Determination of Saffron Volatiles by HS-SBSE-GC in Flavored Cured Ham
Abstract
:1. Introduction
2. Results and Discussion
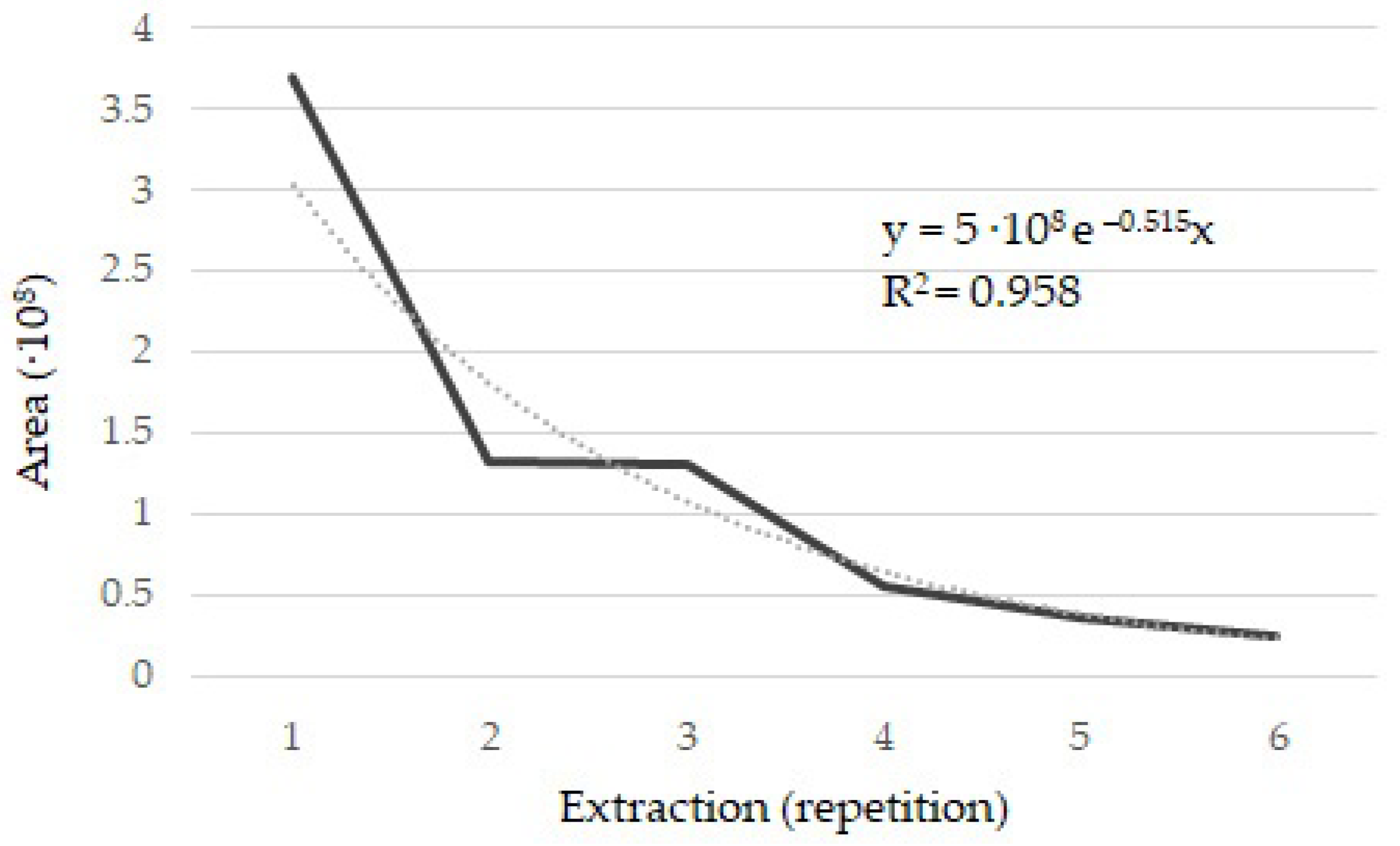
2.1. Conditions for Extraction of Volatile Compounds from Saffron
2.2. Internal Method Validation
2.3. Determination of Saffron Aroma in Cured Ham
3. Materials and Methods
3.1. Samples and Reagents
3.2. Selection of Conditions for Extraction of Volatile Compounds from Saffron
3.3. Analysis of Volatile Compounds by Headspace-Stir Bar Sorptive Extraction-Gas Chromatography/Mass Spectrometry
3.4. Analytical Method Validation
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Čandek-Potokar, M.; Škrlep, M. Factors in Pig Production That Impact the Quality of Dry-Cured Ham: A Review. Animal 2012, 6, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, J.F. N-Alkane Content of Intramuscular Lipids of Iberian Fresh Ham from Different Feeding Systems and Crossbreeding. Meat Sci. 2001, 57, 371–377. [Google Scholar] [CrossRef]
- Flores, M.; Grimm, C.C.; Toldrá, F.; Spanier, A.M. Correlations of Sensory and Volatile Compounds of Spanish “Serrano” Dry-Cured Ham as a Function of Two Processing Times. J. Agric. Food Chem. 1997, 45, 2178–2186. [Google Scholar] [CrossRef]
- Pérez-Santaescolástica, C.; Fraeye, I.; Barba, F.J.; Gómez, B.; Tomasevic, I.; Romero, A.; Moreno, A.; Toldrá, F.; Lorenzo, J.M. Application of Non-Invasive Technologies in Dry-Cured Ham: An Overview. Trends Food Sci. Technol. 2019, 86, 360–374. [Google Scholar] [CrossRef]
- Jin, S.-K.; Kim, C.-W.; Chung, K.-H.; Jo, K.-K.; Jeong, J.-Y.; Hur, I.-C.; Jung, E.-Y.; Joo, S.-T.; Yang, H.-S. Physicochemical and Sensory Properties of Irradiated Dry-Cured Ham. Radiat. Phys. Chem. 2012, 81, 208–215. [Google Scholar] [CrossRef]
- Martínez-Onandi, N.; Rivas-Cañedo, A.; Picon, A.; Nuñez, M. Influence of Physicochemical Parameters and High Pressure Processing on the Volatile Compounds of Serrano Dry-Cured Ham after Prolonged Refrigerated Storage. Meat Sci. 2016, 122, 101–108. [Google Scholar] [CrossRef]
- Hui, T.; Zhang, Y.; Jamali, M.A.; Peng, Z. Incorporation of Pig Back Fat in Restructured Dry Cured Ham to Facilitate the Release of Unsaturated Fatty Acids and Generation of Volatile Compounds: Pig Back Fat in RDH Impact on FFA and Volatile Compound. Eur. J. Lipid Sci. Technol. 2017, 119, 1600025. [Google Scholar] [CrossRef]
- García-Blázquez, A.; Moratalla-López, N.; Lorenzo, C.; Salinas, M.R.; Alonso, G.L. Effect of Crocus Sativus L. Stigmas Microwave Dehydration on Picrocrocin, Safranal and Crocetin Esters. Foods 2021, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- del Campo, C.P.; Carmona, M.; Maggi, L.; Kanakis, C.D.; Anastasaki, E.G.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Picrocrocin Content and Quality Categories in Different (345) Worldwide Samples of Saffron (Crocus Sativus L.). J. Agric. Food Chem. 2010, 58, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Moratalla-López, N.; Parizad, S.; Habibi, M.K.; Winter, S.; Kalantari, S.; Bera, S.; Lorenzo, C.; García-Rodríguez, M.V.; Dizadji, A.; Alonso, G.L. Impact of Two Different Dehydration Methods on Saffron Quality, Concerning the Prevalence of Saffron Latent Virus (SaLV) in Iran. Food Chem. 2021, 337, 127786. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Zalacain, A.; Salinas, M.R.; Alonso, G.L. A New Approach to Saffron Aroma. Crit. Rev. Food Sci. Nutr. 2007, 47, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthou, A.; Pouliou, E.; Kyriakoudi, A.; Tsimidou, M.Z. Sensory Threshold Studies of Picrocrocin, the Major Bitter Compound of Saffron. J. Food Sci. 2016, 81, S189–S198. [Google Scholar] [CrossRef] [PubMed]
- Berruga, M.I.; Licón, C.C.; Carmona, M.; Molina, A.M.; Román, M.; Olivares, V.; Olivares, F.J.; Olivares, S. Procedimiento de Elaboración de Queso de Oveja Con Azafrán y Queso Obtenido Mediante Dicho Procedimiento. ES2364933A1, 19 September 2011. [Google Scholar]
- Licón, C.C.; de Mendoza, J.H.; Maggi, L.; Berruga, M.I.; Aranda, R.M.M.; Carmona, M. Optimization of Headspace Sorptive Extraction for the Analysis of Volatiles in Pressed Ewes’ Milk Cheese. Int. Dairy J. 2012, 23, 9. [Google Scholar] [CrossRef]
- Licón, C.C.; Carmona, M.; Berruga, M.I. Volatile Compounds in Pressed Ewes’ Milk Cheese with Saffron Spice (Crocus Sativus L.). Int. J. Dairy Technol. 2015, 68, 399–408. [Google Scholar] [CrossRef]
- Buettner, A. A Selective and Sensitive Approach to Characterize Odour-Active and Volatile Constituents in Small-Scale Human Milk Samples. Flavour Fragr. J. 2007, 22, 465–473. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Ma, W.-J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.-P. Characterization of the Key Aroma Compounds in Longjing Tea Using Stir Bar Sorptive Extraction (SBSE) Combined with Gas Chromatography-Mass Spectrometry (GC–MS), Gas Chromatography-Olfactometry (GC-O), Odor Activity Value (OAV), and Aroma Recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Feyzi, S.; Varidi, M.; Housaindokht, M.R.; Es’haghi, Z. Innovative Method for Analysis of Safranal under Static and Dynamic Conditions through Combination of HS-SPME-GC Technique with Mathematical Modelling. Phytochem. Anal. 2020, 31, 564–574. [Google Scholar] [CrossRef]
- Pérez-Juan, M.; Flores, M.; Toldrá, F. Model Studies on the Efficacy of Protein Homogenates from Raw Pork Muscle and Dry-Cured Ham in Binding Selected Flavor Compounds. J. Agric. Food Chem. 2006, 54, 4802–4808. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Verzera, A. Bioactive Volatiles in Sicilian (South Italy) Saffron: Safranal and Its Related Compounds. J. Essent. Oil Res. 2017, 29, 221–227. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Di Pietro, L.; Maggi, M.A.; Rossi, L. Optimization Using Chemometrics of HS-SPME/GC–MS Profiling of Saffron Aroma and Identification of Geographical Volatile Markers. Eur. Food Res. Technol. 2018, 244, 1605–1613. [Google Scholar] [CrossRef]
- Alonso, G.L.; Salinas, M.R.; Esteban-Infantes, F.J.; Sánchez-Fernández, M.A. Determination of Safranal from Saffron (Crocus Sativus L.) by Thermal Desorption−Gas Chromatography. J. Agric. Food Chem. 1996, 44, 185–188. [Google Scholar] [CrossRef]
- Carmona, M.; Martínez, J.; Zalacain, A.; Rodríguez-Méndez, M.L.; de Saja, J.A.; Alonso, G.L. Analysis of Saffron Volatile Fraction by TD–GC–MS and e-Nose. Eur. Food Res. Technol. 2006, 223, 96–101. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; 2014; ISBN 978-91-87461-59-0. Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 4 April 2021).
- International Organization for Standardization (ISO). ISO 3632-1 Saffron (Crocus Sativus L.)—Part 1 (Specification) and Part 2 (Test Methods); ISO: Geneva, Switzerland, 2011. [Google Scholar]

| Standard | Linear Range (µg Standard/20 mL vial) | Regression Equation | R2 |
|---|---|---|---|
| Safranal | 0–5.109 | y = (1.405 × 107 ± 4.224 × 105)x | 0.999 |
| Isophorone | 0–5.982 | y = (4.382 × 106 ± 5.432 × 105)x | 0.991 |
| Linalool | 0–6.455 | y = (1.560 × 106 ± 1.344 × 105)x | 0.996 |
| tMCHdO 1 | 0–4.517 | y = (4.875 × 106 ± 1.131 × 106)x | 0.985 |
| Standard | LOD (µg Standard/20 mL vial) | LOQ (µg Standard/20 mL vial) | Repeatability (% RSD) | Reproducibility (% RSD) |
|---|---|---|---|---|
| Safranal | 3.992 × 10−4 | 1.210 × 10−3 | 2.338 | 4.050 |
| Isophorone | 1.628 × 10−3 | 4.935 × 10−3 | 1.861 | 3.223 |
| Linalool | 2.590 × 10−2 | 7.847 × 10−2 | 2.200 | 3.811 |
| tMCHdO 1 | 2.650 × 10−3 | 8.029 × 10−3 | 1.723 | 2.984 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Sáez, E.M.; Moratalla-López, N.; Lorenzo, C.; Vergara, H.; Alonso, G.L. Determination of Saffron Volatiles by HS-SBSE-GC in Flavored Cured Ham. Molecules 2021, 26, 2073. https://doi.org/10.3390/molecules26072073
Gómez-Sáez EM, Moratalla-López N, Lorenzo C, Vergara H, Alonso GL. Determination of Saffron Volatiles by HS-SBSE-GC in Flavored Cured Ham. Molecules. 2021; 26(7):2073. https://doi.org/10.3390/molecules26072073
Chicago/Turabian StyleGómez-Sáez, Elena M., Natalia Moratalla-López, Cándida Lorenzo, Herminia Vergara, and Gonzalo L. Alonso. 2021. "Determination of Saffron Volatiles by HS-SBSE-GC in Flavored Cured Ham" Molecules 26, no. 7: 2073. https://doi.org/10.3390/molecules26072073






