The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD)
Abstract
:1. Introduction
2. The Pharmacological Effects of Clopidogrel
3. Definition of Clopidogrel Resistance
4. Factors Associated with CR
4.1. Gene Polymorphism
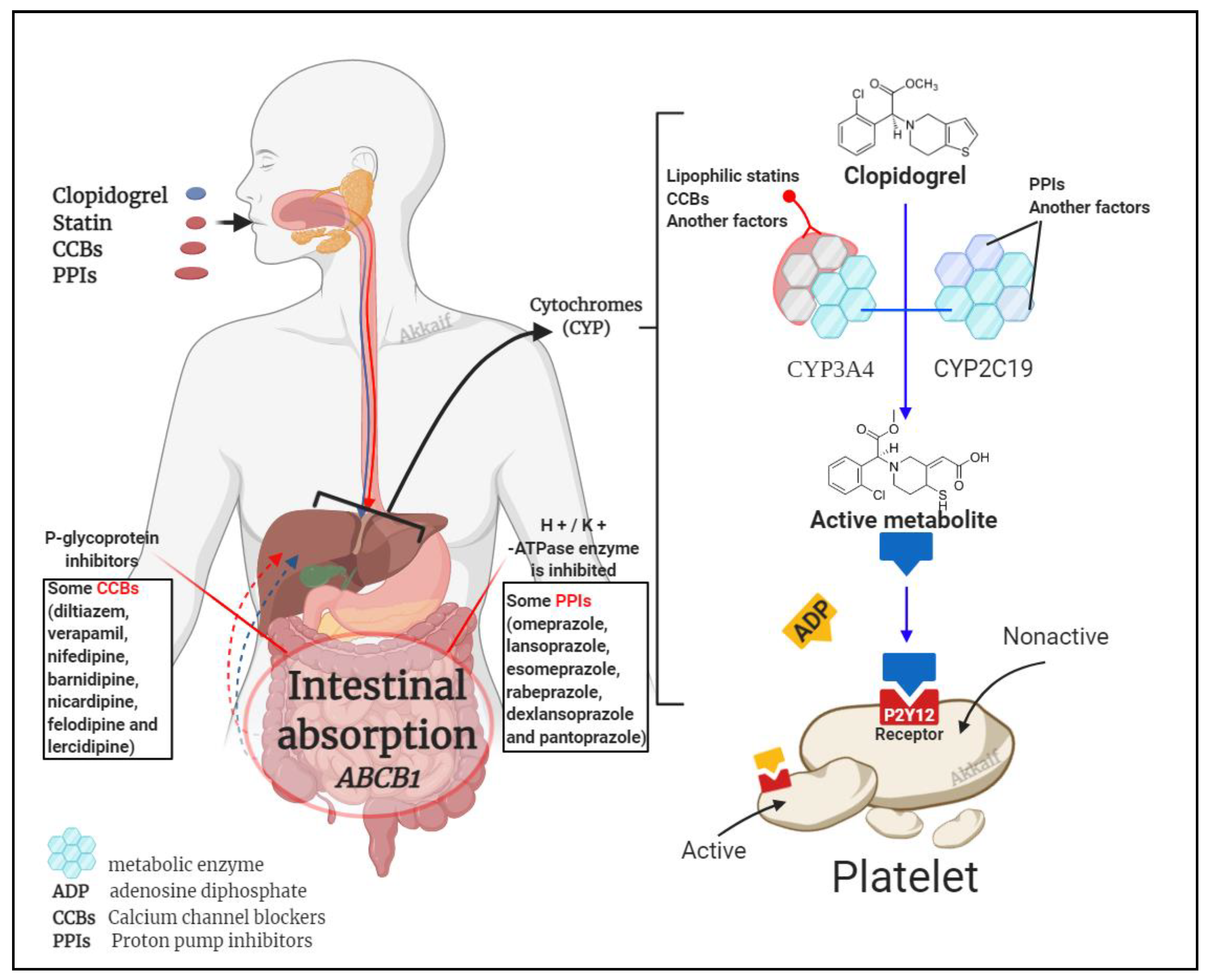
4.2. Drug Interactions
4.2.1. Clopidogrel Interaction with Statins
4.2.2. Calcium Channel Blockers
4.2.3. Proton Pump Inhibitors (PPIs)
4.3. Dose Factors
4.4. Other Factors
5. Strategies to Overcome CR
5.1. Increase the Dose of Clopidogrel
5.2. Combined Use of Other Antiplatelet Drugs
5.3. Replacement of New P2Y12 Receptor Antagonists
5.4. Other Management of CR
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasan, M.S.; Basri, H.B.; Hin, L.P.; Stanslas, J. Genetic polymorphisms and drug interactions leading to clopidogrel resistance: Why the Asian population requires special attention. Int. J. Neurosci. 2013, 123, 143–154. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. J. Cardio-Thora. Surg. 2018, 53, 34–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-L. De-escalation of anti-platelet therapy in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A narrative review. Chin. Med. J. 2019, 132, 197–210. [Google Scholar] [CrossRef]
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Shen, L.; Hockett, R.D.; Brandt, J.T.; Walker, J.R.; Antman, E.M.; Macias, W.L.; Braunwald, E. CLINICAL PERSPECTIVE. Circulation 2009, 119, 2553–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.-G.; Zou, J.-J.; Hu, Z.-Y.; Zhang, J.-J.; Ye, F.; Chen, S.-L. Individual variability in the disposition of and response to clopidogrel: Pharmacogenomics and beyond. Pharmacol. Ther. 2011, 129, 267–289. [Google Scholar] [CrossRef]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [PubMed] [Green Version]
- Chen, Z.; Jiang, L.; Chen, Y. COMMIT (ClOpidogrel and Metoprolol in Myorardial Infarction Trial). Dobavleniye klopidogrelya k aspirinu u 45,852 patsiyentov s ostrym infarktom miokarda: Randomizirovannoyep latsebo-kontroliruyemoyeissledovaniye. Lancet 2005, 366, 1607–1621. [Google Scholar]
- Michelson, A.D.; Bhatt, D.L. How I use laboratory monitoring of antiplatelet therapy. Blood J. Am. Soc. Hematol. 2017, 130, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Gorog, D.A.; Fuster, V. Platelet function tests in clinical cardiology: Unfulfilled expectations. J. Am. Coll. Cardiol. 2013, 61, 2115–2129. [Google Scholar] [CrossRef] [Green Version]
- Bonello, L.; Tantry, U.S.; Marcucci, R.; Blindt, R.; Angiolillo, D.J.; Becker, R.; Bhatt, D.L.; Cattaneo, M.; Collet, J.P.; Cuisset, T. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010, 56, 919–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnasser, S.; Huang, W.; Gore, J.; Steg, P.; Eagle, K.; Anderson, F.; Fox, K.; Gurfinkel, E.; Brieger, D.; Klein, W.; et al. Late Consequences of Acute Coronary Syndromes: Global Registry of Acute Coronary Events (GRACE) Follow-up. Am. J. Med. 2015, 128, 766–775. [Google Scholar] [CrossRef]
- Patti, G.; Micieli, G.; Cimminiello, C.; Bolognese, L. The Role of Clopidogrel in 2020: A Reappraisal. Cardiovasc. Ther. 2020, 8703627. [Google Scholar] [CrossRef] [Green Version]
- Gori, A.M.; Marcucci, R.; Migliorini, A.; Valenti, R.; Moschi, G.; Paniccia, R.; Buonamici, P.; Gensini, G.F.; Vergara, R.; Abbate, R. Incidence and clinical impact of dual nonresponsiveness to aspirin and clopidogrel in patients with drug-eluting stents. J. Am. Coll. Cardiol. 2008, 52, 734–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraf, S.; Christopoulos, C.; Salha, I.B.; Stott, D.J.; Gorog, D.A. Impaired endogenous thrombolysis in acute coronary syndrome patients predicts cardiovascular death and nonfatal myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 2107–2115. [Google Scholar] [CrossRef]
- Matetzky, S.; Shenkman, B.; Guetta, V.; Shechter, M.; Beinart, R.; Goldenberg, I.; Novikov, I.; Pres, H.; Savion, N.; Varon, D. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004, 109, 3171–3175. [Google Scholar] [CrossRef]
- Müller, I.; Besta, F.; Schulz, C.; Massberg, S.; Schönig, A.; Gawaz, M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. J. Thromb. Haemost. 2003, 89, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Chen, G.-Z.; Zhang, Y.-H.; Zhang, L.; Huang, L.-A. Clinical outcomes and predictive model of platelet reactivity to clopidogrel after acute ischemic vascular events. Chin. Med. J. 2019, 132, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Pareed, S.A.; Vijayaraghavan, G.; Kartha, C.; Manoj, M. Antiplatelet drug resistance in Indians. Ann. Clin. Cardiol. 2020, 2, 36. [Google Scholar]
- Namazi, S.; Kojuri, J.; Khalili, A.; Azarpira, N. The impact of genetic polymorphisms of P2Y12, CYP3A5 and CYP2C19 on clopidogrel response variability in Iranian patients. Biochem. Pharmacol. 2012, 83, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Sahib, H.A.; Mohammad, B.I.; Abdul-Majid, B.A. Therapeutic Effectiveness of Clopidogrel-Induced Platelets Inhibition: An Inter-Individual Response Variability among Iraqi Patients. World Heart J. 2016, 8, 23–28. [Google Scholar]
- Park, K.-J.; Chung, H.-S.; Kim, S.-R.; Kim, H.-J.; Han, J.-Y.; Lee, S.-Y. Clinical, pharmacokinetic, and pharmacogenetic determinants of clopidogrel resistance in Korean patients with acute coronary syndrome. Korean J. Lab. Med. 2011, 31, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M.; Chin, L.S.; Noor, D.A.M.; Mostafa, H.; Kader, M.A.S.A.; Hay, Y.K.; Ibrahim, B. The effect of CYP2C19 genetic polymorphism and non-genetic factors on clopidogrel platelets inhibition in East Asian coronary artery disease patients. Thromb. Res. 2017, 158, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Sakr, H.I.; Alamri, H.S.; Almoghairi, A.M.; Alkhudair, A.A.; AlMasood, A.S. Prevalence and risk factors of clopidogrel non-response among Saudi patients undergoing coronary angiography. Saudi Med. J. 2016, 37, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Tekkeşin, A.İ.; Kaya, A.; Çakıllı, Y.; Türkkan, C.; Hayıroğlu, M.İ.; Borklu, E.B.; Kalenderoğlu, K.; Gümüşdağ, A.; Yıldırımtürk, Ö.; Bozbeyoğlu, E. The first six-month clinical outcomes and risk factors associated with high on-treatment platelet reactivity of clopidogrel in patients undergoing coronary interventions. Anatol. J. Cardiol. 2016, 16, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Bhatt, D.L.; Topol, E.J. Aspirin and clopidogrel resistance: An emerging clinical entity. Eur. Heart J. 2006, 27, 647–654. [Google Scholar] [CrossRef]
- Lock, E.; Saveliev, A.; Kennedy, L. Methanol and dimethyl sulfide removal by pulsed corona part I: Experiment. Plasma Chem. Plasma Process. 2006, 26, 527–542. [Google Scholar] [CrossRef]
- Bates, E.R.; Lau, W.C.; Angiolillo, D.J. Clopidogrel–drug interactions. J. Am. Coll. Cardiol. 2011, 57, 1251–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, L.A.; Stouffer, G.A.; Polasek, M.; Rossi, J.S. Review of clopidogrel dose escalation in the current era of potent P2Y12 inhibitors. Expert Rev. Clin. Pharmacol. 2015, 8, 411–421. [Google Scholar] [CrossRef]
- Ding, Z.; Kim, S.; Dorsam, R.T.; Jin, J.; Kunapuli, S.P. Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood J. Am. Soc. Hematol. 2003, 101, 3908–3914. [Google Scholar] [CrossRef] [Green Version]
- Patrono, C.; Coller, B.; Dalen, J.E.; Gerald, G.A.F.; Fuster, V.; Gent, M.; Hirsh, J.; Roth, G. Platelet-active drugs: The relationships among dose, effectiveness, and side effects. Chest 2001, 119, 39S–63S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.-L.; Samant, S.; Lesko, L.J.; Schmidt, S. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin. Pharmacokinet. 2015, 54, 147–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuisset, T.; Morange, P.-E.; Alessi, M.-C. Recent advances in the pharmacogenetics of clopidogrel. Human genetics 2012, 131, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Tantry, U.S.; Hennekens, C.H.; Zehnder, J.L.; Gurbel, P.A. Clopidogrel Resistance and Clopidogrel Treatment Failure; Leung, L.L.K., Cutlip, D., Eds.; UpToDate Inc.: Waltham, MA, USA, 2018. [Google Scholar]
- Cay, S.; Cagirci, G.; Aydogdu, S.; Balbay, Y.; Sen, N.; Maden, O.; Demir, A.D.; Erbay, A.R. Safety of clopidogrel in older patients. Drugs Aging 2011, 28, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Carlquist, J.F.; Knight, S.; Horne, B.D.; Huntinghouse, J.A.; Rollo, J.S.; Muhlestein, J.B.; May, H.; Anderson, J.L. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. J. Thromb. Haemost. 2013, 109, 744–754. [Google Scholar]
- Park, J.J.; Park, K.W.; Kang, J.; Jeon, K.-H.; Kang, S.-H.; Ahn, H.S.; Han, J.-K.; Koh, J.-S.; Lee, S.E.; Yang, H.-M. Genetic determinants of clopidogrel responsiveness in Koreans treated with drug-eluting stents. Int. J. Cardiol. 2013, 163, 79–86. [Google Scholar] [CrossRef]
- Fontana, P.; Roffi, M.; Reny, J.-L. Platelet function test use for patients with coronary artery disease in the early 2020s. Med. Clin. Med. 2020, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.S.; Anderson, J.L.; Adams, C.D.; Bridges, C.R.; Casey, D.E.; Ettinger, S.M.; Fesmire, F.M.; Ganiats, T.G.; Jneid, H.; Lincoff, A.M. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2011, 57, e215–e367. [Google Scholar]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 2016, 134, e123–e155. [Google Scholar]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J. 2018 ESC/EACTS Guidelines on myocardial revascularisation. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Lin, J.; Li, H.; Johnston, S.C.; Lin, Y.; Pan, Y.; Liu, L.; Wang, D.; Wang, C. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. Jama 2016, 316, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarno, G.; Garg, S.; Onuma, Y.; Buszman, P.; Linke, A.; Ischinger, T.; Klauss, V.; Eberli, F.; Corti, R.; Wijns, W. The impact of body mass index on the one year outcomes of patients treated by percutaneous coronary intervention with Biolimus-and Sirolimus-eluting stents (from the LEADERS Trial). Am. J. Cardiol. 2010, 105, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Weisz, G.; Smilowitz, N.R.; Kirtane, A.J.; Rinaldi, M.J.; Parvataneni, R.; Xu, K.; Stuckey, T.D.; Maehara, A.; Witzenbichler, B.; Neumann, F.-J. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: The ADAPT-DES study. Circ. Cardiovasc. Interv. 2015, 8, e001952. [Google Scholar] [CrossRef] [PubMed]
- Mizobe, M.; Hokimoto, S.; Akasaka, T.; Arima, Y.; Kaikita, K.; Morita, K.; Miyazaki, H.; Oniki, K.; Nakagawa, K.; Ogawa, H. Impact of CYP2C19 polymorphism on clinical outcome following coronary stenting is more important in non-diabetic than diabetic patients. Thromb. Res. 2014, 134, 72–77. [Google Scholar] [CrossRef]
- Elkind, M.S.; Luna, J.M.; McClure, L.A.; Zhang, Y.; Coffey, C.S.; Roldan, A.; Del Brutto, O.H.; Pretell, E.J.; Pettigrew, L.C.; Meyer, B.C. C-reactive protein as a prognostic marker after lacunar stroke: Levels of inflammatory markers in the treatment of stroke study. Stroke 2014, 45, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.; Schutt, R.C.; Hannawi, B.; DeLao, T.; Barker, C.M.; Kleiman, N.S. Association of immature platelets with adverse cardiovascular outcomes. J. Am. Coll. Cardiol. 2014, 64, 2122–2129. [Google Scholar] [CrossRef] [Green Version]
- Chirumamilla, A.P.; Maehara, A.; Mintz, G.S.; Mehran, R.; Kanwal, S.; Weisz, G.; Hassanin, A.; Hakim, D.; Guo, N.; Baber, U. High platelet reactivity on clopidogrel therapy correlates with increased coronary atherosclerosis and calcification: A volumetric intravascular ultrasound study. JACC: Cardiovasc. Imaging 2012, 5, 540–549. [Google Scholar]
- Rinfret, S.; Rodés-Cabau, J.; Bagur, R.; Déry, J.-P.; Dorais, M.; Larose, É.; Barbeau, G.; Gleeton, O.; Nguyen, C.-M.; Noël, B. Telephone contact to improve adherence to dual antiplatelet therapy after drug-eluting stent implantation. Heart 2013, 99, 562–569. [Google Scholar] [CrossRef]
- Zoheir, N.; Abd Elhamid, S.; Abulata, N.; Sobky, M.E.; Khafagy, D.; Mostafa, A. P2Y12 receptor gene polymorphism and antiplatelet effect of clopidogrel in patients with coronary artery disease after coronary stenting. Blood Coagul. Fibrinolysis 2013, 24, 525–531. [Google Scholar] [CrossRef]
- Xie, C.; Ding, X.; Gao, J.; Wang, H.; Hang, Y.; Zhang, H.; Zhang, J.; Jiang, B.; Miao, L. The effects of CES1A2 A (− 816) C and CYP2C19 loss-of-function polymorphisms on clopidogrel response variability among Chinese patients with coronary heart disease. Pharmacogenetics Genom. 2014, 24, 204–210. [Google Scholar] [CrossRef]
- Tang, X.-F.; Wang, J.; Zhang, J.-H.; Meng, X.-M.; Xu, B.; Qiao, S.-B.; Wu, Y.-J.; Chen, J.; Wu, Y.; Chen, J.-L. Effect of the CYP2C19* 2 and* 3 genotypes, ABCB1 C3435T and PON1 Q192R alleles on the pharmacodynamics and adverse clinical events of clopidogrel in Chinese people after percutaneous coronary intervention. Eur. J. Clin. Pharmacol. 2013, 69, 1103–1112. [Google Scholar] [CrossRef]
- Zhuo, Z.-L.; Xian, H.-P.; Long, Y.; Liu, C.; Sun, Y.-Y.; Ma, Y.-T.; Gao, H.; Zhao, J.-Z.; Zhao, X.-T. Association between CYP2C19 and ABCB1 polymorphisms and clopidogrel resistance in clopidogrel-treated Chinese patients. Anatol. J. Cardiol. 2018, 19, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.C.; Gurbel, P.A.; Watkins, P.B.; Neer, C.J.; Hopp, A.S.; Carville, D.G.; Guyer, K.E.; Tait, A.R.; Bates, E.R. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 2004, 109, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaev, K.; Samsonova, K.; Potapov, P.; Andreev, D.; Grishina, E.; Ryzhikova, K.; Sychev, D. Genotyping and phenotyping CYP3A4\CYP3A5: No association with antiplatelet effect of clopidogrel. Mjol. Biol. Resp. 2019, 46, 4195–4199. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.T.; Close, S.; Iturria, S.; Payne, C.; Farid, N.; Ernest, C.; Lachno, D.; Salazar, D.; Winters, K. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 2007, 5, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Kazui, M.; Nishiya, Y.; Ishizuka, T.; Hagihara, K.; Farid, N.A.; Okazaki, O.; Ikeda, T.; Kurihara, A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 2010, 38, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Kuliczkowski, W.; Witkowski, A.; Polonski, L.; Watala, C.; Filipiak, K.; Budaj, A.; Golanski, J.; Sitkiewicz, D.; Pregowski, J.; Gorski, J. Interindividual variability in the response to oral antiplatelet drugs: A position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur. Heart J. 2009, 30, 426–435. [Google Scholar]
- Simon, T.; Verstuyft, C.; Mary-Krause, M.; Quteineh, L.; Drouet, E.; Méneveau, N.; Steg, P.G.; Ferrières, J.; Danchin, N.; Becquemont, L. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 2009, 360, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.Y. Clopidogrel pharmacogenetics of east, south and other Asian populations. Eur. Heart J. Suppl. 2012, 14, A41–A42. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.-A.; Pereira, N. Pharmacogenomic impact of CYP2C19 variation on clopidogrel therapy in precision cardiovascular medicine. J. Pers. Med. 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Dong, W.; Yang, D.; Sun, L.; He, X.; Hu, H.; Zhang, J.; Wang, C.; Li, Y.; Zhao, M. Body weight, CYP2C19, and P2Y12 receptor polymorphisms relate to clopidogrel resistance in a cohort of Chinese ischemic stroke patients with aspirin intolerance. Eur. J. Clin. Pharmacol. 2020, 76, 1517–1527. [Google Scholar] [CrossRef]
- Al-Azzam, S.I.; Alzoubi, K.H.; Khabour, O.F.; Nusair, M.B.; Al-Hadidi, H.; Awidi, A.; Saleh, A. Factors that contribute to clopidogrel resistance in cardiovascular disease patients: Environmental and genetic approach. Int. J. Clin. Pharmacol. Ther. 2013, 51, 179–186. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, S.; Shin, D.-J.; Choi, D.; Shim, C.Y.; Ko, Y.-G.; Kim, J.-S.; Shin, E.-S.; Chang, C.W.; Lee, J.-E. Relation of genetic polymorphisms in the cytochrome P450 gene with clopidogrel resistance after drug-eluting stent implantation in Koreans. Am. J. Cardiol. 2009, 104, 46–51. [Google Scholar] [CrossRef]
- Alhazzani, A.A.; Munisamy, M.; Karunakaran, G. Pharmacogenetics of CYP2C19 genetic polymorphism on clopidogrel response in patients with ischemic stroke from Saudi Arabia. Neurosciences 2017, 22, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lai, X.; Li, W.; Jing, Z.; Xiong, Z.; Xu, A.; Ruan, Y. VASP phosphorylation and genetic polymorphism for clopidogrel resistance in Chinese patients with non-cardioembolic ischemic stroke. Thromb. Res. 2014, 134, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Al-Husein, B.A.; Al-Azzam, S.I.; Alzoubi, K.H.; Khabour, O.F.; Nusair, M.B.; Alzayadeen, S. Investigating the effect of demographics, clinical characteristics, and polymorphism of MDR-1, CYP1A2, CYP3A4, and CYP3A5 on clopidogrel resistance. J. Cardiovasc. Pharmacol. 2018, 72, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Zhang, A.; Zhang, X.; You, T.; Xie, D.; Yang, W.; Chen, Y.; Zhang, X.; Di, C. A SNP involved in alternative splicing of ABCB1 is associated with clopidogrel resistance in coronary heart disease in Chinese population. Aging (Albany NY) 2020, 12, 25684–25699. [Google Scholar] [PubMed]
- Chen, F.; Zhang, J.; Bian, C.-X.; Zhang, J.; Xin, X.-B.; Pan, Y.-Y.; Zhang, X. A Study on the Correlation Between MDR1 Polymorphism and Clopidogrel Resistance in Hui Patients Treated with Percutaneous Coronary Intervention. Int. J. Gen. Med. 2021, 14, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Malagoli Tagliazucchi, G.; Crocamo, A. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: The PHARMCLO trial. J. Am. Coll. Cardiol. 2018, 71, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; You, J.H. CYP2C19 LOF and GOF-guided antiplatelet therapy in patients with acute coronary syndrome: A cost-effectiveness analysis. Cardiovasc. Drug Ther. 2017, 31, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Máchal, J.; Hlinomaz, O. Efficacy of P2Y12 receptor blockers after myocardial infarction and genetic variability of their metabolic pathways. Curr. Vasc. Pharmacol. 2019, 17, 35–40. [Google Scholar] [CrossRef]
- Martin, J.; Williams, A.K.; Klein, M.D.; Sriramoju, V.B.; Madan, S.; Rossi, J.S.; Clarke, M.; Cicci, J.D.; Cavallari, L.H.; Weck, K.E. Frequency and clinical outcomes of CYP2C19 genotype-guided escalation and de-escalation of antiplatelet therapy in a real-world clinical setting. Genet. Med. 2020, 22, 160–169. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Wang, C.-Y.; Wen, M.-S.; Lee, T.-H.; Chu, Y.; Hsieh, M.-J.; Chang, S.-H.; Lee, C.-H.; Wang, J.-L.; Chen, C.-C. Paraoxonase-1 is not a major determinant of stent thrombosis in a Taiwanese population. PLoS ONE 2012, 7, e39178. [Google Scholar] [CrossRef]
- Scott, S.; Sangkuhl, K.; Stein, C.; Hulot, J.S.; Mega, J.; Roden, D.; Klein, T.; Sabatine, M.; Johnson, J.; Shuldiner, A. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 2013, 94, 317–323. [Google Scholar] [CrossRef]
- Desta, Z.; Zhao, X.; Shin, J.-G.; Flockhart, D.A. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin. Pharmacokinet. 2002, 41, 913–958. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Jeong, Y.-H.; Kim, I.-S.; Koh, J.-S.; Kang, M.-K.; Park, Y.; Kwak, C.H.; Hwang, J.-Y. The cytochrome 2C19* 2 and* 3 alleles attenuate response to clopidogrel similarly in East Asian patients undergoing elective percutaneous coronary intervention. Thromb. Res. 2011, 127, 23–28. [Google Scholar] [CrossRef]
- Sim, S.C.; Risinger, C.; Dahl, M.L.; Aklillu, E.; Christensen, M.; Bertilsson, L.; Ingelman-Sundberg, M. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin. Pharmacol. Ther. 2006, 79, 103–113. [Google Scholar] [CrossRef]
- Sugimoto, K.; Uno, T.; Yamazaki, H.; Tateishi, T. Limited frequency of the CYP2C19* 17 allele and its minor role in a Japanese population. Br. J. Clin. Pharmacol. 2008, 65, 437–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Z.; Hou, J.; Li, B.; Zhang, Q.; Liu, S.; Li, C.; Liu, Z.; Yang, M.; Zhong, W.; Zhao, P. Analysis of CYP2C19 genetic polymorphism in a large ethnic Hakka population in southern China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 6186–6192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Zhao, T.; Bao, S.; Jia, L.; Feng, J.; Yu, A.; Sun, L.; Guo, X.; Li, H.; Yu, L. CYP2C19, PON1, and ABCB1 gene polymorphisms in Han and Uygur populations with coronary artery disease in Northwestern Xinjiang, China, From 2014 Through 2019. Medicine 2020, 99, e20582. [Google Scholar] [CrossRef] [PubMed]
- Anichavezhi, D.; Chakradhara Rao, U.; Shewade, D.; Krishnamoorthy, R.; Adithan, C. Distribution of CYP2C19* 17 allele and genotypes in an Indian population. J. Clin. Pharmacol. Ther. 2012, 37, 313–318. [Google Scholar] [CrossRef]
- Dehbozorgi, M.; Kamalidehghan, B.; Hosseini, I.; Dehghanfard, Z.; Sangtarash, M.H.; Firoozi, M.; Ahmadipour, F.; Meng, G.Y.; Houshmand, M. Prevalence of the CYP2C19* 2 (681 G> A),* 3 (636 G> A) and* 17 (-806 C> T) alleles among an Iranian population of different ethnicities. Mjol. Med. Rep. 2018, 17, 4195–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahib, H.A.; Mohammed, B.I.; Abdul-Majid, B.A. Genetic Polymorphism of CYP2C19 in A sample of Iraqi Population. Int. J. Pharm. Biol. Sci. 2015, 5, 54–60. [Google Scholar]
- Sviri, S.; Shpizen, S.; Leitersdorf, E.; Levy, M.; Caraco, Y. Phenotypic-genotypic analysis of CYP2C19 in the Jewish Israeli population. Clin. Pharmacol. Ther. 1999, 65, 275–282. [Google Scholar] [CrossRef]
- Rjoub, M.; Saleh, A.; Hakooz, N.; Imraish, A.; Jarrar, Y.; Zihlif, M. Allelic frequency of PON1 Q192R, CYP2C19* 2 and CYP2C19* 17 among Jordanian patients taking clopidogrel. Trop. J. Pharm. Res. 2018, 17, 2275–2280. [Google Scholar] [CrossRef]
- Kim, K.A.; Song, W.K.; Kim, K.R.; Park, J.Y. Assessment of CYP2C19 genetic polymorphisms in a Korean population using a simultaneous multiplex pyrosequencing method to simultaneously detect the CYP2C19* 2, CYP2C19* 3, and CYP2C19* 17 alleles. J. Clin. Pharm. Ther. 2010, 35, 697–703. [Google Scholar] [CrossRef]
- Amin Mostafa, A.M.; Chin, L.S.; Mohamed Noor, D.A.; Amin Mostafa, H.M.; Ali SK Abdul Kader, M.; Hay, Y.K.; Ibrahim, B. TCT-841 Integrative Pharmacometabonomics-Pharmacogenetics approach to predict clopidogrel response in Coronary Artery Disease (CAD) patients undergoing Interventional Angiographic Procedure (IAP). J. Am. Coll. Cardiol. 2017, 70, B340. [Google Scholar] [CrossRef]
- Riaz, S.; Din, S.M.; Tareen, M.U.; Tariq, F.; Latif, Y.; Siddiqi, S.; Sultan, A.; Mansoor, A. Genetic Polymorphism of CYP2C19 in Pakistani Population. Iran. J. Pharm. Res. IJPR 2019, 18, 1097–1102. [Google Scholar] [PubMed]
- Ayesh, B.M.; Al-Astal, I.R.; Yassin, M.M. The clinical effects of CYP2C19* 2 allele frequency on Palestinian patients receiving clopidogrel after percutaneous coronary intervention. Int. J. Clin. Pharm. 2019, 41, 96–103. [Google Scholar] [CrossRef]
- Elewa, H.; Ali, Z.O.; Bader, L. CYP2C19 Genetic Polymorphism Prevalence in Qataris. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings, Doha, Qatar, 19–20 March 2018. Issue 2; HBPD660. [Google Scholar]
- Mirzaev, K.B.; Zelenskaya, E.M.; Barbarash, O.L.; Ganyukov, V.I.; Apartsin, K.A.; Saraeva, N.O.; Nikolaev, K.Y.; Ryzhikova, K.A.; Lifshits, G.I.; Sychev, D.A. CYP2C19 polymorphism frequency in Russian patients in Central Russia and Siberia with acute coronary syndrome. Pharm. Pers. Med. 2017, 10, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Al-Jenoobi, F.I.; Alkharfy, K.M.; Alghamdi, A.M.; Bagulb, K.M.; Al-Mohizea, A.M.; Al-Muhsen, S.; Halwani, R.; Parvez, M.K.; Al-Dosari, M.S. CYP2C19 genetic polymorphism in Saudi Arabians. Basic Clin. Pharmacol. Toxicol. 2013, 112, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Sukasem, C.; Tunthong, R.; Chamnanphon, M.; Santon, S.; Jantararoungtong, T.; Koomdee, N.; Prommas, S.; Puangpetch, A.; Vathesatogkit, P. CYP2C19 polymorphisms in the Thai population and the clinical response to clopidogrel in patients with atherothrombotic-risk factors. Pharmacogenomics Pers. Med. 2013, 6, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Arici, M.; Özhan, G. CYP2C9, CYPC19 and CYP2D6 gene profiles and gene susceptibility to drug response and toxicity in Turkish population. Saudi Pharm. J. 2017, 25, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.P.; Nguyen, H.T.T.; Tran, N.T.B.; Nguyen, T.D.; Huynh, H.T.T.; Nguyen, X.T.; Nguyen, D.T.; Nong, H.V.; Nguyen, H.H. CYP2C19 genetic polymorphism in the Vietnamese population. Ann. Hum. Biol. 2019, 46, 491–497. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Chen, M.; Zhu, L.-L.; Yu, L.-S.; Zeng, S.; Xiang, M.-X.; Zhou, Q. Pharmacokinetic drug interactions with clopidogrel: Updated review and risk management in combination therapy. Ther. Clin. Risk Manag. 2015, 11, 449–467. [Google Scholar]
- El Rouby, N.; Lima, J.J.; Johnson, J.A. Proton pump inhibitors: From CYP2C19 pharmacogenetics to precision medicine. Expert Opin. Drug Metab. Toxicol. 2018, 14, 447–460. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Tantry, U.S.; Jeong, Y.-H.; Gurbel, P.A. The Clopidogrel-Statin Interaction–Reopening Pandora’s Box–. Circulation 2014, 78, 592–594. [Google Scholar] [CrossRef] [Green Version]
- Mansour, V.; Murdico, A.T.; Fudin, J. Do Statins Interfere With Clopidogrel During Platelet Therapy? Pharm. Times 2019, 85. [Google Scholar]
- Lau, W.C.; Carville, D.; Bates, E.R. Clinical significance of the atorvastatin-clopidogrel drug-drug interaction. Circulation 2004, 110, e66. [Google Scholar] [CrossRef] [Green Version]
- Brophy, J.M.; Babapulle, M.N.; Costa, V.; Rinfret, S. A pharmacoepidemiology study of the interaction between atorvastatin and clopidogrel after percutaneous coronary intervention. Am. Heart J. 2006, 152, 263–269. [Google Scholar] [CrossRef]
- Gulec, S.; Ozdol, C.; Rahimov, U.; Atmaca, Y.; Kumbasar, D.; Erol, C. Myonecrosis after elective percutaneous coronary intervention: Effect of clopidogrel-statin interaction. J. Of Invasive Cardiol. 2005, 17, 589–593. [Google Scholar]
- Albadr, Y.; Bohassan, A.K.; Ming, L.C.; Khan, T.M. An exploratory study investigating the potential drug–drug interactions in internal medicine department, Alahsa, Saudi Arabia. J. Pharm. Health Serv. Res. 2014, 5, 237–241. [Google Scholar] [CrossRef]
- Wenaweser, P.; Eshtehardi, P.; Abrecht, L.; Zwahlen, M.; Schmidlin, K.; Windecker, S.; Meier, B.; Haeberli, A.; Hess, O.M. A randomised determination of the Effect of Fluvastatin and Atorvastatin on top of dual antiplatelet treatment on platelet aggregation after implantation of coronary drug-eluting stents. J. Thromb. Haemost. 2010, 104, 554–562. [Google Scholar]
- Pelliccia, F.; Rosano, G.; Marazzi, G.; Vitale, C.; Spoletini, I.; Franzoni, F.; Speziale, G.; Polacco, M.; Greco, C.; Gaudio, C. Pharmacodynamic effects of atorvastatin versus rosuvastatin in coronary artery disease patients with normal platelet reactivity while on dual antiplatelet therapy—The PEARL randomised cross-over study. Eur. J. Pharmacol. 2014, 725, 18–22. [Google Scholar] [CrossRef]
- An, K.; Huang, R.; Tian, S.; Guo, D.; Wang, J.; Lin, H.; Wang, S. Statins significantly reduce mortality in patients receiving clopidogrel without affecting platelet activation and aggregation: A systematic review and meta-analysis. Lipids Health Dis. 2019, 18, 121. [Google Scholar] [CrossRef] [Green Version]
- Nafasi, L.; Rahmani, R.; Shafiee, A.; Salari, A.; Abdollahi, A.; Meysamie, A. Can a high reloading dose of atorvastatin prior to percutaneous coronary intervention reduce periprocedural myocardial infarction? Curr. Res. Med. Opin. 2014, 30, 381–386. [Google Scholar] [CrossRef]
- Karaźniewicz-Łada, M.; Rzeźniczak, J.; Główka, F.; Gumienna, A.; Dolatowski, F.; Słomczyński, M.; Burchardt, P. Influence of statin treatment on pharmacokinetics and pharmacodynamics of clopidogrel and its metabolites in patients after coronary angiography/angioplasty. Biomed. Pharmacother. 2019, 116, 108991. [Google Scholar] [CrossRef]
- Katoh, M.; Nakajima, M.; Shimada, N.; Yamazaki, H.; Yokoi, T. Inhibition of human cytochrome P450 enzymes by 1, 4-dihydropyridine calcium antagonists: Prediction of in vivo drug–drug interactions. Eur. J. Clin. Pharmacol. 2000, 55, 843–852. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Lang, I.; Christ, G.; Jilma, B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J. Am. Coll. Cardiol. 2008, 52, 1557–1563. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.-D.; Kim, Y.D.; Yoon, Y.-W.; Kim, J.-Y.; Lee, K.-Y. Antiplatelet effect of clopidogrel can be reduced by calcium-channel blockers. Yonsei Med. J. 2014, 55, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Loomba, R.S.; Arora, R.R. Effects of concurrent calcium channel blocker on antiplatelet efficacy of clopidogrel therapy: A systematic review. Am. J. Ther. 2016, 23, e29–e36. [Google Scholar] [CrossRef]
- Lee, C.H.; Franchi, F.; Angiolillo, D.J. Clopidogrel drug interactions: A review of the evidence and clinical implications. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Horn, J. Clinical pharmacology of proton pump inhibitors. Drugs 2003, 63, 2739–2754. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Teeluck, A.R.; Bhurtu, A.; Huang, W.-Q. Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: A systematic review and meta-analysis of recently published studies (2012–2016). BMC Cardiovasc. Disord. 2017, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrono, C.; Bachmann, F.; Baigent, C.; Bode, C.; De Caterina, R.; Charbonnier, B.; Fitzgerald, D.; Hirsh, J.; Husted, S.; Kvasnicka, J. Expert consensus document on the use of antiplatelet agents: The Task Force on the Use of Antiplatelet Agents in Patients With Atherosclerotic Cardiovascular Disease of the European Society of Cardiology. Eur. Heart J. 2004, 25, 166–181. [Google Scholar] [CrossRef] [Green Version]
- L’Allier, P.L.; Ducrocq, G.; Pranno, N.; Noble, S.; Ibrahim, R.; Grégoire, J.C.; Azzari, F.; Nozza, A.; Berry, C.; Doucet, S. Clopidogrel 600-mg double loading dose achieves stronger platelet inhibition than conventional regimens: Results from the PREPAIR randomized study. J. Am. Coll. Cardiol. 2008, 51, 1066–1072. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, A.; Ibanez, B.; Badimón, J.J. Clinical implications of clopidogrel resistance. J. Thromb. Haemost. 2008, 100, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Jakubowski, J.A.; Ferreiro, J.L.; Tello-Montoliu, A.; Rollini, F.; Franchi, F.; Ueno, M.; Darlington, A.; Desai, B.; Moser, B.A. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Capodanno, D.; Angiolillo, D.J. Antithrombotic Therapy for Atherosclerotic Cardiovascular Disease Risk Mitigation in Patients With Coronary Artery Disease and Diabetes Mellitus. Circulation 2020, 142, 2172–2188. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Cesari, F.; Marcucci, R.; Giusti, B.; Paniccia, R.; Antonucci, E.; Gensini, G.; Abbate, R. The balance between pro-and anti-inflammatory cytokines is associated with platelet aggregability in acute coronary syndrome patients. Atherosclerosis 2009, 202, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Zhou, Y.; Liu, X.; Nie, X.; Wang, Z.; Guo, Y.; Chen, W.; Yang, Q. Relationship between plasma inflammatory markers and platelet aggregation in patients with clopidogrel resistance after angioplasty. Angiology 2012, 63, 62–66. [Google Scholar] [CrossRef]
- Cirillo, P.; Taglialatela, V.; Pellegrino, G.; Morello, A.; Conte, S.; Di Serafino, L.; Cimmino, G. Effects of colchicine on platelet aggregation in patients on dual antiplatelet therapy with aspirin and clopidogrel. J. Thromb. Thrombolysis 2020, 50, 468–472. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Ryu, J.; Seo, H.; Kang, M.; Kim, E. Is a high maintenance dose of clopidogrel suitable for overcoming clopidogrel resistance in patients? Int. J. Clin. Pharm. 2015, 37, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Sideris, G.; Meuleman, C.; Bal-dit-Sollier, C.; Lellouche, N.; Steg, P.G.; Slama, M.; Milleron, O.; Collet, J.-P.; Henry, P. A randomised comparison of high clopidogrel loading doses in patients with non–ST-segment elevation acute coronary syndromes: The ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) trial. J. Am. Coll. Cardiol. 2006, 48, 931–938. [Google Scholar] [PubMed] [Green Version]
- Snoep, J.D.; Hovens, M.M.; Eikenboom, J.C.; van der Bom, J.G.; Jukema, J.W.; Huisman, M.V. Clopidogrel non-responsiveness in patients undergoing percutaneous coronary intervention with stenting: A systematic review and meta-analysis. Am. Heart J. 2007, 154, 221–231. [Google Scholar] [CrossRef]
- Powers, W.J.; Clarke, W.R.; Grubb, R.L.; Videen, T.O.; Adams, H.P.; Derdeyn, C.P.; COSS Investigators, F.T. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: The Carotid Occlusion Surgery Study randomised trial. Jama 2011, 306, 1983–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Wang, Z.; Shi, D.; Liu, Y.; Zhao, Y.; Han, H.; Li, Y.; Liu, W.; Zhang, L.; Yang, L. High clopidogrel dose in patients with chronic kidney disease having clopidogrel resistance after percutaneous coronary intervention. Angiology 2015, 66, 319–325. [Google Scholar] [CrossRef]
- Stellbaum, C.; Ayral, Y.; Morguet, A.; Schultheiss, H.-P.; Rauch, U. Doubling the clopidogrel dose in patients with reduced responsiveness to the standard dose is associated with a limited effectiveness as evaluated by impedance aggregometry. Cardiovasc. Revascularization Med. 2012, 13, 159–166. [Google Scholar] [CrossRef]
- Aĭnetdinova, D.; Udovichenko, A.; Sulimov, V. Resistance to antiplatelet drugs in patients with non ST elevation acute coronary syndrome. Kardiologiia 2008, 48, 35–39. [Google Scholar] [PubMed]
- Fong, A.Y.Y.; Ling, H.S. Dual Antiplatelet and Glycoprotein Inhibitors in Emergency PCI. In Primary Angioplasty: A Practical Guide; Watson, T., Ong, P., Tcheng, J., Eds.; Springer: Gateway East, Singapore, 2018; Chapter 8; pp. 99–108. [Google Scholar]
- Schneider, D.J. Anti-platelet therapy: Glycoprotein IIb-IIIa antagonists. Br. J. Clin. Pharmacol. 2011, 72, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Jernberg, T.; Payne, C.D.; Winters, K.J.; Darstein, C.; Brandt, J.T.; Jakubowski, J.A.; Naganuma, H.; Siegbahn, A.; Wallentin, L. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur. Heart J. 2006, 27, 1166–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamatsu, J.; Sasaki, K.-I.; Katsuki, Y.; Kawasaki, T.; Murasato, Y.; Ajisaka, H.; Yokoi, H.; Tashiro, H.; Harada, A.; Hirakawa, Y. Prasugrel effectively reduces the platelet reactivity units in patients with genetically metabolic dysfunction of cytochrome P450 2C19 who are treated with long-term dual antiplatelet therapy after undergoing drug-eluting stent implantation. Heart Vessels 2020, 35, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, K.; Hamad, B.; Kirkpatrick, P. Ticagrelor. Nar. Rev. Drug Discov. 2011, 10, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-D.; Lee, M.-J.; Baek, Y.-S.; Kwon, S.-W.; Shin, S.-H.; Woo, S.-I.; Kim, D.-H.; Kwan, J.; Park, K.-S. Randomised trial to compare a protective effect of Clopidogrel Versus TIcagrelor on coronary Microvascular injury in ST-segment Elevation myocardial infarction (CV-TIME trial). EuroIntervention J. Eur. Colab. Work. Group Interv. Cadriol. Eur. Soc. Cadriol. 2016, 12, e964–e971. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Jacobshagen, C. TROPICAL-ACS Investigators. A randomised trial on platelet function-guided de-escalation of antiplatelet treatment in ACS patients undergoing PCI. Rationale and design of the Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) Trial. J. Thromb. Haemost. 2017, 117, 188–195. [Google Scholar]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef] [Green Version]
- Boersma, E.; Pieper, K.S.; Steyerberg, E.W.; Wilcox, R.G.; Chang, W.-C.; Lee, K.L.; Akkerhuis, K.M.; Harrington, R.A.; Deckers, J.W.; Armstrong, P.W. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation: Results from an international trial of 9461 patients. Circulation 2000, 101, 2557–2567. [Google Scholar] [CrossRef] [Green Version]
- Eagle, K.A.; Lim, M.J.; Dabbous, O.H.; Pieper, K.S.; Goldberg, R.J.; Van de Werf, F.; Goodman, S.G.; Granger, C.B.; Steg, P.G.; Gore, J.M. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. Jama 2004, 291, 2727–2733. [Google Scholar] [CrossRef]
- Larmore, C.; Effron, M.B.; Molife, C.; DeKoven, M.; Zhu, Y.; Lu, J.; Karkare, S.; Lieu, H.D.; Lee, W.C.; Vetrovec, G.W. “Real-world” comparison of prasugrel with ticagrelor in patients with acute coronary syndrome treated with percutaneous coronary intervention in the United States. Catherter. Cardiovasc. Interv. 2016, 88, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Clin. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibbing, D.; Gross, L.; Trenk, D.; Jacobshagen, C.; Geisler, T.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; Komócsi, A.; Parma, R. Age and outcomes following guided de-escalation of antiplatelet treatment in acute coronary syndrome patients undergoing percutaneous coronary intervention: Results from the randomised TROPICAL-ACS trial. Eur. Heart J. 2018, 39, 2749–2758. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Investigators | Country | Number of Patients | Clopidogrel Loading Dose (mg) | CR |
|---|---|---|---|---|
| Ma et al. 2019 [18] | China | 441 | 300 | 17.2% |
| Pareed et al. 2020 [19] | India | 200 | 300 | 32% |
| Namazi et al. 2012 [20] | Iran | 112 | 600 | 25.90% |
| Sahib et al. 2016 [21] | Iraq | 127 | 300 | 24% |
| Park et al. 2011 [22] | Korea | 114 | 75/150 | 46% |
| Amin et al., 2017 [23] | Malaysia | 71 | 600 | 38% |
| Sakr et al., 2016 [24] | Saudi Arabia | 49 172 83 | 75 300 600 | 81.6% 66.3% 55.4% |
| Tekkeşin et al. 2016 [25] | Turkish | 1.238 | 600 | 30.2% |
| Range | 17.2–81.6% |
| Author | Population | Population Sample | Gene | SNP | Genotype | Allele Frequencies | Total (n/%) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| CR Group (n/%) | NCR Group (n/%) | ||||||||
| Li et al., 2020 [62] | China | 126 | CYP2C19*2 | rs4244285 | GG (*1/*1) GT (*1/*2) TT (*2/*2) | 9 (23.1%) 21 (53.8%) 9 (23.1%) | 48 (55.2%) 30 (34.5%) 9 (10.3%) | 57 (45.2%) 51 (40.5%) 18 (14.3%) | 0.001 0.041 0.093 |
| CYP2C19*3 | rs4986893 | GG (*1/*1) GT (*1/*3) TT (*3/*3) | 27 (69.2%) 10 (25.6%) 2 (5.1%) | 75 (86.2%) 11 (12.6%) 1 (1.2%) | 102 (80.9%) 21 (16.7%) 3 (2.4%) | 0.025 0.070 0.176 | |||
| Al-Azzam et al., 2013 [63] | Jordan | 240 | CYP2C19*2 | rs4244285 | GG (*1/*1) GT (*1/*2) TT (*2/*2) | 22 (22.9%) 38 (31.7%) 16 (67.7%) | 74 (77.1%) 82 (68.3%) 8 (33.3%) | 96(40%) 120(50%) 24(10%) | <0.001 |
| Lee et al., 2009 [64] | Korean | 387 | CYP2C19*2 | rs4244285 | GG GA AA | 55(49.1%) 40(35.7%) 13(11.6%) | 155(56.4%) 93(33.8%) 26(9.5%) | 210(54.3%) 133(34.4%) 39(10.1%) | 0.287 |
| CYP2C19*3 | rs4986893 | GG GA AA | 80(71.4%) 31(27.7%) 1(0.9%) | 236(85.8%) 37(13.5%) 1(0.4%) | 316(81.7%) 68(17.6%) 2(0.5%) | 0.001 | |||
| Amin et al., 2017 [23] | Malaysia | 71 | CYP2C19*2 | rs4244285 | GG (*1/*1) GT (*1/*2) TT (*2/*2) | 11 (40.7%) 8 (29.6%) 8 (29.6%) | 19 (43.2%) 22 (50.0%) 3 (6.8%) | 30 (42.3%) 30 (42.3%) 11(15.5) | 0.026 |
| Alhazzani, et al., 2017 [65] | Saudi Arabia | 50 | CYP2C19*2 | rs4244285 | GG GA + AA | 21(84%) 4(16%) | 10(40%) 15(60%) | 31(62%) 19((38%) | 0.001 |
| CYP2C19*3 | rs4986893 | GG GA + AA | 20(80%) 5(20%) | 13(52%) 12((48%) | 33(66%) 17(34%) | 0.036 | |||
| Shijun et al., 2014 [66] | China | 95 | CYP3A4*G1 | rs2242480 | (GG) (GA + AA) | 24 (61.50%) 15 (38.50%) | 33 (58.90%) 23 (41.10%) | 57 (60.00%) 38 (40.00) | 0.798 |
| Namazi, et al. 2012 [20] | Iran | 112 | CYP3A5 | rs776746 | (*1/*1) (*1/*3) (*3/*3) | - | - | 9 (8.00%) 42 (37.50%) 61(54.50%) | >0.05 |
| Al-Husein et al., 2018 [67] | Jordan | 280 | CYP3A4 | rs2242480 | (*1/*1) (*1/*3+ *3/*3) | 80(28.6%) 1 (0.4%) | 196 (70%) 3 (1.1%) | 276 (98.6%) 4 (1.4%) | >0.9999 |
| CYP3A5 | rs776746 | (*1/*1) (*1/*3) (*3/*3) | 57 (20.4%) 23(8.2%) 119(42.5%) | 24(8.6%) 10(3.6%) 47(16.8%) | 81(28.9%) 33(11.8%) 166(59.3%) | 0.961 | |||
| Lee et al., 2009 [64] | Korean | 387 | CYP3A4 | rs2246709 | TT TC CC | 42(37.5%) 57(50.9%) 12(10.7%) | 103(37.5%) 139(50.5%) 28(10.2%) | 145(37.5%) 196(50.6%) 40(10.3%) | 0.925 |
| rs2242480 | GG GA AA | 74(66.1%) 32(28.6%) 6(5.4%) | 172(62.5%) 90(32.7%) 13(4.7%) | 246(63.6%) 122(31.5%) 19(4.9%) | 0.568 | ||||
| CYP3A5 | rs776746 | GG GA AA | 61(54.5%) 41(36.6%) 6(5.4%) | 154(56.0%) 102(37.1%) 12(4.4%) | 215(55.6%) 143(37.0%) 18(4.7%) | 0.808 | |||
| Shasha et al., 2020 [68] | China | 741 | ABCB1 | rs1045642 | GG GA + AA | 94(38.5%) 222(70.3%) | 161(44.4%) 264(62.1%) | 255(34.4%) 486(65.6%) | 0.021 |
| Chen et al., 2021 [69] | China | 204 | MDR1 | rs 1128503 | CC CT TT | 12 (24%) 17 (34.7%) 20 (40.8%) | 40 (25.8%) 65 (41.9%) 50 (32.3%) | 52 (25.5%) 82 (40.2%) 70 (34.3%) | 0.521 |
| Li et al., 2020 [62] | China | 126 | P2Y12 | rs6809699 | GG GT TT | 15 (38.5%) 21 (53.8%) 3 (7.7%) | 67 (79.3%) 18 (20.7%) 2 (2.3%) | 82 (66.7%) 39 (30.9%) 5 (2.4%) | 0.000 0.000 0.152 |
| Namazi et al., 2012 [20] | Iran | 112 | rs2046934 | CC CT + TT | - | - | 104(92.9%) 8 (7.1%) | >0.05 | |
| Lee et al., 2009 [64] | Korean | 387 | rs2046934 | TT TC CC | 81(72.3%) 26(23.2%) 4(3.6%) | 177(64.4%) 89(32.4%) 8(2.9%) | 258(66.7%) 115(29.7%) 12(3.1%) | 0.139 | |
| Likely Phenotype | Genotypes | Examples of Diplotypes |
|---|---|---|
| Ultrarapid metaboliser: Normal or increased activity (−5–30% of patients) | An individual carrying two increased activity alleles (*17) or one functional allele (*1) plus one increased-activity allele (*17) | *1/*17, *17/ *17 |
| Extensive metaboliser: Homozygous wild-type or normal activity (~35–50% of patients) | An individual carrying two functional (*1) alleles. | *1/*1 |
| Intermediate metaboliser: Heterozygote or intermediate activity (~18–45% of patients) | An individual carrying one functional allele (*1) plus one loss-of-function allele (*2-*8) or one loss-of-function allele (*2-*8) plus one increased-activity allele (*17) | *1/ *2, *1/*3, *2/*17 |
| Poor metaboliser: Homozygous variant, mutant, low, or deficient activity (~2–15% of patients) | An individual carrying two loss-of-function alleles (*2-*8) | *2/*2, *2/*3, *3/*3 |
| Author | Population | Population Sample | Method | Allele Frequency (%) | ||
|---|---|---|---|---|---|---|
| CYP2C19*2 | CYP2C19*3 | CYP2C19*17 | ||||
| Zhong et al., (2017) [80] | China | 6686 | PCR and DNA Sequencing | 31.06 | 4.61 | ND |
| T. Wang et al., (2020) [81] | China | 1129 | TaqMan-Real-Time PCR | ND | ND | 2.5 |
| (Anichavezhi, Chakradhara Rao, Shewade, Krishnamoorthy, & Adithan, (2012) [82] | India | 206 | PCR-RFLP | 40.2 | 0 | 19.2 |
| Dehbozorgi et al., (2018) [83] | Iran | 1,229 | PCR and DNA Sequencing | 21.4 | 1.7 | 27.1 |
| Sahib, Mohammed, & Abdul-Majid, (2015) [84] | Iraq | 221 | PCR and DNA Sequencing | 15.2 | 0.2 | 19.5 |
| Sugimoto, Uno, Yamazaki, & Tateishi, (2008) [79] | Japanese | 265 | PCR-RFLP | 27.9 | 12.8 | 1.13 |
| (Sviri, Shpizen, Leitersdorf, Levy, & Caraco, (1999) [85] | Jewish Israeli | 136 | PCR-RFLP | 15 | 1 | ND |
| Rjoub et al., 2018 [86] | Jordanian | 148 | PCR-RFLP | 9.8 | ND | 28.72 |
| Kim, Song, Kim, & Park, (2010) [87] | Korean | 271 | PCR and pyrosequencing | 28.4 | 10.1 | 1.5 |
| Amin et al., (2017) [88] | Malaysia | 89 | PCR and DNA Sequencing | 59.6 | 6.74 | ND |
| Riaz et al., (2019) [89] | Pakistan | 1028 | ASA-PCR | 29.0 | ND | 23.70 |
| (Ayesh, Al-Astal, & Yassin, (2019) [90] | Palestinian | 110 | PCR-RFLP | 15.5 | 2.3 | ND |
| Elewa, Ali, & Bader, (2018) [91] | Qatar | 129 | TaqMan-Real-Time PCR | 4 | 0 | 10 |
| Mirzaev et al., (2017) [92] | Russia | 512 | TaqMan-Real-Time PCR | 11.25 | 1.2 | 22 |
| Al-Jenoobi et al., 2013 [93] | Saudi Arabia | 192 | PCR and DNA Sequencing | 8.2 | 0 | 26.9 |
| Sukasem et al., (2013) [94] | Thai | 1051 | AmpliChip CYP450 test | 41.95 | 13.03 | 4.30 |
| (Arici & Özhan, (2017) [95] | Turkish | 160 | PCR-RFLP | 12 | 13 | 25 |
| Vu et al., (2019) [96] | Vietnam | 100 | PCR-RFLP | 20.5 | 2.5 | 1 |
| Total | 13662 | |||||
| Average | 23.00 | 4.61 | 15.18 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkaif, M.A.; Daud, N.A.A.; Sha’aban, A.; Ng, M.L.; Abdul Kader, M.A.S.; Noor, D.A.M.; Ibrahim, B. The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules 2021, 26, 1987. https://doi.org/10.3390/molecules26071987
Akkaif MA, Daud NAA, Sha’aban A, Ng ML, Abdul Kader MAS, Noor DAM, Ibrahim B. The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules. 2021; 26(7):1987. https://doi.org/10.3390/molecules26071987
Chicago/Turabian StyleAkkaif, Mohammed Ahmed, Nur Aizati Athirah Daud, Abubakar Sha’aban, Mei Li Ng, Muhamad Ali Sk Abdul Kader, Dzul Azri Mohamed Noor, and Baharudin Ibrahim. 2021. "The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD)" Molecules 26, no. 7: 1987. https://doi.org/10.3390/molecules26071987








