 3861–3874. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Razmjou, A.; Ghaedi, M.; Jannesar, R. Nanoporous solid-state membranes modified with multi-wall carbon nanotubes with anti-biofouling property. Int. J. Nanomed. 2019, 14, 1669–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, I.; Schaber, C.F.; Heinlein, T.; Schneider, J.J.; Gorb, S.N.; Schmitz, R.A. Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J. Mater. Chem. B 2016, 4, 5228–5235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, B.D.; Ali, M.A. Functionalized carbon nanomaterials for biosensors. In Nanomaterials for Biosensors, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2018; pp. 75–103. [Google Scholar]
- Klupp, G.; Margadonna, S.; Prassides, K. Fullerenes. In Reference Module in Materials Science and Materials Engineering, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2016. [Google Scholar]
- Hsu, S.-H.; Luo, P.-W. From nanoarchitectonics to tissue architectonics: Nanomaterials for tissue engineering. In Advanced Supramolecular Nanoarchitectonics, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2019; pp. 277–288. [Google Scholar]
- El-Sayed, N.M.; Reda, F.M.; Farag, O.F.; Nasrallah, D.A. Surface analysis of nitrogen plasma-treated C60/PS nanocomposite films for antibacterial activity. J. Biol. Phys. 2017, 43, 211–224. [Google Scholar] [CrossRef]
- Darabpour, E.; Doroodmand, M.M.; Halabian, R.; Fooladi, A.A.I. Sulfur-functionalized fullerene nanoparticle as an inhibitor and eliminator agent on Pseudomonas aeruginosa biofilm and rxpression of toxA gene. Microb. Drug Resist. 2019, 25, 594–602. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Yan, X.; Tang, Y.; Mazumder, A.; Wu, S.; Liang, Y. Antimicrobial nanomaterials against biofilms: An alternative strategy. Environ. Rev. 2017, 25, 225–244. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Nakano, H.; Tetsuka, H.; Spencer, M.J.S.; Morishita, T. Chemical modification of group IV graphene analogs. Sci. Technol. Adv. Mater. 2018, 19, 76–100. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [Green Version]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15. [Google Scholar] [CrossRef]
- Ramalingam, V.; Raja, S.; Sundaramahalingam, S.; Rajaram, R. Chemical fabrication of graphene oxide nanosheets attenuates biofilm formation of human clinical pathogens. Bioorg. Chem. 2018, 83, 326–335. [Google Scholar] [CrossRef]
- Dybowska-Sarapuk, Ł.; Kotela, A.; Krzemiński, J.; Wróblewska, M.; Marchel, H.; Romaniec, M.; Łęgosz, P.; Jakubowska, M. Graphene nanolayers as a new method for bacterial biofilm prevention: Preliminary results. J. AOAC Int. 2017, 100, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Ellepola, K.; Decroix, F.E.D.; Morin, J.L.P.; Neto, A.H.C.; Seneviratne, C.J.; Rosa, V. Graphene onto medical grade titanium: An atom thick multimodal coating that promotes osteoblast maturation and inhibits biofilm formation from distinct species. Nanotoxicology 2018, 12, 274–289. [Google Scholar] [CrossRef]
- Tao, B.; Chen, M.; Lin, C.; Lu, L.; Yuan, Z.; Liu, J.; Liao, Q.; Xia, Z.; Peng, Z.; Cai, K. Zn-incorporation with graphene oxide on Ti substrates surface to improve osteogenic activity and inhibit bacterial adhesion. J. Biomed. Mater. Res. Part A 2019, 107, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, R.; Assadian, H.; Chiniforush, N.; Parker, S.; Pourakbari, B.; Ehsani, B.; Alikhani, M.Y.; Bahador, A. Modulation of virulence in Enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: An ex vivo biofilm model. Photodiagnosis Photodyn. Ther. 2020, 29, 101643. [Google Scholar] [CrossRef]
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Wang, C.; Jiang, C.; Sun, J.; Yu, C.; Jiao, T. Graphene oxide enables the reosteogenesis of previously contaminated titanium in vitro. J. Dent. Res. 2020, 99, 922–929. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; Al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene induces formation of pores that kill spherical and rod-shaped bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Fei, D.; Zhang, Y.; Wang, Q. Functionalized titanium implant in regulating bacteria and cell response. Int. J. Nanomed. 2019, 14, 1433–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-Q.; Chen, M.; Lam, R.; Xu, X.; Osawa, E.; Ho, D. Polymer-functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 2009, 3, 2609–2616. [Google Scholar] [CrossRef]
- Chen, M.; Pierstorff, E.D.; Lam, R.; Li, S.-Y.; Huang, H.; Osawa, E.; Ho, D. Nanodiamond-mediated delivery of water-insoluble therapeutics. ACS Nano 2009, 3, 2016–2022. [Google Scholar] [CrossRef]
- Chang, Y.-R.; Lee, H.-Y.; Chen, K.; Chang, C.-C.; Tsai, D.-S.; Fu, C.-C.; Lim, T.-S.; Tzeng, Y.-K.; Fang, C.-Y.; Han, C.-C.; et al. Mass production and dynamic imaging of fluorescent nanodiamonds. Nat. Nanotechnol. 2008, 3, 284–288. [Google Scholar] [CrossRef]
- Szunerits, S.; Barras, A.; Boukherroub, R. Antibacterial applications of nanodiamonds. Int. J. Environ. Res. Public Health 2016, 13, 413. [Google Scholar] [CrossRef] [PubMed]
- Khanal, M.; Larsonneur, F.; Raks, V.; Barras, A.; Baumann, J.-S.; Martin, F.A.; Boukherroub, R.; Ghigo, J.-M.; Mellet, C.O.; Zaistev, V.; et al. Inhibition of Type 1 fimbriae-mediated Escherichia coli adhesion and biofilm formation by trimeric cluster thiomannosides conjugated to diamond nanoparticles. Nanoscale 2015, 7, 2325–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Zhang, Y.; Wang, X.; Li, Q.; Xiao, Y.; Li, P.; Wang, L.; Ye, Z.; Xing, X. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 162. [Google Scholar] [CrossRef]
- Mangal, U.; Kim, J.-Y.; Seo, J.-Y.; Kwon, J.-S.; Choi, S.-H. Novel poly (methyl methacrylate) containing and fungal resistance. Materials 2019, 12, 3438. [Google Scholar] [CrossRef] [Green Version]
- Nimesh, S. Dendrimers. In Gene Therapy: Potential Applications of Nanotechnology, 1st ed.; Woodhead Publishing Limited: Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2013; pp. 259–285. [Google Scholar]
- Tsai, H.-C.; Imae, T. Fabrication of dendrimers toward biological application. Prog. Mol. Biol. Transl. Sci. 2011, 104, 101–140. [Google Scholar] [CrossRef]
- Johansson, E.M.V.; Crusz, S.A.; Kolomiets, E.; Buts, L.; Kadam, R.U.; Cacciarini, M.; Bartels, K.-M.; Diggle, S.P.; Camara, M.; Williams, P.; et al. Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptide dendrimers targeting the fucose-specific lectin LecB. Chem. Biol. 2008, 15, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ren, B.; Zhou, X.; Xu, H.H.K.; Wang, S.; Li, M.; Weir, M.D.; Feng, M.; Cheng, L. Novel dental adhesive with biofilm-regulating and remineralization capabilities. Materials 2017, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Liu, Y.; Ma, Y.; Zhang, M.; He, Z.; Siriwardena, T.N.; Xu, H.; Bai, Y.; Zhang, X.; Reymond, J.-L.; et al. Peptide dendrimers G3KL and TNS18 inhibit Pseudomonas aeruginosa biofilms. Appl. Microbiol. Biotechnol. 2019, 103, 5821–5830. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Chai, M.; Li, X.; Deng, Y.; Jin, Q.; Ji, J. Size and charge adaptive clustered nanoparticles targeting the biofilm microenvironment for chronic lung infection management. ACS Nano 2020, 14, 5686–5699. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Pednekar, P.P.; Godiyal, S.C.; Jadhav, K.R.; Kadam, V.J. Mesoporous silica nanoparticles: A promising multifunctional drug delivery system. In Nanostructures for Cancer Therapy, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2017; pp. 593–621. [Google Scholar]
- Kuthati, Y.; Kankala, R.K.; Lin, S.-X.; Weng, C.-F.; Lee, C.-H. pH-Triggered controllable release of silver-indole-3 acetic acid complexes from mesoporous silica nanoparticles (IBN-4) for effectively killing malignant bacteria. Mol. Pharm. 2015, 12, 2289–2304. [Google Scholar] [CrossRef]
- Lu, M.-M.; Ge, Y.; Qiu, J.; Shao, D.; Zhang, Y.; Bai, J.; Zheng, X.; Chang, Z.-M.; Wang, Z.; Dong, W.-F.; et al. Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int. J. Nanomed. 2018, 13, 7697–7709. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.-M.; Mao, J.; Li, D.-X.; Luo, X.-J.; Chen, J.; Tay, F.R.; Niu, L.-N. Bimodal antibacterial system based on quaternary ammonium silane-coupled core-shell hollow mesoporous silica. Acta Biomater. 2019, 85, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Paramanantham, P.; Siddhardha, B.; Sruthil Lal, S.B.; Sharan, A.; Alyousef, A.A.; Al Dosary, M.S.; Arshad, M.; Syed, A. Antimicrobial photodynamic therapy on Staphylococcus aureus and Escherichia coli using malachite green encapsulated mesoporous silica nanoparticles: An in vitro study. PeerJ 2019, 7, e7454. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, C.J.; Leung, K.C.F.; Wong, C.H.; Lee, S.F.; Li, X.; Leung, P.C.; Lau, C.B.S.; Wat, E.; Jin, L. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS ONE 2014, 9, e103234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Yang, H.; Li, K.; Ren, H.; Lei, J.; Huang, C. Development of epigallocatechin-3-gallate-encapsulated nanohydroxyapatite/mesoporous silica for therapeutic management of dentin surface. ACS Appl. Mater. Interfaces 2017, 9, 25796–25807. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Asli, A.; Brouillette, E.; Ster, C.; Ghinet, M.G.; Brzezinski, R.; Lacasse, P.; Jacques, M.; Malouin, F. Antibiofilm and antibacterial effects of specific chitosan molecules on Staphylococcus aureus isolates associated with bovine mastitis. PLoS ONE 2017, 12, e0176988. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Shi, Z.; Neoh, K.G.; Kishen, A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J. Endod. 2010, 36, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Gondim, B.L.C.; Castellano, L.R.C.; de Castro, R.D.; Machado, G.; Carlo, H.L.; Valença, A.M.G.; de Carvalho, F.G. Effect of chitosan nanoparticles on the inhibition of Candida spp. biofilm on denture base surface. Arch. Oral Biol. 2018, 94, 99–107. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Veiga, M.; Tavaria, F.K.; Pintado, M.M. Exploring chitosan nanoparticles as effective inhibitors of antibiotic resistant skin microorganisms—From in vitro to ex vitro testing. Carbohydr. Polym. 2018, 201, 340–346. [Google Scholar] [CrossRef]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguayo, P.R.; Larenas, T.B.; Godoy, C.A.; Rivas, B.C.; González-Casanova, J.; Rojas-Gómez, D.; Fuentes, N.C. Antimicrobial and antibiofilm capacity of chitosan nanoparticles against wild type strain of Pseudomonas sp. isolated from milk of cows diagnosed with bovine mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef]
- Elshinawy, M.I.; Al-Madboly, L.A.; Ghoneim, W.M.; El-Deeb, N.M. Synergistic effect of newly introduced root canal medicaments; ozonated olive oil and chitosan nanoparticles, against persistent endodontic pathogens. Front. Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-P.; Chen, C.-T.; Tsai, T. Chitosan nanoparticles for antimicrobial photodynamic inactivation: Characterization and in vitro investigation. Photochem. Photobiol. 2012, 88, 570–576. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan nanoparticles enhance the efficiency of methylene blue-mediated antimicrobial photodynamic inactivation of bacterial biofilms: An in vitro study. Photodiagnosis Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, W.; Zhang, H.; Guo, Y.; Chen, W.; Jin, Y.; Chen, H.; Du, K.; Dai, H.; Ji, J.; et al. Photosensitizer-loaded multifunctional chitosan nanoparticles for simultaneous in situ imaging, highly efficient bacterial biofilm eradication, and tumor ablation. ACS Appl. Mater. Interfaces 2019, 11, 2302–2316. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Hosseini, N.; Boluki, E.; Chiniforush, N.; Bahador, A. Photoelimination potential of chitosan nanoparticles-indocyanine green complex against the biological activities of Acinetobacter baumannii strains: A preliminary in vitro study in burn wound infections. J. Lasers Med. Sci. 2020, 11, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, L.L.R.; Tedesco, A.C.; Takahashi, L.A.U.; Curylofo-Zotti, F.A.; Souza-Gabriel, A.E.; Corona, S.A.M. Conjugate of chitosan nanoparticles with chloroaluminium phthalocyanine: Synthesis, characterization and photoinactivation of Streptococcus mutans biofilm. Photodiagnosis Photodyn. Ther. 2020, 3, 101709. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Giuntini, F. Sonodynamic antimicrobial chemotherapy: First steps towards a sound approach for microbe inactivation. J. Photochem. Photobiol. B 2015, 150, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Rokn, A.R.; Barikani, H.R.; Bahador, A. Photosonodynamic antimicrobial chemotherapy via chitosan nanoparticles-indocyanine green against polymicrobial periopathogenic biofilms: Ex vivo study on dental implants. Photodiagnosis Photodyn. Ther. 2020, 31, 101834. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Sahu, S.K.; Pramanik, P.; Roy, S. In vitro antimicrobial activity of nanoconjugated vancomycin against drug resistant Staphylococcus aureus. Int. J. Pharm. 2012, 436, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ma, S.; Liu, C.; Yu, W.; Han, F. Enhancing the stability and antibiofilm activity of DspB by immobilization on carboxymethyl chitosan nanoparticles. Microbiol. Res. 2015, 178, 35–41. [Google Scholar] [CrossRef]
- Panwar, R.; Pemmaraju, S.C.; Sharma, A.K.; Pruthi, V. Efficacy of ferulic acid encapsulated chitosan nanoparticles against Candida albicans biofilm. Microb. Pathog. 2016, 95, 21–31. [Google Scholar] [CrossRef]
- Almaaytah, A.; Mohammed, G.K.; Abualhaijaa, A.; Al-Balas, Q. Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des. Dev. Ther. 2017, 11, 3159–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbi, S.; Nimal, T.R.; Rajan, V.K.; Baranwal, G.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Fucoidan coated ciprofloxacin loaded chitosan nanoparticles for the treatment of intracellular and biofilm infections of Salmonella. Colloids Surf. B Biointerfaces 2017, 160, 40–47. [Google Scholar] [CrossRef]
- Maghsoudi, A.; Yazdian, F.; Shahmoradi, S.; Ghaderi, L.; Hemati, M.; Amoabediny, G. Curcumin-loaded polysaccharide nanoparticles: Optimization and anticariogenic activity against Streptococcus mutans. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Siddalingam, R.P.; Yuen, K.H.; Ambu, S.P.; Davamani, F. Chitosan-propolis nanoparticle formulation demonstrates anti-bacterial activity against Enterococcus faecalis biofilms. PLoS ONE 2017, 12, e0174888. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Haselmann, G.M.; Wang, J.; Eder, D.; Schneider-Stickler, B. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr. Polym. 2018, 200, 35–42. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Rashed, M.M.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Barik, S.; Muralitharan, G.; Busi, S. Ferulic acid encapsulated chitosan-tripolyphosphate nanoparticles attenuate quorum sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa PAO1. IET Nanobiotechnol. 2018, 12, 1056–1061. [Google Scholar] [CrossRef]
- Subhaswaraj, P.; Barik, S.; Macha, C.; Chiranjeevi, P.V.; Siddhardha, B. Anti quorum sensing and anti biofilm efficacy of cinnamaldehyde encapsulated chitosan nanoparticles against Pseudomonas aeruginosa PAO1. LWT 2018, 97, 752–759. [Google Scholar] [CrossRef]
- Ajdnik, U.; Zemljič, L.F.; Bračič, M.; Maver, U.; Plohl, O.; Rebol, J. Functionalisation of silicone by drug-embedded chitosan nanoparticles for potential applications in otorhinolaryngology. Materials 2019, 12, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, Y.; Li, X.; Lee, B.S.; Jung, S.; Lee, M.-S. Enhancing the thermo-stability and anti-biofilm activity of alginate lyase by immobilization on low molecular weight chitosan nanoparticles. Int. J. Mol. Sci. 2019, 20, 4565. [Google Scholar] [CrossRef] [Green Version]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Ling, C.C.S.; Ambu, S.P.; Davamani, F. Cationic chitosan-propolis nanoparticles alter the zeta potential of S. epidermidis, inhibit biofilm formation by modulating gene expression and exhibit synergism with antibiotics. PLoS ONE 2019, 14, e0213079. [Google Scholar] [CrossRef]
- Patel, K.K.; Tripathi, M.; Pandey, N.; Agrawal, A.K.; Gade, S.; Anjum, M.M.; Tilak, R.; Singh, S. Alginate lyase immobilized chitosan nanoparticles of ciprofloxacin for the improved antimicrobial activity against the biofilm associated mucoid P. aeruginosa infection in cystic fibrosis. Int. J. Pharm. 2019, 563, 30–42. [Google Scholar] [CrossRef]
- Siddhardha, B.; Pandey, U.; Kaviyarasu, K.; Pala, R.; Syed, A.; Bahkali, A.H.; Elgorban, A.M. Chrysin-loaded chitosan nanoparticles potentiates antibiofilm activity against Staphylococcus aureus. Pathogens 2020, 9, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Rêgo, M.; Gláucia-Silva, F.; Rocha Soares, K.S.R.; de Souza, L.B.F.C.; Damasceno, I.Z.; Santos-Silva, E.D.; Lacerda, A.F.; Chaves, G.M.; da Silva-Júnior, A.A.; de Freitas Fernandes-Pedrosa, M. Biodegradable cross-linked chitosan nanoparticles improve anti-Candida and anti-biofilm activity of TistH, a peptide identified in the venom gland of the Tityus stigmurus scorpion. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109830. [Google Scholar] [CrossRef]
- Parolia, A.; Kumar, H.; Ramamurthy, S.; Davamani, F.; Pau, A. Effectiveness of chitosan-propolis nanoparticle against Enterococcus faecalis biofilms in the root canal. BMC Oral Health 2020, 20, 339. [Google Scholar] [CrossRef]
- Saberpour, M.; Bakhshi, B.; Najar-Peerayeh, S. Evaluation of the antimicrobial and antibiofilm effect of chitosan nanoparticles as carrier for supernatant of mesenchymal stem cells on multidrug-resistant Vibrio cholerae. Infect. Drug Resist. 2020, 13, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Ludwig, R.; Schneider-Stickler, B. Co-immobilization of cellobiose dehydrogenase and deoxyribonuclease I on chitosan nanoparticles against fungal/bacterial polymicrobial biofilms targeting both biofilm matrix and microorganisms. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110499. [Google Scholar] [CrossRef]
- Yeon, K.-M.; You, J.; Adhikari, M.D.; Hong, S.-G.; Lee, I.; Kim, H.S.; Na Kim, L.; Nam, J.; Kwon, S.-J.; Kim, M.I.; et al. Enzyme-immobilized chitosan nanoparticles as environmentally friendly and highly effective antimicrobial agents. Biomacromolecules 2019, 20, 2477–2485. [Google Scholar] [CrossRef]
- Ma, S.; Moser, D.; Han, F.; Leonhard, M.; Schneider-Stickler, B.; Tan, Y. Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydr. Polym. 2020, 241, 116254. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Sung, J.H. Microtechnology-based multi-organ models. Bioengineering 2017, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, K.; Fan, Z. Circulating tumor cell isolation and analysis. In Advances in Applied Microbiology; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2016; Volume 75, pp. 1–31. [Google Scholar]
- Lee, J.-H.; Kaplan, J.B.; Lee, W.Y. Microfluidic devices for studying growth and detachment of Staphylococcus epidermidis biofilms. Biomed. Microdevices 2008, 10, 489–498. [Google Scholar] [CrossRef]
- Bahar, O.; De La Fuente, L.; Burdman, S. Assessing adhesion, biofilm formation and motility of Acidovorax citrulli using microfluidic flow chambers. FEMS Microbiol. Lett. 2010, 312, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.P.; Kim, Y.-G.; Choi, C.-H.; Kim, H.-E.; Lee, S.-H.; Chang, W.-S.; Lee, C.-S. In situ monitoring of antibiotic susceptibility of bacterial biofilms in a microfluidic device. Lab Chip 2010, 10, 3296–3299. [Google Scholar] [CrossRef]
- Kim, Y.W.; Mosteller, M.P.; Subramanian, S.; Meyer, M.T.; Bentley, W.E.; Ghodssi, R. An optical microfluidic platform for spatiotemporal biofilm treatment monitoring. J. Micromechanics Microengineering 2015, 26, 015013. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.H.; Hegde, M.; Kim, J.; Wang, X.; Jayaraman, A.; Wood, T.K. Synthetic quorum-sensing circuit to control consortial biofilm for-mation and dispersal in a microfluidic device. Nat. Commun. 2012, 3, 613. [Google Scholar] [CrossRef] [PubMed]
- Shumi, W.; Kim, S.H.; Lim, J.; Cho, K.-S.; Han, H.; Park, S. Shear stress tolerance of Streptococcus mutans aggregates determined by microfluidic funnel device (μFFD). J. Microbiol. Methods 2013, 93, 85–89. [Google Scholar] [CrossRef]
- Molina, A.; González, J.; Laborda, E.; Compton, R. Analytical solutions for fast and straightforward study of the effect of the electrode geometry in transient and steady state voltammetries: Single- and multi-electron transfers, coupled chemical reactions and electrode kinetics. J. Electroanal. Chem. 2015, 756, 1–21. [Google Scholar] [CrossRef]
- Moya, A.; Guimerà, X.; Del Campo, F.J.; Alfonso, E.P.; Dorado, A.D.; Baeza, M.; Villa, R.; Gabriel, D.; Gamisans, X.; Gabriel, G. Biofilm oxygen profiling using an array of microelectrodes on a microfabricated needle. Procedia Eng. 2014, 87, 256–259. [Google Scholar] [CrossRef] [Green Version]
- Moya, A.; Guimerà, X.; Del Campo, F.J.; Prats-Alfonso, E.; Dorado, A.D.; Baeza, M.; Villa, R.; Gabriel, D.; Gamisans, X.; Gabriel, G. Profiling of oxygen in biofilms using individually addressable disk microelectrodes on a microfabricated needle. Microchim. Acta 2014, 182, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Becerro, S.; Paredes, J.; Arana, S. Multiparametric biosensor for detection and monitoring of bacterial biofilm adhesion and growth. In Proceedings of the 6th European Conference of the International Federation for Medical and Biological Engineering, IFMBE Proceedings, Dubrovnik, Croatia, 7–11 September 2014; Springer: Cham, Switzerland; Volume 45, pp. 333–336.
- Hou, J.; Liu, Z.; Zhou, Y.; Chen, W.; Li, Y.; Sang, L. An experimental study of pH distributions within an electricity-producing biofilm by using pH microelectrode. Electrochim. Acta 2017, 251, 187–194. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Z.; Zang, Y.; Zhang, D.; Xin, Q. Detection of respiration changes inside biofilms with microelectrodes during exposure to antibiotics. J. Environ. Sci. Health Part A 2019, 54, 202–207. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Chew, C.L.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Wei, L.C. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef] [Green Version]
- Yousefzade, O.; Ugartemendia, J.M.; Sangroniz, L.; Hernandez, R.; Puiggali, J.; Garmabi, H. Reactive melt processing of poly (l-lactide) in the presence of thermoplastic polyurethane and carboxylated carbon nanotubes. J. Mater. Sci. 2019, 54, 14961–14974. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; Martinez, E.S.M.; Pimentel, R.C.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. 2019, 69, 85–126. [Google Scholar] [CrossRef]
- Martinelli, A.; Bakry, A.; D’Ilario, L.; Francolini, I.; Piozzi, A.; Taresco, V. Release behavior and antibiofilm activity of usnic acid-loaded carboxylated poly(l-lactide) microparticles. Eur. J. Pharm. Biopharm. 2014, 88, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.S.; Bettencourt, A.F.; Gonçalves, L.M.D.; Kasper, S.; Bétrisey, B.; Kikhney, J.; Moter, A.; Trampuz, A.; Almeida, A.J. Activity of daptomycin- and vancomycin-loaded poly-epsilon-caprolactone microparticles against mature staphylococcal biofilms. Int. J. Nanomed. 2015, 10, 4351–4366. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.; Thorn, C.; Richter, K.; Thierry, B.; Prestidge, C. Efficacy of poly-lactic-co-glycolic acid micro- and nanoparticles of ciprofloxacin against bacterial biofilms. J. Pharm. Sci. 2016, 105, 3115–3122. [Google Scholar] [CrossRef]
- Porsio, B.; Cusimano, M.G.; Schillaci, D.; Craparo, E.F.; Giammona, G.; Cavallaro, G. Nano into Micro Formulations of Tobramycin for the Treatment of Pseudomonas aeruginosa Infections in Cystic Fibrosis. Biomacromolecules 2017, 18, 3924–3935. [Google Scholar] [CrossRef]
- Hasan, S.; Thomas, N.; Thierry, B.; Prestidge, C.A. Biodegradable nitric oxide precursor-loaded micro- and nanoparticles for the treatment of Staphylococcus aureus biofilms. J. Mater. Chem. B 2017, 5, 1005–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, I.S.; Kikhney, J.; Kursawe, L.; Kasper, S.; Gonçalves, L.M.D.; Trampuz, A.; Moter, A.; Bettencourt, A.F.; Almeida, A.J. Encapsulation in polymeric microparticles improves daptomycin activity against mature staphylococci Biofilms—A ihermal and imaging Study. AAPS PharmSciTech 2018, 19, 1625–1636. [Google Scholar] [CrossRef]
- Woischnig, A.-K.; Gonçalves, L.M.; Ferreira, M.; Kuehl, R.; Kikhney, J.; Moter, A.; Ribeiro, I.A.; Almeida, A.J.; Khanna, N.; Bettencourt, A.F. Acrylic microparticles increase daptomycin intracellular and in vivo anti-biofilm activity against Staphylococcus aureus. Int. J. Pharm. 2018, 550, 372–379. [Google Scholar] [CrossRef]
- Kang, M.; Kim, S.; Kim, H.; Song, Y.; Jung, D.; Kang, S.; Seo, J.-H.; Nam, S.; Lee, Y. Calcium-Binding Polymer-Coated Poly(lactide-co-glycolide) Microparticles for Sustained Release of Quorum Sensing Inhibitors to Prevent Biofilm Formation on Hydroxyapatite Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 7686–7694. [Google Scholar] [CrossRef]
- Kawakita, E.R.H.; Ré, A.C.S.; Peixoto, M.P.G.; Ferreira, M.P.; Ricomini-Filho, A.P.; Freitas, O.; Aires, C.P. Effect of chitosan dispersion and microparticles on older Streptococcus mutans biofilms. Molecules 2019, 24, 1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarshini, B.M.; Selvan, S.T.; Narayanan, K.; Fawzy, A.S. Characterization of chlorhexidine-loaded calcium-hydroxide microparticles as a potential dental pulp-capping material. Bioengineering 2017, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, W.; Li, F.; Morrow, B.R.; Garcia-Godoy, F.; Hong, L. Sustained release of minocycline from minocycline-calcium-dextran sulfate complex microparticles for periodontitis treatment. J. Pharm. Sci. 2018, 107, 3134–3142. [Google Scholar] [CrossRef]
- Yang, C.; Mavelli, G.V.; Nacharaju, P.; Li, K.; Cleare, L.G.; Nosanchuk, J.D.; Friedman, J.M.; Abuzeid, W.M. Novel nitric oxide-generating platform using manuka honey as an anti-biofilm strategy in chronic rhinosinusitis. Int. Forum. Allergy Rhinol. 2020, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Melake, N.A.; Mahmoud, H.A.; Al-Semary, M.T. Bactericidal activity of various antibiotics versus tetracycline-loaded chitosan microspheres against Pseudomonas aeruginosa biofilms. Afr. J. Microbiol. Res. 2012, 6, 5387–5398. [Google Scholar] [CrossRef]
- Romić, M.D.; Klarić, M. Šegvić; Lovrić, J.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef]
- Reinbold, J.; Hierlemann, T.; Hinkel, H.; Müller, I.; Maier, M.E.; Weindl, T.; Schlensak, C.; Wendel, H.P.; Krajewski, S. Development and in vitro characterization of poly(lactide-co-glycolide) microspheres loaded with an antibacterial natural drug for the treatment of long-term bacterial infections. Drug Des. Devel. Ther. 2016, 10, 2823–2832. [Google Scholar] [CrossRef] [Green Version]
- Thaya, R.; Vaseeharan, B.; Sivakamavalli, J.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-Anbr, M.N.; Khaled, J.M.; Benelli, G. Synthesis of chitosan-alginate microspheres with high antimicrobial and antibiofilm activity against multidrug resistant microbial pathogens. Microb. Pathog. 2018, 114, 17–24. [Google Scholar] [CrossRef]
- Qiao, Z.; Yuan, Z.; Zhang, W.; Wei, D.; Hu, N. Preparation, in vitro release and antibacterial activity evaluation of rifampicin and moxifloxacin-loaded poly(D,l-lactide-co-glycolide) microspheres. Artif. Cells Nanomed. Biotechnol. 2019, 47, 790–798. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-J.; Long, T.; Chen, W.; Ning, C.-Q.; Zhu, Z.-A.; Guo, Y.-P. Bactericidal property and biocompatibility of gentamicin-loaded mesoporous carbonated hydroxyapatite microspheres. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3583–3591. [Google Scholar] [CrossRef]
- Long, T.; Guo, Y.-P.; Tang, S.; Guo, Y.-J.; Zhu, Z.-A. Emulsion fabrication of magnetic mesoporous carbonated hydroxyapatite microspheres for treatment of bone infection. RSC Adv. 2014, 4, 11816–11825. [Google Scholar] [CrossRef]
- Mishra, S.; Rautray, T.R. Silver-incorporated hydroxyapatite–albumin microspheres with bactericidal effects. J. Korean Ceram. Soc. 2020, 57, 175–183. [Google Scholar] [CrossRef]
- Song, H.; Zhang, J.; Qu, J.; Liu, J.; Yin, P.; Zhang, G.; Shang, D. Lactobacillus rhamnosus GG microcapsules inhibit Escherichia coli biofilm formation in coculture. Biotechnol. Lett. 2019, 41, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Sima, F.; Ristoscu, C.; Duta, L.; Gallet, O.; Anselme, K.; Mihailescu, I. Laser thin films deposition and characterization for biomedical applications. In Laser Surface Modification of Biomaterials; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2016; pp. 77–125. [Google Scholar]
- Grumezescu, V.; Socol, G.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Trușcǎ, R.; Bleotu, C.; Balaure, P.C.; Cristescu, R.; Chifiriuc, M.C. Functionalized antibiofilm thin coatings based on PLA-PVA microspheres loaded with usnic acid natural compounds fabricated by MAPLE. Appl. Surf. Sci. 2014, 302, 262–267. [Google Scholar] [CrossRef]
- Grumezescu, V.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Ficai, A.; Vasile, B.S.; Truscă, R.; Bleotu, C.; Lazar, V.; Chifiriuc, C.M.; et al. Usnic acid-loaded biocompatible magnetic PLGA-PVA microsphere thin films fabricated by MAPLE with increased resistance to staphylococcal colonization. Biofabrication 2014, 6, 035002. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Holban, A.M.; Iordache, F.; Socol, G.; Mogoşanu, G.D.; Grumezescu, A.M.; Ficai, A.; Vasile, B.S.; Truşcă, R.; Chifiriuc, M.C.; et al. MAPLE fabricated magnetite@eugenol and (3-hidroxybutyric acid-co-3-hidroxyvaleric acid)–polyvinyl alcohol microspheres coated surfaces with anti-microbial properties. Appl. Surf. Sci. 2014, 306, 16–22. [Google Scholar] [CrossRef]
- Jennings, J.A.; Pulgarin, D.A.V.; Kunwar, D.L.; Babu, J.; Mishra, S.; Bumgardner, J. Bacterial inhibition by chitosan coatings loaded with silver-decorated calcium phosphate microspheres. Thin Solid Films 2015, 596, 83–86. [Google Scholar] [CrossRef]
- Grumezescu, V.; Holban, A.; Sima, L.; Chiritoiu, M.; Chiritoiu, G.; Grumezescu, A.; Ivan, L.; Safciuc, F.; Antohe, F.; Florica, C.; et al. Laser deposition of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid)—Lysozyme microspheres based coatings with anti-microbial properties. Int. J. Pharm. 2017, 521, 184–195. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K. Usnic acid. Phytochem 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Thukkaram, M.; Sitaram, S.; Kannaiyan, S.K.; Subbiahdoss, G. Antibacterial efficacy of iron-oxide nanoparticles against biofilms on different biomaterial surfaces. Int. J. Biomater. 2014, 2014, 716080. [Google Scholar] [CrossRef] [Green Version]
- Latifah-Munirah, B.; Himratul-Aznita, W.H.; Mohd Zain, N. Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Front. Life Sci. 2015, 8, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Frayssinet, P.; Trouillet, J.; Rouquet, N.; Azimus, E.; Autefage, A. Osseointegration of macroporous calcium phosphate ceramics having a different chemical composition. Biomaterials 1993, 14, 423–429. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Gerard, P.D.; Bergin, P.; Chestnutt, B.; Marini, M.; Ramsey, V.; Elder, S.H.; Gilbert, J.A. Chitosan: Potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J. Biomater. Sci. Polym. Ed. 2003, 14, 423–438. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Reddy, S.T.; Chung, K.K.; McDaniel, C.J.; Darouiche, R.O.; Landman, J.; Brennan, A.B. Micropatterned surfaces for reducing the risk of catheter-associated urinary tract infection: An in vitro study on the effect of sharklet micropatterned surfaces to inhibit bacterial colonization and migration of uropathogenic Escherichia coli. J. Endourol. 2011, 25, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- May, R.M.; Hoffman, M.G.; Sogo, M.J.; Parker, A.E.; O’Toole, G.A.; Brennan, A.B.; Reddy, S.T. Micropatterned surfaces reduce bacterial colonization and biofilm formation in vitro: Potential for enhancing endotracheal tube designs. Clin. Transl. Med. 2014, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Wei, Q.; Mettetal, M.R.; Han, J.; Rau, L.; Tie, J.; May, R.M.; Pathe, E.T.; Reddy, S.T.; Sullivan, L.; et al. Surface micropattern reduces colonization and medical device-associated infections. J. Med. Microbiol. 2017, 66, 1692–1698. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, R.; Kennedy, A.J.; Merritt, M.; Crocker, F.H.; Baney, R.H. Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloids Surf. B Biointerfaces 2014, 117, 225–232. [Google Scholar] [CrossRef]
- Chien, H.-W.; Chen, X.-Y.; Tsai, W.-P.; Lee, M. Inhibition of biofilm formation by rough shark skin-patterned surfaces. Colloids Surf. B Biointerfaces 2020, 186, 110738. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Brambilla, E.; Azzola, F.; Ottobelli, M.; Pellegrini, G.; Francetti, L.A. Laser microtextured titanium implant surfaces reduce in vitro and in situ oral biofilm formation. PLoS ONE 2018, 13, e0202262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayón, B.; Cacicedo, M.L.; Álvarez, V.A.; Castro, G.R. Self-assembly stereo-specific synthesis of silver phosphate microparticles on bacterial cellulose membrane surface for antimicrobial applications. Colloid Interface Sci. Commun. 2018, 26, 7–13. [Google Scholar] [CrossRef]
- Fürsatz, M.; Skog, M.; Sivlér, P.; Palm, E.; Aronsson, C.; Skallberg, A.; Greczynski, G.; Khalaf, H.; Bengtsson, T.; Aili, D. Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide ε -poly-L-Lysine. Biomed. Mater. 2017, 13, 025014. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Thinakaran, S.; Loordhuswamy, A.; Rengaswami, G.V. Electrophoretic deposition of chitosan/nano silver embedded micro sphere on centrifugal spun fibrous matrices—A facile biofilm resistant biocompatible material. Int. J. Biol. Macromol. 2020, 148, 68–78. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Lalani, R.; Liu, L. Electrospun zwitterionic poly(sulfobetaine methacrylate) for nonadherent, superabsorbent, and antimicrobial wound dressing applications. Biomacromolecules 2012, 13, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Thakral, G.; Lafontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4. [Google Scholar] [CrossRef]
- Rmaile, A.; Carugo, D.; Capretto, L.; Aspiras, M.; De Jager, M.; Ward, M.; Stoodley, P. Removal of interproximal dental biofilms by high-velocity water microdrops. J. Dent. Res. 2014, 93, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Rmaile, A.; Carugo, D.; Capretto, L.; Wharton, J.A.; Thurner, P.J.; Aspiras, M.; Ward, M.; De Jager, M.; Stoodley, P. An experimental and com-putational study of the hydrodynamics of high-velocity water microdrops for interproximal tooth cleaning. J. Mech. Behav. Biomed. Mater. 2015, 46, 148–157. [Google Scholar] [CrossRef]
- Fabbri, S.; Johnston, D.; Rmaile, A.; Gottenbos, B.; De Jager, M.; Aspiras, M.; Starke, E.; Ward, M.; Stoodley, P. Streptococcus mutans biofilm transient viscoelastic fluid behaviour during high-velocity microsprays. J. Mech. Behav. Biomed. Mater. 2016, 59, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, S.; Johnston, D.A.; Rmaile, A.; Gottenbos, B.; De Jager, M.; Aspiras, M.; Starke, E.M.; Ward, M.T.; Stoodley, P. High-velocity microsprays enhance antimicrobial activity in Streptococcus mutans biofilms. J. Dent. Res. 2016, 95, 1494–1500. [Google Scholar] [CrossRef] [Green Version]
- Mair, L.O.; Nacev, A.; Hilaman, R.; Stepanov, P.Y.; Chowdhury, S.; Jafari, S.; Hausfeld, J.; Karlsson, A.J.; Shirtliff, M.E.; Shapiro, B.; et al. Biofilm disruption with rotating microrods enhances antimicrobial efficacy. J. Magn. Magn. Mater. 2017, 427, 81–84. [Google Scholar] [CrossRef]
- Houry, A.; Gohar, M.; Deschamps, J.; Tischenko, E.; Aymerich, S.; Gruss, A.; Briandet, R. Bacterial swimmers that infiltrate and take over the biofilm matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 13088–13093. [Google Scholar] [CrossRef] [Green Version]
- Stanton, M.M.; Park, B.-W.; Vilela, D.; Bente, K.; Faivre, D.; Sitti, M.; Sánchez, S. Magnetotactic bacteria powered biohybrids target E. coli biofilms. ACS Nano 2017, 11, 9968–9978. [Google Scholar] [CrossRef]
- Xu, J.; Danehy, R.; Cai, H.; Ao, Z.; Pu, M.; Nusawardhana, A.; Rowe-Magnus, D.; Guo, F. Microneedle patch-mediated treatment of bacterial biofilms. ACS Appl. Mater. Interfaces 2019, 11, 14640–14646. [Google Scholar] [CrossRef]
- Yager, D.R.; Nwomeh, B.C. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999, 7, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. X 2020, 2, 100047. [Google Scholar] [CrossRef]


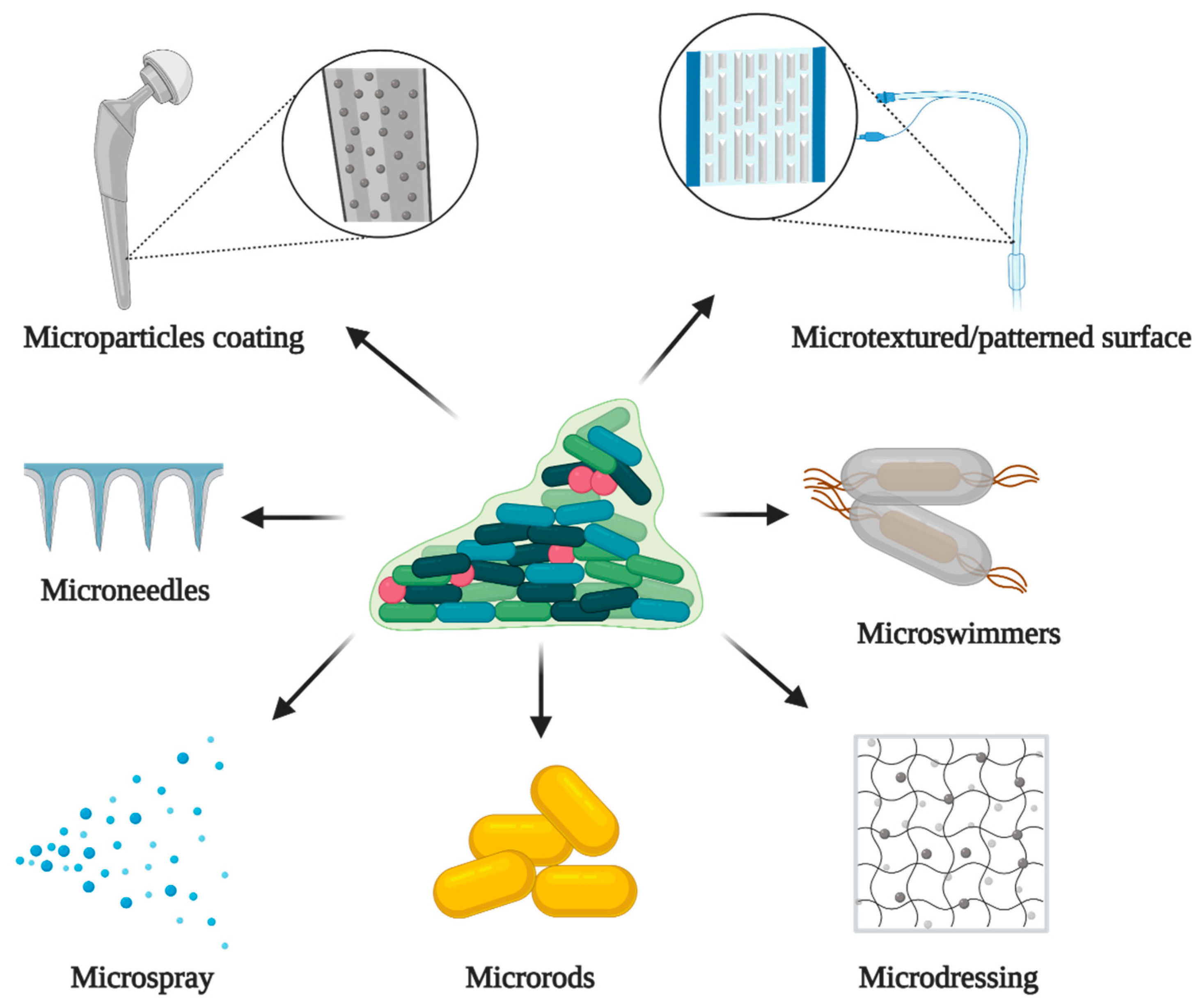
| Species | Matrix Components | References |
|---|---|---|
| Candida species: | ||
| Glucose, DNA, small amounts of hexosamine, small amounts of protein, phosphorous, and uronic acid. | [35] |
| Polysaccharide complex of mannan and glucan. | [36] |
| High concentration of carbohydrate and less protein than C. parapsilosis biofilms. | [37] |
| High concentration of carbohydrates with less protein. | |
| Hexosamine, small amounts of protein, phosphorous, and more uronic acid than C. albicans. | [35] |
| Cryptococcus species: | ||
| Glucurunoxylomannan and sugars such as xylose, mannose, glucose, and galactoxylomannan. | [38] |
| Aspergillusspecies: | ||
| Galactomannan, β-1,3 glucan, monosaccharides, galactose, polyols, melanin, a small amount of protein, N-acetyl-galactosamine (galactosaminogalactan [GAG]) and eDNA. | [39,40,41] |
| Species | ABC Transporters Genes | References | MFS Transporters Genes | References |
|---|---|---|---|---|
| Candida species | ||||
| CaCDR1 CaCDR2 | [56] | CaMDR1 | [56] |
| CgCDR1 CgCDR2 | [57] | - | - |
| SNQ 1 | [58] | - | - | |
| CDR | [59] | MDR | [59] |
| CtCDR1 | [30] | CtMDR1 | [30] |
| Aspergillus species | ||||
| - | - | MDR | [30] |
| Chitosan Nanoparticles (CHNPs) Based Formulation | Microorganism(s) Tested | References |
|---|---|---|
| Vancomycin-loaded Carboxymethyl chitosan-2,2′-ethylenedioxy bis ethylamine-folate nanoparticles | S. aureus | [223] |
| β-N-acetyl-glucosaminidase immobilized linoleic acid carboxymethyl chitosan nanoparticles | S. aureus, S. epidermidis, A. actinomycetemcomitans | [224] |
| Ferulic acid-encapsulated CHNPs | C. albicans | [225] |
| RBRBR-CN (Potent ultrashort antimicrobial peptide—RBRBR) | S. aureus | [226] |
| Ciprofloxacin-loaded CHNPs | S. Paratyphi A | [227] |
| Fucoidan coated ciprofloxacin-loaded CHPNs | S. Paratyphi A | [227] |
| Curcumin-loaded CHNPs | S. mutans | [228] |
| Chitosan-propolis nanoparticles | E. faecalis | [229] |
| Oxacillin and Deoxyribonuclease I-loaded CHNPs | S. aureus | [230] |
| Clove oil-loaded CHNPs | E. coli | [231] |
| Ferulic acid encapsulated chitosan-tripolyphosphate nanoparticles | P. aeruginosa | [232] |
| Cinnamaldehyd- encapsulated CHNPs | P. aeruginosa | [233] |
| Co-amoxiclav embedded CHNPs | S. aureus | [234] |
| Alginate lyase immobilized low molecular weight CHNPs | P. aeruginosa | [235] |
| Chitosan-propolis nanoparticles | S. epidermis | [236] |
| Alginate lyase functionalized CHNPs of Ciprofloxacin | P. aeruginosa | [237] |
| Chrysin-loaded CHNPs | S. aureus | [238] |
| Tityus stigmurus Hypotensin-loaded cross-linked CHNPs | C. tropicalis, C. krusei, C. albicans | [239] |
| Chitosan-propolis nanoparticles | E. faecalis | [240] |
| Mesenchymal stem cells derived conditioned media incorporated CHNPs | V. cholerae | [241] |
| Cellobiose dehydrogenase and deoxyribonuclease I co-immobilized CHNPs | C. albicans, S. aureus (Mono- and polymicrobial) | [242] |
| Glucose oxidase immobilized CHNPs | S. aureus | [243] |
| Curcumin-loaded CHNPs | C. albicans, S. aureus (Mono- and polymicrobial) | [244] |
| Formulation | Matrix Material(s) | Active Ingredient(s) | Microorganism(s) Tested | References |
|---|---|---|---|---|
| Organic Polymeric Microparticles | ||||
| UA-loaded CPLLA MP | Carboxylated poly(l-lactide) | Usnic acid (UA) | S. epidermidis | [265] |
| DAP-loaded PCL MP | Poly-epsilon-caprolactone | Daptomycin | MRSA, S. epidermis | [266] |
| Ciprofloxacin-loaded PLGA MP | PLGA | Ciprofloxacin | P. aeruginosa, S. aureus | [267] |
| PTC-loaded Man-Cyst MP | Mannitol | Polyanion tobramycin complex, Cysteamine | P. aeruginosa | [268] |
| ISMN-loaded PLGA MP | PLGA | Isosorbide mononitrate | S. aureus | [269] |
| DAP-loaded PMMA-EUD MP | Poly (methyl methacrylate) -Eudragit RL 100 | Daptomycin | MRSA, S. epidermis | [270] |
| DAP-MP | Poly (methyl methacrylate)-Eudragit RL 100 | Daptomycin | MRSA | [271] |
| PBMP-coated PLGA MP | Poly (butyl methacrylate-co-methacryloyloxyethyl phosphate), PLGA | Furanone C-30 | S. mutans | [272] |
| Chitosan MP | Chitosan | Chitosan | S. mutans | [273] |
| Inorganic Microparticles | ||||
| CHX-loaded Ca(OH)2 MP | Calcium hydroxide | Chlorhexidine | S. mutans | [274] |
| Hybrid Microparticles | ||||
| Mino-Ca-DS MP | Calcium chloride, dextran sulfate | Minocycline | A. actinomycetemcomitans, S. mutans | [275] |
| SNO MP | Porous organosilica | Nitrosylated thiol groups | P. aeruginosa | [276] |
| Organic Polymeric Microspheres | ||||
| Tetracycline-loaded chitosan MS | Chitosan | Tetracycline, chitosan | P. aeruginosa | [277] |
| MCP MS | Chitosan, Pluronic® F127 | Melatonin, chitosan | MRSA | [278] |
| Totarol-loaded PLGA MS | PLGA | Totarol | S. aureus | [279] |
| Chitosan-alginate MS | Chitosan, alginate | Chitosan | E. faecalis, P. aeruginosa, P. vulgaris, S. aureus | [280] |
| RIF-MOX PLGA MS | PLGA | Rifampicin, moxifloxacin | S. aureus | [281] |
| Inorganic Microspheres | ||||
| Gentamicin-loaded MCH MS | Mesoporous carbonated hydroxyapatite | Gentamicin | S. epidermidis | [282] |
| Gentamicin-loaded MEH MS | Magnetic mesoporous carbonated hydroxyapatite | Gentamicin | S. epidermidis | [283] |
| Hybrid Microspheres | ||||
| Ag–HA–Alb MS | Hydroxyapatite, albumin | Silver | S. aureus | [284] |
| Organic Polymeric Microcapsule | ||||
| Lactobacillus rhamnosus GG alginate-chitosan MC | Alginate, chitosan | L. rhamnosus GG | E. coli | [285] |
| Coating Formulation | Coating Surface | Coating Method | Microorganism(s) Tested | References |
|---|---|---|---|---|
| UA loaded-PLA-PVA MS | Titanium | MAPLE | S. aureus | [288] |
| UA loaded-Magnetic PLGA-PVA MS | Titanium | MAPLE | S. aureus | [289] |
| Magnetite and eugenol loaded P(3HB-3HV)-PVA MS | Glass | MAPLE | P. aeruginosa, S. aureus | [290] |
| Chitosan loaded with silver-decorated calcium phosphate MS | Titanium | Alkyloxysilane | P. denticola, P. gingivalis, S. aureus | [291] |
| P(3HB-3HV)-PEG-Lys MS | Titanium | MAPLE | P. aeruginosa, S. aureus | [292] |
| P(3HB-3HV)-Lys MS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, H.; Choo, S.; Rajeswari Mahalingam, S.R.; Adisuri, D.S.; Madhavan, P.; Md. Akim, A.; Chong, P.P. Approaches for Mitigating Microbial Biofilm-Related Drug Resistance: A Focus on Micro- and Nanotechnologies. Molecules 2021, 26, 1870. https://doi.org/10.3390/molecules26071870
Rao H, Choo S, Rajeswari Mahalingam SR, Adisuri DS, Madhavan P, Md. Akim A, Chong PP. Approaches for Mitigating Microbial Biofilm-Related Drug Resistance: A Focus on Micro- and Nanotechnologies. Molecules. 2021; 26(7):1870. https://doi.org/10.3390/molecules26071870
Chicago/Turabian StyleRao, Harinash, Sulin Choo, Sri Raja Rajeswari Mahalingam, Diajeng Sekar Adisuri, Priya Madhavan, Abdah Md. Akim, and Pei Pei Chong. 2021. "Approaches for Mitigating Microbial Biofilm-Related Drug Resistance: A Focus on Micro- and Nanotechnologies" Molecules 26, no. 7: 1870. https://doi.org/10.3390/molecules26071870
APA StyleRao, H., Choo, S., Rajeswari Mahalingam, S. R., Adisuri, D. S., Madhavan, P., Md. Akim, A., & Chong, P. P. (2021). Approaches for Mitigating Microbial Biofilm-Related Drug Resistance: A Focus on Micro- and Nanotechnologies. Molecules, 26(7), 1870. https://doi.org/10.3390/molecules26071870









