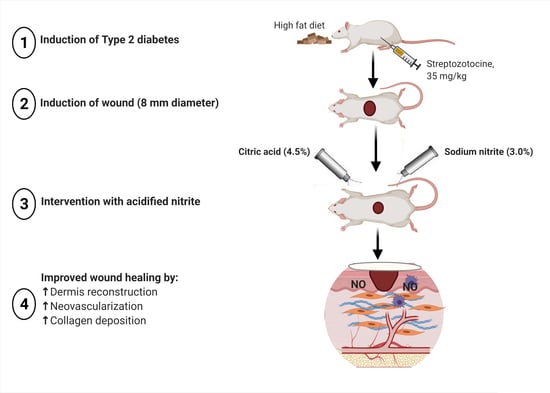
Acidified Nitrite Accelerates Wound Healing in Type 2 Diabetic Male Rats: A Histological and Stereological Evaluation
Abstract
:1. Introduction
2. Results
2.1. Changes in Body Weight
2.2. Effect of Acidified Nitrite on Indices of Re-Epithelialization
2.2.1. Numerical Density of Basal Cells
2.2.2. Epidermal Thickness
2.3. Effect of Acidified Nitrite on Indices of Dermis Structure
2.3.1. Total Volumes of the Granular Tissue and Dermis
2.3.2. Numerical Density of Neutrophils, Macrophages, and Fibroblasts
2.4. Effect of Acidified Nitrite on New Blood Vessel Formation (Neovascularization)
2.4.1. Wound Levels of Vascular Endothelial Growth Factor (VEGF)
2.4.2. Number of Blood Vessels
2.5. Effect of Topical Acidified Nitrite Application on Collagen Deposition
2.5.1. Total Volume of fibrous Tissue
2.5.2. Hydroxyproline Content
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ethical Approval
4.3. Animals and Diet
4.4. Groups and Study Design
4.5. Wound Induction
4.6. Preparation and Application of Acidified Nitrite
4.7. Measurement of Serum Glucose Concentration
4.8. Tissue Collection
4.9. Histological and Stereological Evaluations
4.10. Indices of Re-Epithelialization
4.10.1. Estimation of the Numerical Density of Basal Cells
4.10.2. Measurement of Epidermal Thickness
4.11. Indices of Dermis Structure
4.11.1. Estimation of the Volume of the Granular Tissue and Dermis
4.11.2. Estimation of the Numerical Density of Neutrophils, Macrophages, and Fibroblasts
4.12. Indices of New Blood Vessel Formation (Neovascularization)
4.12.1. Measurement of VEGF
4.12.2. Counting the Number of Blood Vessels
4.13. Indices of Collagen Deposition
4.13.1. Estimation of the Volume of the Fibrous Tissue
4.13.2. Measurement of Hydroxyproline Content
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavi, A.; Sibbald, R.G.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Siitonen, O.I.; Niskanen, L.K.; Laasko, M.; Siitonen, J.T.; Pyörälä, K. Lower-extremity amputations in diabetic and non-diabetic patients. A population-based study in eastern Finland. Diabetes Care 1993, 16, 16–20. [Google Scholar] [CrossRef]
- Trautner, C.; Haastert, B.; Giani, G.; Berger, M. Incidence of lower limb amputations and diabetes. Diabetes Care 1996, 19, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Iversen, M.M.; Tell, G.S.; Riise, T.; Hanestad, B.R.; Østbye, T.; Graue, M.; Midthjell, K. History of foot ulcer increases mortality among individuals with diabetes: Ten-year follow-up of the Nord-Trøndelag Health Study, Norway. Diabetes Care 2009, 32, 2193–2199. [Google Scholar] [CrossRef] [Green Version]
- Margolis, D.J.; Kantor, J.; Berlin, J.A. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care 1999, 22, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W.J.; Chipchase, S.Y.; Ince, P.; Game, F.L. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care 2006, 29, 1784–1787. [Google Scholar] [CrossRef] [Green Version]
- Schwentker, A.; Vodovotz, Y.; Weller, R.; Billiar, T.R. Nitric oxide and wound repair: Role of cytokines? Nitric Oxide 2002, 7, 1–10. [Google Scholar] [CrossRef]
- Marcilli, R.H.; de Oliveira, M.G. Nitric oxide-releasing poly(vinyl alcohol) film for increasing dermal vasodilation. Colloids Surf. B Biointerfaces 2014, 116, 643–651. [Google Scholar] [CrossRef]
- Afzali, H.; Khaksari, M.; Norouzirad, R.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Acidified nitrite improves wound healing in type 2 diabetic rats: Role of oxidative stress and inflammation. Nitric Oxide 2020, 103, 20–28. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, D.; Kim, H.; Kim, J.; Hlaing, S.P.; Hasan, N.; Cao, J.; Yoo, J.-Y. Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds. Pharmaceutics 2020, 12, 618. [Google Scholar] [CrossRef]
- Stallmeyer, B.; Kämpfer, H.; Kolb, N.; Pfeilschifter, J.; Frank, S. The function of nitric oxide in wound repair: Inhibition of inducible nitric oxide-synthase severely impairs wound re-epithelialization. J. Invest. Dermatol. 1999, 113, 1090–1098. [Google Scholar] [CrossRef] [Green Version]
- Chin, L.C.; Kumar, P.; Palmer, J.A.; Rophael, J.A.; Dolderer, J.H.; Thomas, G.P.L.; Morrison, W.A.; Penington, A.J.; Stewart, A.G.; Mitchell, G.M. The influence of nitric oxide synthase 2 on cutaneous wound angiogenesis. Br. J. Dermatol. 2011, 165, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Nguyen, L.N.; Macherla, C.; Chi, Y.; Friedman, J.M.; Nosanchuk, J.D.; Martinez, L.R. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am. J. Pathol. 2012, 180, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Burrow, J.W.; Koch, J.A.; Chuang, H.H.; Zhong, W.; Dean, D.D.; Sylvia, V.L. Nitric oxide donors selectively reduce the expression of matrix metalloproteinases-8 and -9 by human diabetic skin fibroblasts. J. Surg. Res. 2007, 140, 90–98. [Google Scholar] [CrossRef]
- Kitano, T.; Yamada, H.; Kida, M.; Okada, Y.; Saika, S.; Yoshida, M. Impaired Healing of a Cutaneous Wound in an Inducible Nitric Oxide Synthase-Knockout Mouse. Dermatol. Res. Pract. 2017, 5, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.C.; Salyapongse, A.N.; Bragdon, G.A.; Shears 2nd, L.L.; Watkins, S.C.; Edington, H.D.; Billiar, T.R. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am. J. Physiol. 1999, 277, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Masters, K.S.B.; Leibovich, S.J.; Belem, P.; West, J.L.; Poole-Warren, L.A. Effects of nitric oxide releasing poly (vinyl alcohol) hydrogel dressings on dermal wound healing in diabetic mice. Wound Repair Regen. 2002, 10, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.P.; Most, D.; Efron, D.T.; Witte, M.B.; Barbul, A. Supplemental L-arginine enhances wound healing in diabetic rats. Wound Repair Regen. 2003, 11, 198–203. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, D.; Wang, F.; Hou, Z.; Fang, Z. In situ eNOS/NO up-regulation—a simple and effective therapeutic strategy for diabetic skin ulcer. Sci. Rep. 2016, 6, 60–75. [Google Scholar]
- Edmonds, M.E.; Bodansky, H.J.; Boulton, A.J.M.; Chadwick, P.J.; Dang, C.N.; D’Costa, R.; Johnston, A.; Kennon, B.; Leese, G.; Rajbhandari, S.M.; et al. Multicenter, randomized controlled, observer-blinded study of a nitric oxide generating treatment in foot ulcers of patients with diabetes-ProNOx1 study. Wound Repair Regen. 2018, 26, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Bahadoran, Z.; Ghasemi, A.; Mirmiran, P.; Azizi, F.; Hadaegh, F. Beneficial effects of inorganic nitrate/nitrite in type 2 diabetes and its complications. Nutr. Metab. 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef]
- Ormerod, A.D.; Shah, A.A.; Li, H.; Benjamin, N.B.; Ferguson, G.P.; Leifert, C. An observational prospective study of topical acidified nitrite for killing methicillin-resistant Staphylococcus aureus (MRSA) in contaminated wounds. BMC Res. Notes 2011, 4, 458. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Wei, X.; Bian, K.; Murad, F. Effects of nitric oxide on skin burn wound healing. J. Burn Care Res. 2008, 29, 804–814. [Google Scholar] [CrossRef]
- Zhu, H.; Ka, B.; Murad, F. Nitric oxide accelerates the recovery from burn wounds. World J. Surg. 2007, 31, 624–631. [Google Scholar] [CrossRef]
- Weller, R.; Finnen, M.J. The effects of topical treatment with acidified nitrite on wound healing in normal and diabetic mice. Nitric Oxide 2006, 15, 395–399. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, 180–210. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Boric, M.; Skopljanak, I.; Ferhatovic, L.; Kadic, A.J.; Banozic, A.; Puljak, L. Reduced epidermal thickness, nerve degeneration and increased pain-related behavior in rats with diabetes type 1 and 2. J. Chem. Neuroanat. 2013, 53, 33–40. [Google Scholar] [CrossRef]
- Okano, J.; Kojima, H.; Katagi, M.; Nakagawa, T.; Nakae, Y.; Terashima, T.; Kurakane, T.; Kubota, M.; Maegawa, H.; Udagawa, J. Hyperglycemia Induces Skin Barrier Dysfunctions with Impairment of Epidermal Integrity in Non-Wounded Skin of Type 1 Diabetic Mice. PLoS ONE 2016, 11, e0166215. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Ganopolsky, J.G.; Labbé, A.; Gilardino, M.; Wahl, C.; Martoni, C.; Prakash, S. Novel nitric oxide producing probiotic wound healing patch: Preparation and in vivo analysis in a New Zealand white rabbit model of ischaemic and infected wounds. Int. Wound J. 2012, 9, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G.; Sprugel, K.H.; Murray, M.J.; Ross, R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am. J. Pathol. 1990, 136, 1235–1246. [Google Scholar]
- Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Mannino, F.; Vaccaro, M.; Arcoraci, V.; Aliquò, F.; Minutoli, L.; Colonna, M.R.; et al. Activation of the EPOR-β common receptor complex by cibinetide ameliorates impaired wound healing in mice with genetic diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 632–639. [Google Scholar] [CrossRef]
- Tan, W.S.; Arulselvan, P.; Ng, S.-F.; Taib, C.N.M.; Sarian, M.N.; Fakurazi, S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement. Altern. Med. 2019, 19, 20. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Zakhaleva, J.; Ren, H.; Liu, J.; Chen, W.; Pan, Y. Noninvasive and high-resolution optical monitoring of healing of diabetic dermal excisional wounds implanted with biodegradable in situ gelable hydrogels. Tissue Eng. Part C Methods 2010, 16, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Samaha, R.; Othman, A.I.; El-Sherbiny, I.M.; Amer, M.A.; Elhusseini, F.; ElMissiry, M.A. Topical Nitric oxide in nanoformulation enhanced wound healing in experimental diabetes in mice. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 499–514. [Google Scholar]
- Cheng, K.Y.; Lin, Z.-H.; Cheng, Y.-P.; Chiu, H.-Y.; Yeh, N.-L.; Wu, T.-K.; Wu, J.-S. Wound Healing in Streptozotocin-Induced Diabetic Rats Using Atmospheric-Pressure Argon Plasma Jet. Sci. Rep. 2018, 8, 12–14. [Google Scholar]
- Kunkemoeller, B.; Kyriakides, T.R. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid. Redox Signal. 2017, 27, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, S.; Yao, F.; Zhang, Y.; Li, T. Increased ratio of serum matrix metalloproteinase-9 against TIMP-1 predicts poor wound healing in diabetic foot ulcers. J. Diabetes Complicat. 2013, 27, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.; Kiyama, T.; Barbul, A.A. Nitric oxide enhances experimental wound healing in diabetes. Br. J. Surg. 2002, 89, 1594–1601. [Google Scholar] [CrossRef] [Green Version]
- Wetzler, C.; Kämpfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: Prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Dubey, M.; Nagarkoti, S.; Awasthi, D.; Singh, A.K.; Chandra, T.; Kumaravelu, J.; Barthwal, M.K.; Dikshit, M. Nitric oxide-mediated apoptosis of neutrophils through caspase-8 and caspase-3-dependent mechanism. Cell Death Dis. 2016, 7, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Stallmeyer, B.; Kämpfer, H.; Kolb, N.; Pfeilschifer, J. Nitric oxide triggers enhanced induction of vascular endothelial growth factor expression in cultured keratinocytes (HaCaT) and during cutaneous wound repair. FASEB J. 1999, 13, 2002–2014. [Google Scholar] [CrossRef]
- Zandifar, A.; Seifabadi, S.; Zandifar, E.; Beheshti, S.S.; Aslani, A.; Javanmard, S.H. Comparison of the effect of topical versus systemic L-arginine on wound healing in acute incisional diabetic rat model. J. Res. Med. Sci. 2015, 20, 233–238. [Google Scholar]
- Chen, Y.J.; Wu, S.-C.; Wang, H.-C.; Wu, T.-H.; Yuan, S.-S.F.; Lu, T.-T.; Liaw, W.-F.; Wang, Y.-M. Activation of Angiogenesis and Wound Healing in Diabetic Mice Using NO-Delivery Dinitrosyl Iron Complexes. Mol. Pharm. 2019, 16, 4241–4251. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, M.; Khaksarhaddad, M.; Amirhasani, A.R. Compression of Efficacy of Topical Phenytoin, Honey and Combination of Honey with Triglyceride Tree Nitrate on Wound Healing in Rat. Indian J. Forensic Med. Toxicol. 2019, 13, 393–398. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L. Nitric oxide and angiogenesis. J. Neurooncol. 2000, 50, 139–148. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Westerweel, P.E.; Terra, M.; Rafii, S.; Jaspers, J.E.; White, I.A.; Hooper, A.T.; Doevendans, P.A.; Verhaar, M.C. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS ONE 2013, 8, e60357. [Google Scholar] [CrossRef] [Green Version]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G. Diabetic foot ulcers: Pathogenesis and management. Am. Fam. Physician 2002, 66, 1655–1662. [Google Scholar]
- Keswani, S.G.; Balaji, S.; Le, L.D.; Leung, A.; Parvadia, J.K.; Frischer, J.; Yamano, S.; Taichman, N.; Crombleholme, T.M. Role of salivary vascular endothelial growth factor (VEGF) in palatal mucosal wound healing. Wound Repair Regen. 2013, 21, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Zhan, R.; He, W.; Wang, F.; Yao, Z.; Tan, J.; Xu, R.; Zhou, J.; Wang, Y.; Li, H.; Luo, G. Nitric oxide promotes epidermal stem cell migration via cGMP-Rho GTPase signalling. Sci. Rep. 2016, 6, 30687. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Wang, W.; Zhou, S.; Zhang, G.; He, J.; Li, Q. A Novel Model for Cutaneous Wound Healing and Scarring in the Rat. Plast. Reconstr. Surg. 2019, 143, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Dorsett-Martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norouzirad, R.; Gholami, H.; Ghanbari, M.; Hedayati, M.; González-Muniesta, P.; Jeddi, S.; Ghasemi, A. Dietary inorganic nitrate attenuates hyperoxia-induced oxidative stress in obese type 2 diabetic male rats. Life Sci. 2019, 230, 188–196. [Google Scholar] [CrossRef]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honnegowda, T.M.; Kumar, P.; Udupa, P.; Rao, P.; Bhandary, S.; Mahato, K.K.; Sharan, A.; Mayya, S.S. Effect of limited access dressing on hydroxyproline and enzymatic antioxidant status in nonhealing chronic ulcers. Indian J. Plast. Surg. 2014, 47, 216–220. [Google Scholar] [CrossRef]
- Mendes, J.J.; Leandro, C.I.; Bonaparte, D.P.; Pinto, A.L. A rat model of diabetic wound infection for the evaluation of topical antimicrobial therapies. Comp. Med. 2012, 62, 37–48. [Google Scholar]
- Amini, A.; Pouriran, R.; Abdollahifar, M.-A.; Abbaszadeh, H.A.; Ghoreishi, S.K.; Chien, S.; Bayat, M. Stereological and molecular studies on the combined effects of photobiomodulation and human bone marrow mesenchymal stem cell conditioned medium on wound healing in diabetic rats. J. Photochem. Photobiol. B 2018, 182, 42–51. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Gholipourmalekabadi, M.; Salimi, M.; Abdollahifar, M.-A.; Bagheri, M.; Samadikuchaksaraei, A.; Ghanbarian, H.; Mozafari, M.; Kazemi, B.; Niknejad, H. The in vivo effect of Lacto-N-neotetraose (LNnT) on the expression of type 2 immune response involved genes in the wound healing process. Sci. Rep. 2020, 10, 997. [Google Scholar] [CrossRef]
- Firouzi, A.; Norozian, M.; Amini, A.; Abdollahifar, M.-A.; Abbaszadeh, H.-A.; Fathabadi, F.F. Combined Effect of Low-Level Laser Treatment and Levothyroxine on Wound Healing in Rats With Hypothyroidism. J. Lasers Med. Sci. 2018, 9, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar]
- Rodgers, K.E.; Roda, N.; Felix, J.C.; Espinoza, T.; Maldonado, S.; DiZerega, G. Histological evaluation of the effects of angiotensin peptides on wound repair in diabetic mice. Exp. Dermatol. 2003, 12, 784–790. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzali, H.; Khaksari, M.; Jeddi, S.; Kashfi, K.; Abdollahifar, M.-A.; Ghasemi, A. Acidified Nitrite Accelerates Wound Healing in Type 2 Diabetic Male Rats: A Histological and Stereological Evaluation. Molecules 2021, 26, 1872. https://doi.org/10.3390/molecules26071872
Afzali H, Khaksari M, Jeddi S, Kashfi K, Abdollahifar M-A, Ghasemi A. Acidified Nitrite Accelerates Wound Healing in Type 2 Diabetic Male Rats: A Histological and Stereological Evaluation. Molecules. 2021; 26(7):1872. https://doi.org/10.3390/molecules26071872
Chicago/Turabian StyleAfzali, Hamideh, Mohammad Khaksari, Sajad Jeddi, Khosrow Kashfi, Mohammad-Amin Abdollahifar, and Asghar Ghasemi. 2021. "Acidified Nitrite Accelerates Wound Healing in Type 2 Diabetic Male Rats: A Histological and Stereological Evaluation" Molecules 26, no. 7: 1872. https://doi.org/10.3390/molecules26071872
APA StyleAfzali, H., Khaksari, M., Jeddi, S., Kashfi, K., Abdollahifar, M. -A., & Ghasemi, A. (2021). Acidified Nitrite Accelerates Wound Healing in Type 2 Diabetic Male Rats: A Histological and Stereological Evaluation. Molecules, 26(7), 1872. https://doi.org/10.3390/molecules26071872