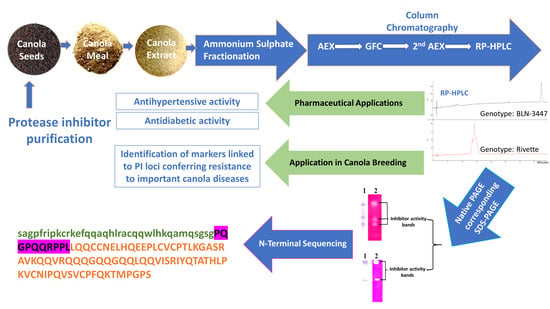
Protease Inhibitors Purified from the Canola Meal Extracts of Two Genetically Diverse Genotypes Exhibit Antidiabetic and Antihypertension Properties
Abstract
1. Introduction
2. Results
2.1. Extraction and Purification
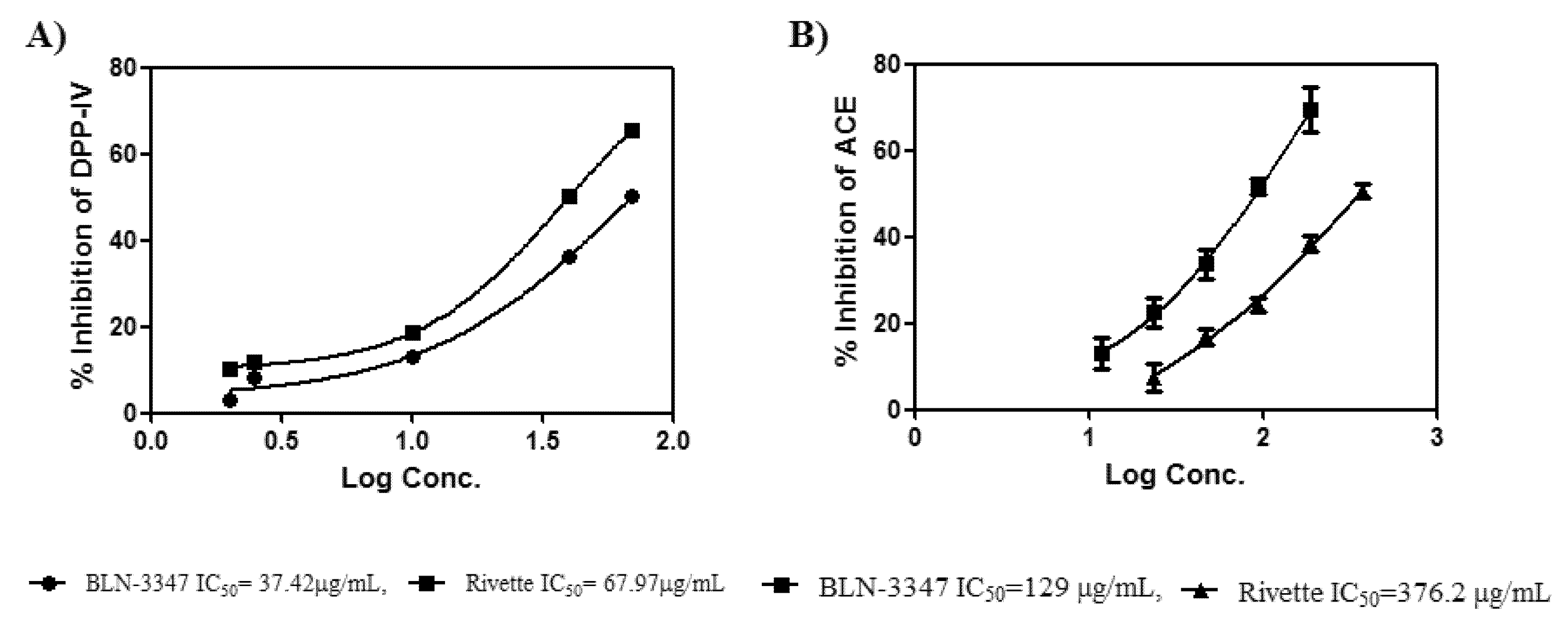
2.2. DPP-IV Inhibition and ACE Inhibition
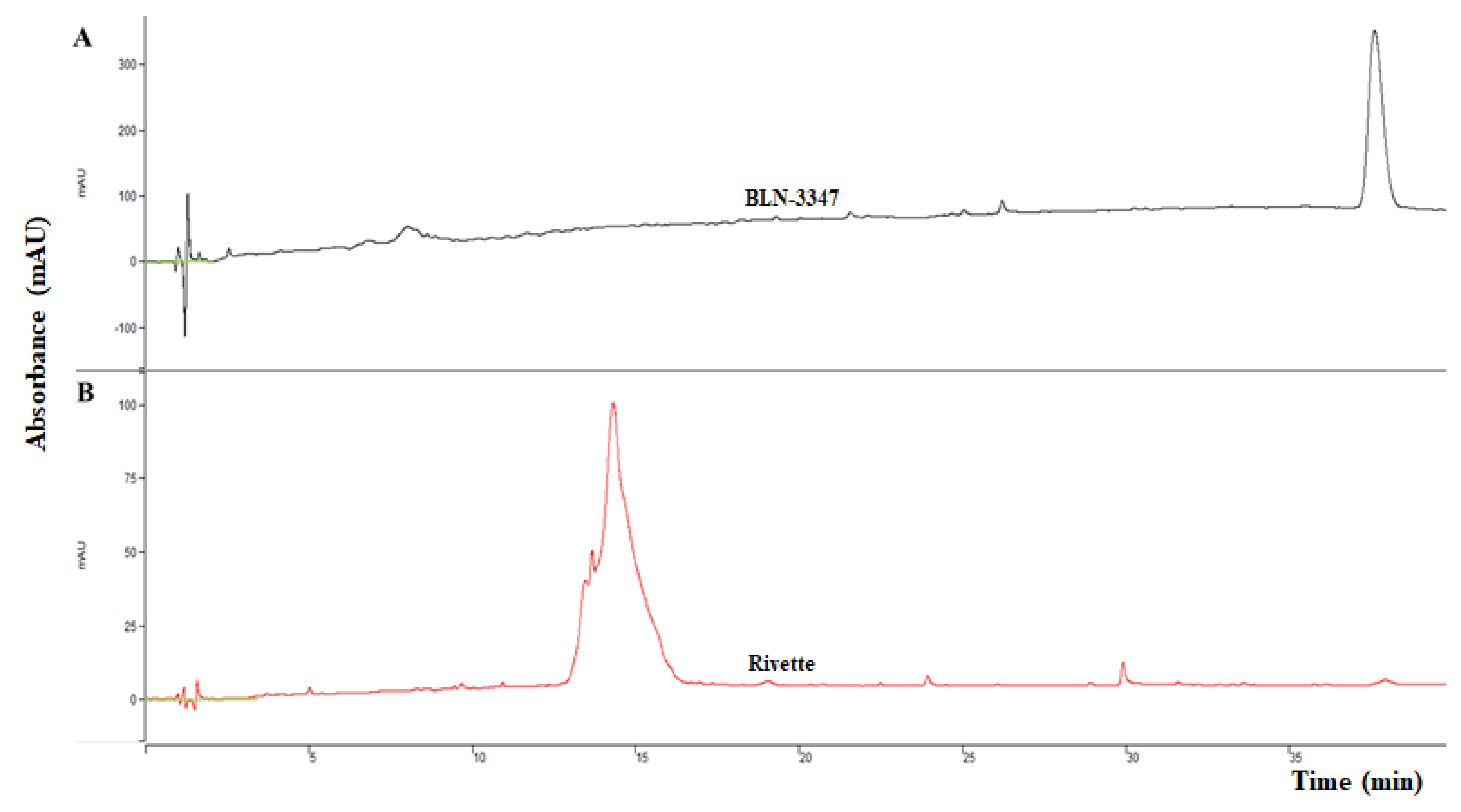
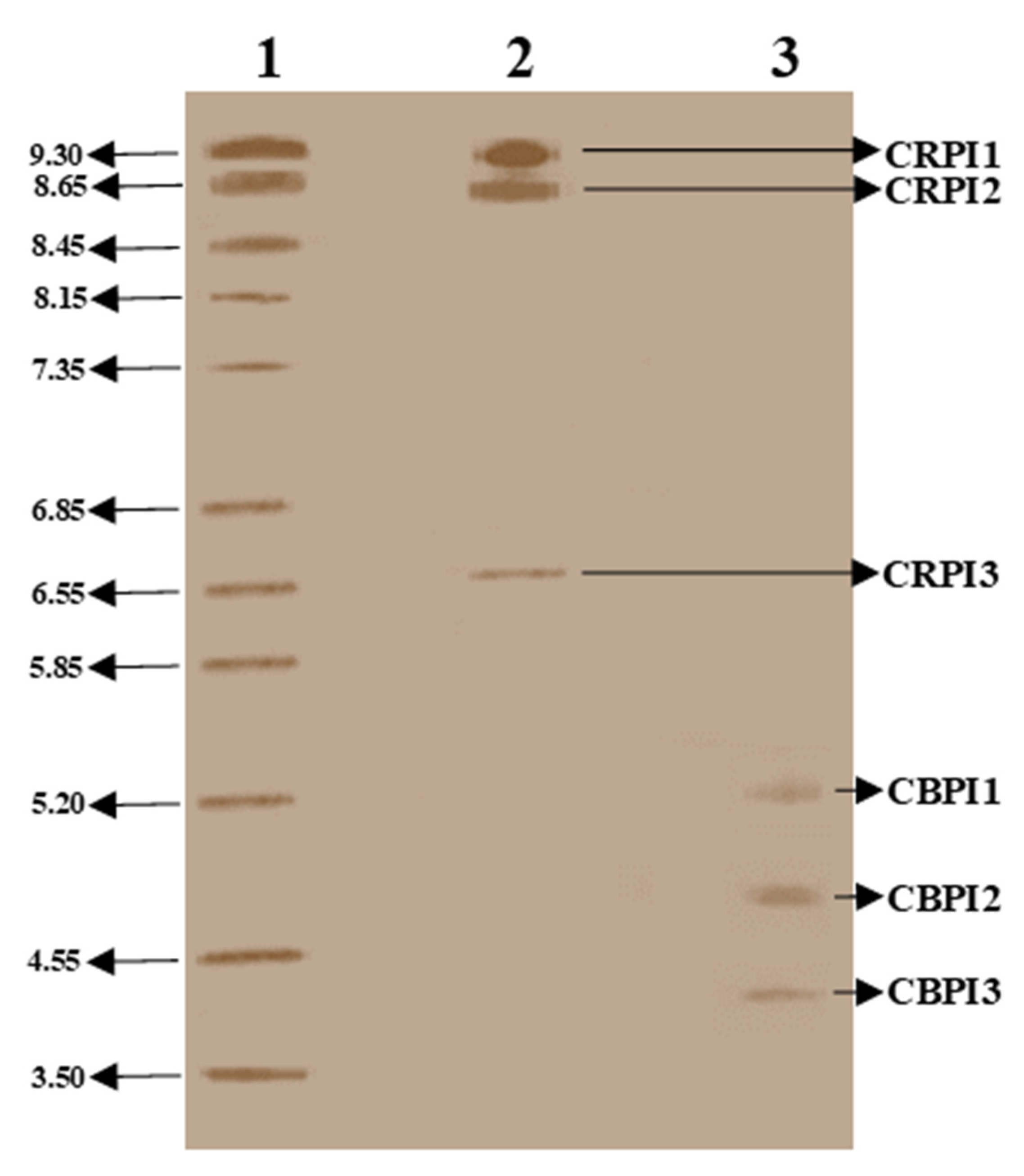
2.3. Molecular Characterization of Purified PIs
3. Discussion
4. Materials and Methods
4.1. Isolation and Purification of Canola Meal Protease Inhibitors
4.1.1. Protease Inhibitor (PI) Activity Assay
4.1.2. In-Gel Trypsin Inhibitor Activity
4.1.3. Isoelectric Focusing (IEF)
4.2. Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Activity
4.3. Angiotensin 1-Converting Enzyme (ACE) Inhibition Activity
4.4. N-Terminal Amino Acid Sequencing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kuhar, K.; Mittal, A.; Kansal, R.; Gupta, V.K. Purification of a protease inhibitor from Dolichos biflorus using immobilized metal affinity chromatography. Indian J. Biochem. Biophys. 2014, 51, 66–74. [Google Scholar] [PubMed]
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Richardson, M. Seed storage proteins: The enzyme inhibitors. Methods Plant Biochem. 1991, 5, 259–305. [Google Scholar]
- Braga-Silva, L.A.; Santos, A.L.S. Aspartic protease inhibitors as potential anti-candida albicans drugs: Impacts on fungal biology, virulence and pathogenesis. Curr. Med. Chem. 2011, 18, 2401–2419. [Google Scholar] [CrossRef] [PubMed]
- Melo, I.R.; Dias, L.P.; Araújo, N.M.; Vasconcelos, I.M.; Martins, T.F.; de Morais, G.A.; Gonçalves, J.F.; Nagano, C.S.; Carneiro, R.F.; Oliveira, J.T. Clcpi, a cysteine protease inhibitor purified from cassia leiandra seeds has antifungal activity against candida tropicalis by inducing disruption of the cell surface. Int. J. Biol. Macromol. 2019, 133, 1115–1124. [Google Scholar] [CrossRef]
- Laparra, J.; Haros, C. Plant seed protease inhibitors differentially affect innate immunity in a tumor microenvironment to control hepatocarcinoma. Food Funct. 2019, 10, 4210–4219. [Google Scholar]
- Salu, B.R.; Pando, S.C.; Brito, M.V.D.; Medina, A.F.; Odei-Addo, F.; Frost, C.; Naude, R.; Sampaio, M.U.; Emsley, J.; Maffei, F.H.A. Improving the understanding of plasma kallikrein contribution to arterial thrombus formation using two plant protease inhibitors. Platelets 2019, 30, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.L.; Souza-Pinto, J.C.; Batista, I.F.; Araujo, M.S.; Silveira, V.F.; Auerswald, E.A.; Mentele, R.; Eckerskorn, C.; Sampaio, M.U.; Sampaio, C.A. Leucaena leucocephala serine proteinase inhibitor: Primary structure and action on blood coagulation, kinin release and rat paw edema. Biochim. Biophys. Acta 2000, 1477, 64–74. [Google Scholar] [CrossRef]
- Hidaka, K.; Kimura, T.; Sankaranarayanan, R.; Wang, J.; McDaniel, K.F.; Kempf, D.J.; Kameoka, M.; Adachi, M.; Kuroki, R.; Nguyen, J.-T. Identification of highly potent human immunodeficiency virus type-1 protease inhibitors against lopinavir and darunavir resistant viruses from allophenylnorstatine-based peptidomimetics with p2 tetrahydrofuranylglycine. J. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Hariharan, A.; Shetty, S.; Shirole, T.; Jagtap, A.G. Potential of protease inhibitor in 3-nitropropionic acid induced huntington’s disease like symptoms: Mitochondrial dysfunction and neurodegeneration. Neurotoxicology 2014, 45, 139–148. [Google Scholar] [CrossRef]
- Waxler, B.; Rabito, S.F. Aprotinin: A serine protease inhibitor with therapeutic actions: Its interaction with ace inhibitors. Curr. Pharm. Des. 2003, 9, 777–787. [Google Scholar] [CrossRef]
- Cristina Oliveira de Lima, V.; Piuvezam, G.; Leal Lima Maciel, B.; Heloneida de Araújo Morais, A. Trypsin inhibitors: Promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders? J. Enzyme Inhib. Med. Chem. 2019, 34, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.F.d.; Costa, I.d.S.; Carvalho, F.M.C.d.; Kiyota, S.; Souza, B.B.P.d.; Sifuentes, D.N.; Serquiz, R.P.; Maciel, B.L.L.; Uchôa, A.F.; Santos, E.A.d. Biochemical characterisation of a kunitz-type inhibitor from tamarindus indica l. Seeds and its efficacy in reducing plasma leptin in an experimental model of obesity. J. Enzyme Inhib. Med. Chem. 2018, 33, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R. Food protein-derived peptides: Production, isolation, and purification. Proteins in Food Processing 2017, 389. [Google Scholar]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. The complex world of plant protease inhibitors: Insights into a kunitz-type cysteine protease inhibitor of arabidopsis thaliana. Commun. Integr. Biol. 2018, 11, e1368599. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Rehman, A.U.; Luckett, D.J.; Blanchard, C.L.; Obied, H.K.; Strappe, P. Phenolic compounds with antioxidant properties from canola meal extracts inhibit adipogenesis. Int. J. Mol. Sci. 2019, 21, 1. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine (Baltimore) 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Bhavtaran, S.; Narendra, P.; Varghese, N.; Vanchhawng, L.; Shihabudeen H, M.S.; Thirumurgan, K. Dipeptidyl peptidase-iv inhibitory activity of Berberis aristata. J. Nat. Prod. 2011, 4, 158–163. [Google Scholar]
- Silveira, S.T.; Martinez-Maqueda, D.; Recio, I.; Hernandez-Ledesma, B. Dipeptidyl peptidase-iv inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef]
- Jadav, P.; Bahekar, R.; Shah, S.R.; Patel, D.; Joharapurkar, A.; Kshirsagar, S.; Jain, M.; Shaikh, M.; Sairam, K.V. Long-acting peptidomimetics based dpp-iv inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3516–3521. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl peptidase iv inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef]
- Yogisha, S.; Ravisha, K. Dipeptidyl peptidase iv inhibitory activity of mangifera indica. J. Nat. Prod. 2010, 3, 76–79. [Google Scholar]
- Ali, K.M.; Jana, K.; Bera, T.K.; De, D.; Chatterjee, K.; Ghosh, A.; Maiti, S.; Ghosh, D. In-vitro testing of antioxidant efficacy of the methanol extract of seed of holarrhena antidysenterica: A correlative study with in-vivo bioactivity on oxidative stress in streptozotocin induced diabetic wistar rat. J. Herb. Med. Toxicol. 2012, 6, 123–131. [Google Scholar]
- Eriksson, U.; Danilczyk, U.; Penninger, J.M. Just the beginning: Novel functions for angiotensin-converting enzymes. Curr. Biol. 2002, 12, R745–R752. [Google Scholar] [CrossRef]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin i-converting enzyme (ace) inhibitory peptides from cuttlefish (sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Malomo, S.A.; Girgih, A.T.; Aluko, R.E. Blood pressure lowering effects of australian canola protein hydrolysates in spontaneously hypertensive rats. Food Res. Int. 2014, 55, 281–287. [Google Scholar] [CrossRef]
- Aider, M.; Barbana, C. Canola proteins: Composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity—A practical and critical review. Trends Food Sci. Technol. 2011, 22, 21–39. [Google Scholar] [CrossRef]
- Josefsson, L.-G.; Lenman, M.; Ericson, M.L.; Rask, L. Structure of a gene encoding the 1.7 s storage protein, napin, from brassica napus. J. Biol. Chem. 1987, 262, 12196–12201. [Google Scholar] [CrossRef]
- Konrad, B.; Anna, D.; Marek, S.; Marta, P.; Aleksandra, Z.; Józefa, C. The Evaluation of Dipeptidyl peptidase (DPP)-IV, α-Glucosidase and Angiotensin Converting Enzyme (ACE) inhibitory activities of Whey proteins Hydrolyzed with Serine Protease Isolated from Asian Pumpkin (Cucurbita ficifolia). Int. J. Peptide Res. Therapeut. 2014, 20, 483–491. [Google Scholar] [CrossRef]
- Han, R.; Maycock, J.; Murray, B.S.; Boesch, C. Identification of angiotensin converting enzyme and dipeptidyl peptidase-iv inhibitory peptides derived from oilseed proteins using two integrated bioinformatic approaches. Food Res. Int. 2019, 115, 283–291. [Google Scholar] [CrossRef]
- Xu, F.; Mejia, E.G.d.; Chen, H.; Rebecca, K.; Pan, M.; He, R.; Yao, Y.; Wang, L.; Ju, X. Assessment of the DPP-IV inhibitory activity of a novel octapeptide derived from rapeseed using Caco_2 cell monolayers and molecular docking analysis. J. Food Biochem. 2020, 44, e13406. [Google Scholar] [CrossRef]
- Ee, K.Y.; Zhao, J.; Rehman, A.U.; Agboola, S.O. Purification and characterization of a kunitz-type trypsin inhibitor from Acacia victoriae bentham seeds. J. Agric. Food Chem. 2009, 57, 7022–7029. [Google Scholar] [CrossRef] [PubMed]
- Birk, Y. Plant Protease Inhibitors: Significance in Nutrition, Plant Protection, Cancer Prevention and Genetic Engineering; Springer Science & Business Media: Berlin, Germany, 2003. [Google Scholar]
- Mosolov, V.; Grigoreva, L.; Valueva, T. Plant proteinase inhibitors as multifunctional proteins (review). Appl. Biochem. Microbiol. 2001, 37, 545–551. [Google Scholar] [CrossRef]
- Aachary, A.A.; Thiyam, U. A pursuit of the functional nutritional and bioactive properties of canola proteins and peptides. Crit. Rev. Food Sci. Nutr. 2012, 52, 965–979. [Google Scholar] [CrossRef]
- Beynon, R.; Bond, J.S. Proteolytic Enzymes, 2nd ed.; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Kollipara, K.P.; Hymowitz, T. Characterization of trypsin and chymotrypsin inhibitors in the wild perennial glycine species. J. Agric. Food Chem. 1992, 40, 2356–2363. [Google Scholar] [CrossRef]
- Nyo, M.K.; Nguyen, L.T. Value-addition of defatted peanut cake by proteolysis: Effects of proteases and degree of hydrolysis on functional properties and antioxidant capacity of peptides. Waste Biomass Valorization 2019, 10, 1251–1259. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Li-Chan, E.C. Inhibition of dipeptidyl peptidase (dpp)-iv and α-glucosidase activities by pepsin-treated whey proteins. J. Agric. Food Chem. 2013, 61, 7500–7506. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Screening of whey protein isolate hydrolysates for their dual functionality: Influence of heat pre-treatment and enzyme specificity. Food Chem. 2013, 136, 1435–1443. [Google Scholar] [CrossRef] [PubMed]

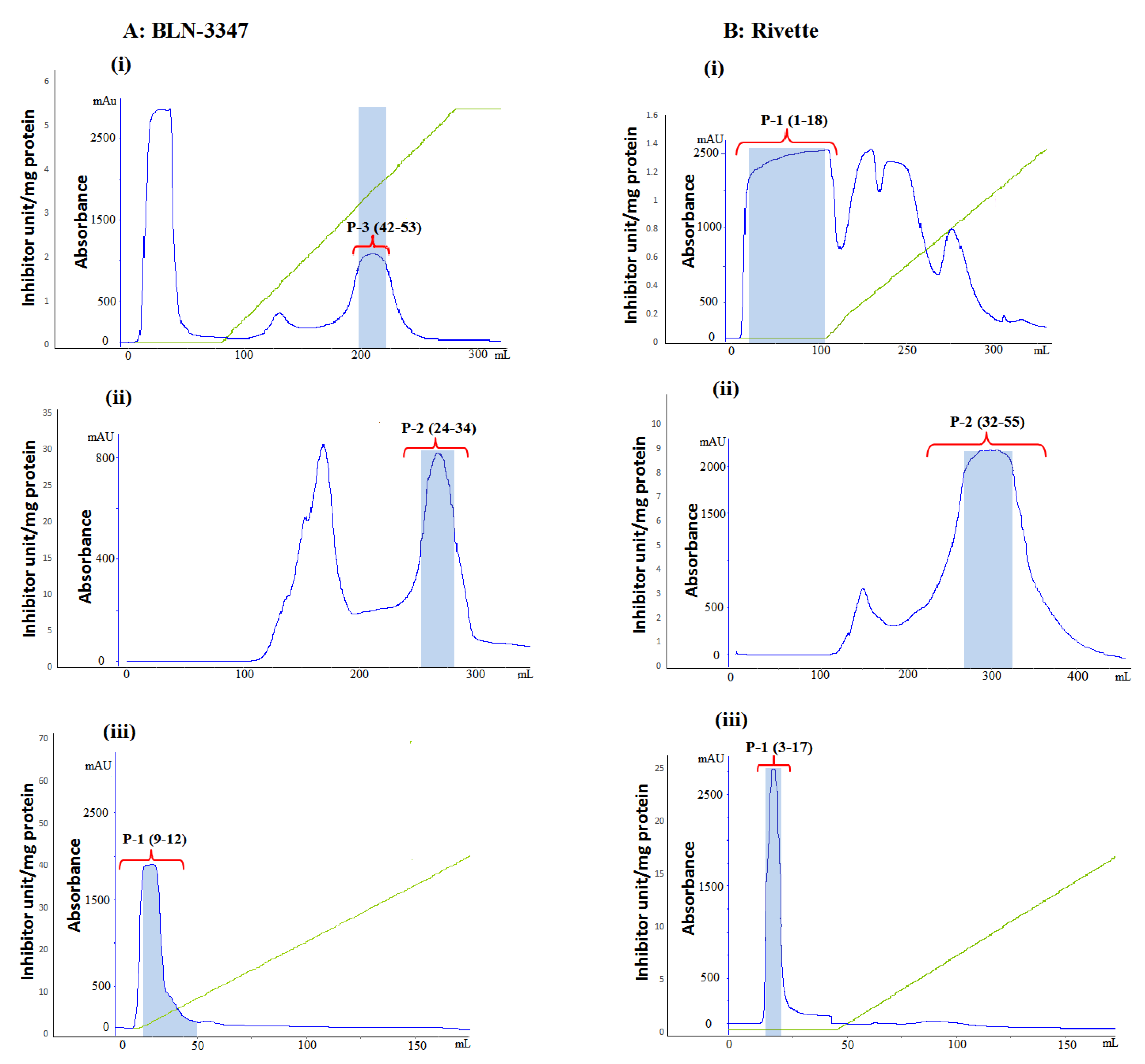

| Steps/Characteristics | Total Protein (mg) | Specific Activity 1 (TIU /mg) | % Recovery 2 | Purification Factor 3 | ||||
|---|---|---|---|---|---|---|---|---|
| BLN-3347 | Rivette | BLN-3347 | Rivette | BLN-3347 | Rivette | BLN-3347 | Rivette | |
| Crude extract | 4406 | 7560 | 0.013 | 0.008 | 100 | 100 | 1 | 1 |
| Ammonium sulphate precipitate (AS) | 1050 | 1498 | 0.44 | 0.06 | 24 | 20 | 33 | 7.5 |
| Anion exchange fraction (AEF) | 279 | 735 | 5.62 | 1.41 | 6.30 | 9.72 | 431 | 176 |
| Gel filtration fraction | 130 | 534 | 32 | 9.26 | 3.00 | 7.06 | 2388 | 1158 |
| Second AEF | 95 | 429 | 61 | 26 | 2.04 | 5.6 | 4633 | 3250 |
| Genotype | N-Terminus | Predicted Sequence | GenBank Accession | pI (cal/obs) | MW (kDa) (cal/obs) |
|---|---|---|---|---|---|
| BLN-3347 | APTLQGE-IK | mnipcfkfthihsvshqfcpasltqvrhasqkfrqashis trslkiaavaAPTLQGEWIKVEQKGGNTLSDRSSHGIAVVGDELYAFGGEFNTNDLHVFDLNTQNCTSL | XP_013643640.1 PREDICTED: epithiospecifier protein-like [Brassica napus] | 4.49/4.00 | 6.4/8 |
| ADLVE-PKKD | maenADLVEWPKKDKRRFLHVVYRVGDLDRTIQFYTECFGMKVLRKRDVPEEKYSNAFLGFGPETSNFVVELTYNYGVSSYDIGTGFGHFAISTQDVSKMVEAVrakggnvtrepgpvkgggsviafvkdpdgytfeliqrgptpeplcqvmlrvgdldraikfyekalgmrllrrierpeykytigmmgyaeeyesivleltynygvteytkgnayaqiaigtddvyksaevvkivnqelggkitreagplpglgtkivsfldpdgwktvlvdnedflkele | XP_013710061 PREDICTED: about 100 amino acids from determined N-terminal sequence of putative lactoylglutathione lyase [Brassica napus] | 5.37/6.13 | 11.5/11 | |
| * GQEHRIDK | No homology could be inferred | None. | --/4.90 | --/18 | |
| RIVETTE | IYPSF-V | mdmatksvsslaaffilflvifempeieaqdseclkeyggdvgfgfcaprIYPSFCVQRCRADKGALSGKCIWGQGGNVKCLCNFCRHEPGQILSGI | XP_013688693 PREDICTED: defensin-like protein 4 [Brassica napus] | 8.92/8.65 | 5.115/7 |
| PQGPQQRPPL | sagpfripkc rkefqqaqhl racqqwlhkq amqsgsgPQGPQQRPPLLQQCCNELHQEEPLCVCPTLKGASRAVKQQVRQQQGQQGQQLQQVISRIYQTATHLPKVCNIPQVSVCPFQKTMPGPS | P80208.1 2SS3_BRANA Napin-3 OS = Brassica napus PE = 1 SV = 1 | 9.15/9.30 | 9.813/15.5 | |
| * D/AA/EE/KGKKMET/A | No homology could be inferred | None. | --/6.55 | --/19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Rehman, A.u.; Luckett, D.J.; Naqvi, S.M.S.; Blanchard, C.L. Protease Inhibitors Purified from the Canola Meal Extracts of Two Genetically Diverse Genotypes Exhibit Antidiabetic and Antihypertension Properties. Molecules 2021, 26, 2078. https://doi.org/10.3390/molecules26072078
Hussain S, Rehman Au, Luckett DJ, Naqvi SMS, Blanchard CL. Protease Inhibitors Purified from the Canola Meal Extracts of Two Genetically Diverse Genotypes Exhibit Antidiabetic and Antihypertension Properties. Molecules. 2021; 26(7):2078. https://doi.org/10.3390/molecules26072078
Chicago/Turabian StyleHussain, Saira, Ata ur Rehman, David J. Luckett, Syed Muhammad Saqlan Naqvi, and Christopher L. Blanchard. 2021. "Protease Inhibitors Purified from the Canola Meal Extracts of Two Genetically Diverse Genotypes Exhibit Antidiabetic and Antihypertension Properties" Molecules 26, no. 7: 2078. https://doi.org/10.3390/molecules26072078
APA StyleHussain, S., Rehman, A. u., Luckett, D. J., Naqvi, S. M. S., & Blanchard, C. L. (2021). Protease Inhibitors Purified from the Canola Meal Extracts of Two Genetically Diverse Genotypes Exhibit Antidiabetic and Antihypertension Properties. Molecules, 26(7), 2078. https://doi.org/10.3390/molecules26072078