Increase in Autoantibodies-Abzymes with Peroxidase and Oxidoreductase Activities in Experimental Autoimmune Encephalomyelitis Mice during the Development of EAE Pathology
Abstract
1. Introduction
2. Results
2.1. Experimental Groups of Mice
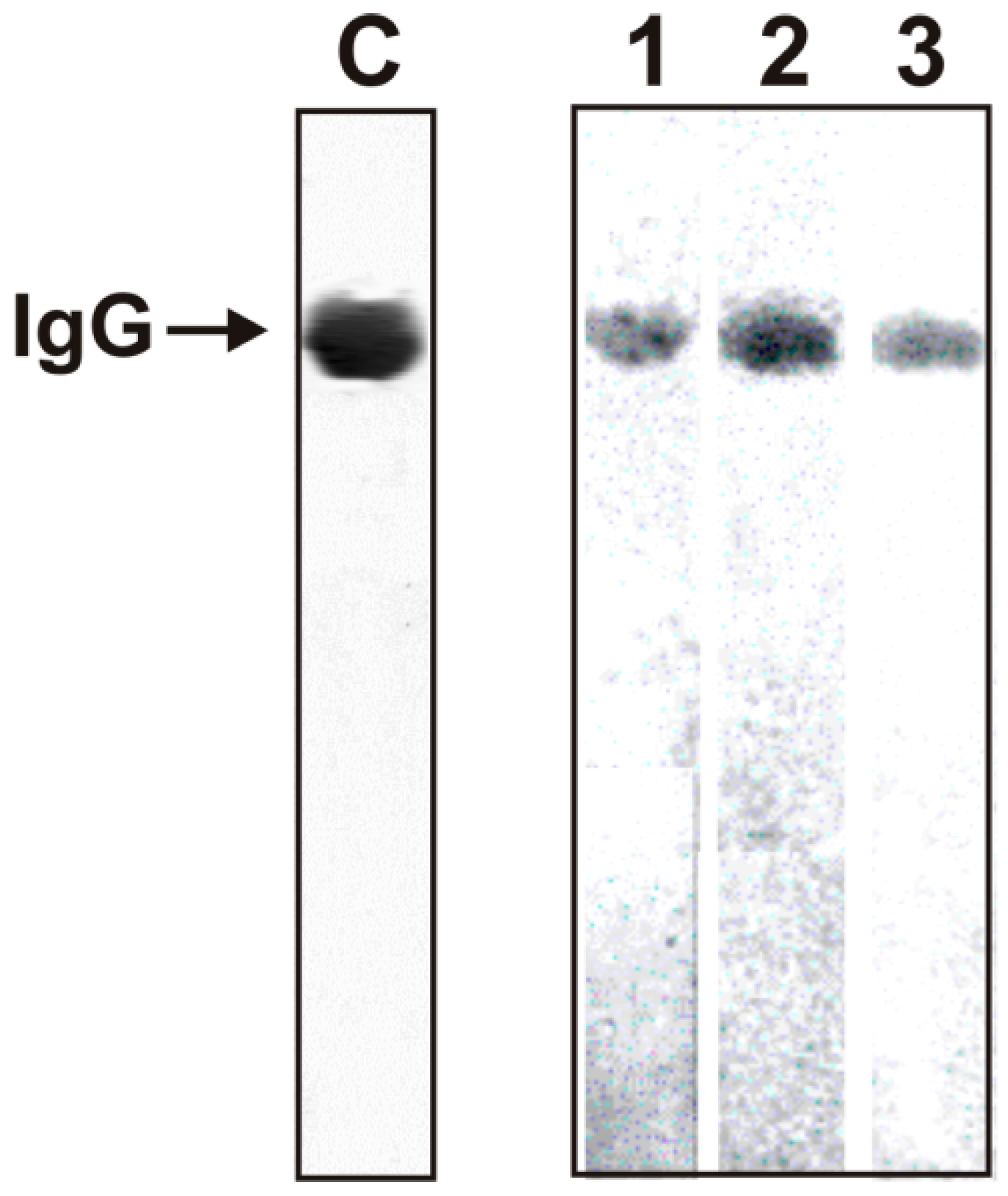
2.2. Criteria Analysis of Catalytic Activities of Antibodies
2.3. Substrates of IgGs
2.4. In Time Changes in the Peroxidase Activity during the Development of EAE
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Experimental Animals
4.3. Immunization of Mice
4.4. IgG Purification
4.5. Assay of Redox Activities
4.6. In Situ Analysis of Peroxidase Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| Abs | antibodies |
| auto-Abs | autoantibodies |
| AI | autoimmune |
| BFU-E | erythroid burst-forming unit (early erythroid colonies) |
| CSF | cerebrospinal fluid |
| CFU-GM | granulocyte-macrophage colony-forming unit |
| CFU-E | erythroid burst-forming unit (late erythroid colonies) |
| CFU-GEMM | granulocyte-erythroid-megacaryocyte-macrophage colony-forming unit |
| CNS | central nervous system |
| HSCs | hematopoietic stem cells |
| DNA-metBSA | complex of polymeric bovine DNA with methylated bovine serum albumin |
| EAE | experimental autoimmune encephalomyelitis |
| MOG35–55 or MOG | mouse myelin oligodendrocyte glycoprotein |
| MTT | tetrazolium dye MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| ROS | reactive oxygen species |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SLE | systemic lupus erythematosus |
| RA | relative activity |
References
- O’Connor, K.C.; Bar-Or, A.; Hafler, D.A. The neuroimmunology of multiple sclerosis: Possible roles of T and B lymphocytes in immunopathogenesis. J. Clin. Immunol. 2001, 21, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Croxford, A.L.; Kurschus, F.C.; Waisman, A. Mouse models for multiple sclerosis: Historical facts and future implications. Bochim. Biophys. Acta 2011, 1812, 177–183. [Google Scholar] [CrossRef]
- Miller, S.D.; Karpus, W.J.; Davidson, T.S. Experimental A. Autoimmune Encephalomyelitis in the Mouse. Curr. Protoc. Immunol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Mouse EAE models. Overview and Model Selection; Hooke Laboratories, Inc.: Lawrence, MA, USA, 2013. [Google Scholar]
- Ikehara, S.; Kawamura, M.; Takao, F. Organ-specific and systemic autoimmune diseases originate from defects in hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 1990, 87, 8341–8344. [Google Scholar] [CrossRef] [PubMed]
- Andryushkova, A.A.; Kuznetsova, I.A.; Orlovskaya, I.A.; Buneva, V.N.; Nevinsky, G.A. Antibodies with amylase activity from the sera of autoimmune-prone MRL/MpJ-lpr mice. FEBS Lett. 2006, 580, 5089–5095. [Google Scholar] [CrossRef] [PubMed]
- Andryushkova, A.S.; Kuznetsova, I.A.; Orlovskaya, I.A.; Buneva, V.N.; Nevinsky, G.A. Nucleotide- hydrolyzing antibodies from the sera of autoimmune-prone MRL-lpr/lpr mice. Int. immunol. 2009, 21, 935–945. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andryushkova, A.S.; Kuznetsova, I.A.; Buneva, V.N.; Toporkova, L.B.; Sakhno, L.V.; Tikhonova, M.A.; Chernykh, E.R.; Orlovskaya, I.A.; Nevinsky, G.A. Formation of different abzymes in autoimmune-prone MRL-lpr/lpr mice is associated with changes in colony formation of haematopoetic progenitors. J. Cell Mol. Med. 2007, 11, 531–551. [Google Scholar] [CrossRef]
- Doronin, V.B.; Parkhomenko, T.A.; Korablev, A.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaja, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; et al. Changes in different parameters, lymphocyte proliferation and hematopoietic progenitor colony formation in EAE mice treated with myelin oligodendrocyte glycoprotein. J. Cell Mol. Med. 2016, 20, 81–94. [Google Scholar] [CrossRef]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sennikov, S.V.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in haematopoietic progenitor colony differentiation and proliferation and the production of different abzymes in EAE mice treated with DNA. J. Cell Mol. Med. 2017, 21, 3795–3809. [Google Scholar] [CrossRef]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sedykh, S.E.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in cell differentiation and proliferation lead to production of abzymes in EAE mice treated with DNA-Histone complexes. J. Cell Mol. Med. 2018, 22, 5816–5832. [Google Scholar] [CrossRef]
- Lerner, R.A.; Tramontano, A. Antibodies as enzymes. Trends Bioch. Sci. 1987, 12, 427–438. [Google Scholar] [CrossRef]
- Stewart, J.D.; Benkovic, S.J. Recent developments in catalytic antibodies. Int. Rev. Immunol. 1993, 10, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.B.; Schultz, P.G. Opportunities at the interface of chemistry and biology. Trends Cell Biol. 1999, 9, 24–28. [Google Scholar] [CrossRef]
- Keinan, E.E. (Ed.) Catalytic Antibodies; Wiley-VCH Verlag GmbH and Co. KgaA: Weinheim, Germany, 2005. [Google Scholar]
- Nevinsky, G.A. Autoimmune processes in multiple sclerosis: Production of harmful catalytic antibodies associated with significant changes in the hematopoietic stem cell differentiation and proliferation. In Multiple Sclerosis; Conzalez-Quevedo, A., Ed.; InTech: Rijeka, Croatia, 2016; pp. 100–147. [Google Scholar]
- Nevinsky, G.A.; Buneva, V.N. Natural catalytic antibodies–abzymes. In Catalytic Antibodies; Keinan, E., Ed.; VCH-Wiley Press: Weinheim, Germany, 2005; pp. 505–569. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in autoimmune diseases. In Autoimmune Diseases: Symptoms, Diagnosis and Treatment; Brenner, K.J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 1–107. [Google Scholar]
- Nevinsky, G.A. Natural catalytic antibodies in norm and in HIV-infected patients. In Understanding HIV/AIDS Management and Care—Pandemic Approaches the 21st Century; Kasenga, F.H., Ed.; InTech: Rijeka, Croatia, 2011; pp. 151–192. [Google Scholar]
- Nevinsky, G.A. Catalytic antibodies in norm and systemic lupus erythematosus. In Lupus; Khan, W.A., Ed.; InTech: Rijeka, Croatia, 2017; pp. 41–101. [Google Scholar]
- Kozyr, A.V.; Kolesnikov, A.V.; Aleksandrova, E.S.; Sashchenko, L.P.; Gnuchev, N.V.; Favorov, P.V.; Kotelnikov, M.A.; Iakhnina, E.I.; Astsaturov, I.A.; Prokaeva, T.B.; et al. Novel functional activities of anti-DNA autoantibodies from sera of patients with lymphoproliferative and autoimmune diseases. Appl. Biochem. Biotechnol. 1998, 75, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Nevinsky, G.A.; Buneva, V.N. Catalytic antibodies in healthy humans and patients with autoimmune and viral pathologies. J. Cell Mol. Med. 2003, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 1983, 221, 1256. [Google Scholar] [CrossRef]
- Cutler, R.G. Antioxidants and aging. Am. J. Clin. Nutr. 1991, 53, 373S. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547. [Google Scholar] [CrossRef]
- Feuers, R.J.; Weindruch, R.; Hart, R.W. Caloric restriction, aging, and antioxidant enzymes. Mutat. Res. 1993, 295, 191. [Google Scholar] [CrossRef]
- Allen, R.G. Free Radicals in Aging; CPC Press: Boca Raton, FL, USA, 1993; pp. 12–23. [Google Scholar]
- Zenkov, N.K.; Lankin, V.Z.; Men’shikova, E.B. Oxidative Stress. Biochemical and Pathophysiological Aspects; MAIK. Nauka/Interperiodica: Moscow, Russia, 2001; pp. 3–343. [Google Scholar]
- Frei, B.; Stoker, R.; Ames, B.N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc. Natl. Acad. Sci. USA 1988, 85, 9748. [Google Scholar] [CrossRef]
- Correia, I.R. Stability of IgG isotypes in serum. MAbs 2010, 2, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ikhmyangan, E.N.; Vasilenko, N.L.; Buneva, V.N.; Nevinsky, G.A. IgG antibodies with peroxidase-like activity from the sera of healthy Wistar rats. FEBS Lett. 2005, 579, 3960. [Google Scholar] [CrossRef] [PubMed]
- Ikhmyangan, E.N.; Vasilenko, N.L.; Buneva, V.N.; Nevinsky, G. Metal ions-dependent peroxidase and oxidoreductase activities of polyclonal IgGs from the sera of Wistar rats. J. Mol. Recognit. 2006, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Ikhmyangan, E.N.; Vasilenko, N.L.; Sinitsina, O.I.; Buneva, V.N.; Nevinsky, G.A. Substrate specificity of rat sera IgG antibodies with peroxidase and oxidoreductase activities. J. Mol. Recognit. 2006, 19, 432. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, A.S.; Zaksas, N.P.; Buneva, V.N.; Vasilenko, N.L.; Nevinsky, G.A. Oxidoreductase activities of polyclonal IgG from the sera of Wistar rats are better activated by combinations of different metal ions. J. Mol. Recognit. 2009, 22, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, A.S.; Vasilenko, N.L.; Zaksas, N.P.; Sinitsina, O.I.; Buneva, V.N.; Nevinsky, G.A. Immunoglobulins a from the blood of healthy Wistar rats oxidize amines. Russ. J. Immunol. 2009, 3, 39–49. [Google Scholar]
- Shcheglova, T.V.; Tolmacheva, A.S.; Ovchinnikova, L.P.; Sinitsina, O.I.; Vasilenko, N.L.; Buneva, V.N.; Nevinsky, G.A. Superoxide dismutase, catalase, peroxidase and H2O2-independent oxidoreductase IgG antibody activity from the blood of healthy Wistar rats. Russ. J. Immunol. 2011, 5, 11–20. [Google Scholar]
- Tolmacheva, A.S.; Blinova, E.A.; Ermakov, E.A.; Buneva, V.N.; Vasilenko, N.L.; Nevinsky, G.A. IgG abzymes with peroxidase and oxidoreductase activities from the sera of healthy humans. J. Mol. Recognit. 2015, 28, 565–580. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Ermakov, E.A.; Buneva, V.N.; Nevinsky, G.A. Substrate specificity of healthy human sera IgG antibodies with peroxidase and oxydoreductase activities. R. Soc. Open Sci. 2018, 5, 171097. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Buneva, V.N.; Nevinsky, G.A. Substrate specificity of IgGs with peroxidase and oxidoreductase activities from sera of patients with systemic lupus erythematosus and multiple sclerosis. J. Mol. Recognit. 2019, 32, e2807. [Google Scholar] [CrossRef]
- Founel, S.; Muller, S. Antinucleosome antibodies and T-cell response in systemic lupus erythematosus. Ann. Med. Interne 2002, 153, 513–519. [Google Scholar]
- Williamson, R.A.; Burgoon, M.P.; Owens, G.P.; Ghausi, O.; Leclerc, E.; Firme, L.; Carlson, S.; Corboy, J.; Parren, P.W.; Sanna, P.P.; et al. Anti-DNA antibodies are a major component of the intrathecal B cell response in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2001, 98, 1793–1798. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Souliotis, V.L.; Vlachogiannis, N.I.; Pappa, M.; Argyriou, A.; Ntouros, P.A.; Sfikakis, P.P. DNA Damage response and oxidative stress in systemic autoimmunity. Int. J. Mol. Sci. 2019, 21, 55. [Google Scholar] [CrossRef]
- Da Fonseca, L.J.S.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative stress in rheumatoid arthritis: What the future might hold regarding novel biomarkers and add-on therapies. Oxid. Med. Cell. Longev. 2019, 7536805. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Li, C. Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med. Sci. Monit. 2019, 25, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Menshchikova, E.B.; Lankin, V.Z.; Zenkov, N.K.; Bondar, I.A.; Krugovykh, N.F.; Trufakin, V.A. Oxidative Stress. Prooxidants and Antioxidants; Slovo: Moscow, Russia, 2006; p. 556. [Google Scholar]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, R.; Kemp, K.; Rice, C.; Hares, K.; Scolding, N.; Wilkins, A. Oxidative stress-related biomarkers in multiple sclerosis: A review. Biomark. Med. 2016, 10, 889–902. [Google Scholar] [CrossRef]
- Van Horssen, J.; Schreibelt, G.; Drexhage, J.; Hazes, T.; Dijkstra, C.D.; van der Valk, P.; de Vries, H.E. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 2008, 45, 1729–1737. [Google Scholar] [CrossRef]
- Paul, S.; Planque, S.A.; Nishiyama, Y.; Hanson, C.V.; Massey, R.J. Nature and nurture of catalytic antibodies. Adv. Exp. Med. Biol. 2012, 750, 56–75. [Google Scholar] [PubMed]
- Planque, S.A.; Nishiyama, Y.; Hara, M.; Sonoda, S.; Murphy, S.K.; Watanabe, K.; Mitsuda, Y.; Brown, E.L.; Massey, R.J.; Pimmeer, S.R.; et al. Physiological IgM class catalytic antibodies selective for transthyretin amyloid. J. Biol. Chem. 2014, 289, 13243–13258. [Google Scholar] [CrossRef]
- Abu-Shakra, M.; Shoenfeld, Y. Chronic infections and autoimmunity. Immunol. Ser. 1991, 55, 285–313. [Google Scholar] [PubMed]
- Yamamoto, K. Possible mechanisms of autoantibody production and the connection of viral infections in human autoimmune diseases. Tohoku J. Exp. Med. 1994, 173, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Van Ghelue, M.; Moens, U.; Bendiksen, S.; Rekvig, O.P. Autoimmunity to nucleosomes related to viral infection: A focus on hapten-carrier complex formation. J. Autoimmun. 2003, 20, 171–182. [Google Scholar] [CrossRef]
- Barzilai, O.; Ram, M.; Shoenfeld, Y. Viral infection can induce the production of autoantibodies. Curr. Opin. Rheumatol. 2007, 19, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. Multiple sclerosis: A two-stage disease. Nat. Immunol. 2001, 2, 2762–2764. [Google Scholar] [CrossRef]
- Parkhomenko, T.A.; Doronin, V.B.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of DNA-hydrolyzing antibodies from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e93001. [Google Scholar] [CrossRef]
- Doronin, V.B.; Parkhomenko, T.A.; Castellazzi, M.; Padroni, M.; Pastore, M.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of antibodies hydrolyzing myelin basic protein from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2014, 9, e107807. [Google Scholar] [CrossRef]
- Doronin, V.B.; Parkhomenko, T.A.; Castellazzi, M.; Cesnik, E.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of antibodies with amylase activity from cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS ONE 2016, 11, e0154688. [Google Scholar] [CrossRef]
- Hachim, M.Y.; Elemam, N.M.; Maghazachi, A.A. The beneficial and debilitating effects of environmental and microbial toxins, drugs, organic solvents and heavy metals on the onset and progression of multiple sclerosis. Toxins 2019, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.M.; Christy, J.M.; Cauvi, D.M.; Kono, D.H. Environmental xenobiotic exposure and autoimmunity. Curr. Opin. Toxicol. 2018, 10, 15–22. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis. Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Agar, N.S.; Sadrzadeh, S.M.; Hallaway, P.E.; Eaton, J.W. Erythrocyte catalase. A somatic oxidant defense? J. Clin. Investig. 1986, 77, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, A.D.; Jones, L.H.; Wentworth, P., Jr.; Janda, K.D.; Lerner, R.A. Antibodies have the intrinsic capacity to destroy antigens. Proc. Natl. Acad. Sci. USA 2000, 97, 10930–10935. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, P., Jr.; Jones, L.H.; Wentworth, A.D.; Zhu, X.; Larsen, N.A.; Wilson, I.A.; Xu, X.; Goddard, W.A., III; Janda., K.D.; Eschenmoser, A.; et al. Antibody catalysis of the oxidation of water. Science 2001, 293, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Fersht, A. Enzyme Structure and Mechanism, 2nd ed.; W.H. Freeman Co.: New York, NY, USA, 1985. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolmacheva, A.S.; Aulova, K.S.; Urusov, A.E.; Orlovskaya, I.A.; Nevinsky, G.A. Increase in Autoantibodies-Abzymes with Peroxidase and Oxidoreductase Activities in Experimental Autoimmune Encephalomyelitis Mice during the Development of EAE Pathology. Molecules 2021, 26, 2077. https://doi.org/10.3390/molecules26072077
Tolmacheva AS, Aulova KS, Urusov AE, Orlovskaya IA, Nevinsky GA. Increase in Autoantibodies-Abzymes with Peroxidase and Oxidoreductase Activities in Experimental Autoimmune Encephalomyelitis Mice during the Development of EAE Pathology. Molecules. 2021; 26(7):2077. https://doi.org/10.3390/molecules26072077
Chicago/Turabian StyleTolmacheva, Anna S., Kseniya S. Aulova, Andrey E. Urusov, Irina A. Orlovskaya, and Georgy A. Nevinsky. 2021. "Increase in Autoantibodies-Abzymes with Peroxidase and Oxidoreductase Activities in Experimental Autoimmune Encephalomyelitis Mice during the Development of EAE Pathology" Molecules 26, no. 7: 2077. https://doi.org/10.3390/molecules26072077
APA StyleTolmacheva, A. S., Aulova, K. S., Urusov, A. E., Orlovskaya, I. A., & Nevinsky, G. A. (2021). Increase in Autoantibodies-Abzymes with Peroxidase and Oxidoreductase Activities in Experimental Autoimmune Encephalomyelitis Mice during the Development of EAE Pathology. Molecules, 26(7), 2077. https://doi.org/10.3390/molecules26072077






