Novel Foods and Sustainability as Means to Counteract Malnutrition in Madagascar
Abstract
:1. Introduction
2. Food Habits in Madagascar
3. Traditional Cooking
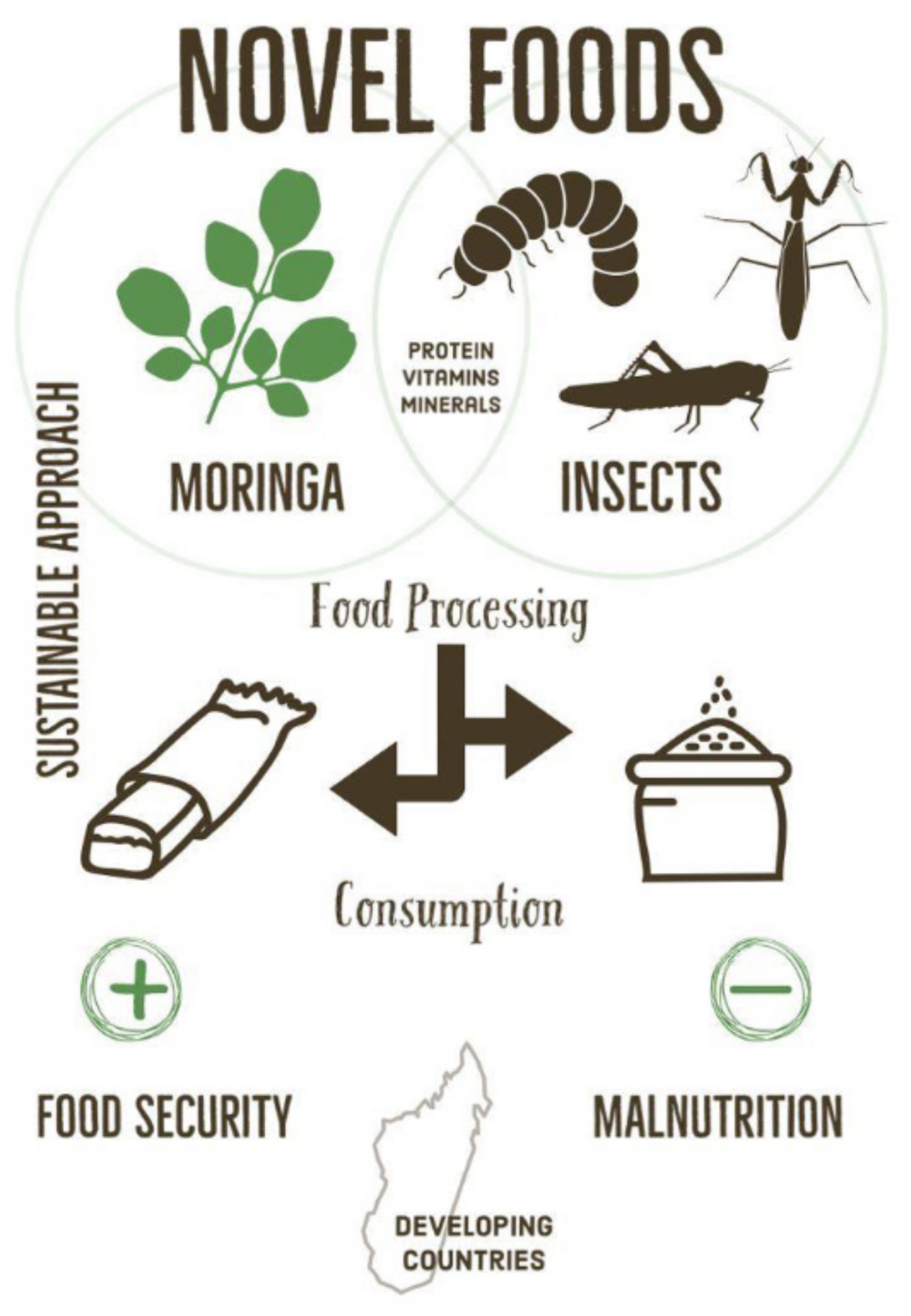
4. Sustainable Solutions and Novel Food
5. Novel Foods
5.1. Moringa
5.2. Edible Insects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- WHO Definition. 2020. Available online: https://www.who.int/news-room/factsheets/detail/malnutrition (accessed on 22 March 2021).
- FAO; WHO. Sustainable Healthy Diets—Guiding Principles; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Scrinis, G. Reframing malnutrition in all its forms: A critique of the tripartite classification of malnutrition. Glob. Food Secur. 2020, 26, 100396. [Google Scholar] [CrossRef]
- Garcia, M. Malnutrition and Food Insecurity Projections, 2020; No. 567-2016-38977; International Food Policy Research Institute: Washington, DC, USA, 1994. [Google Scholar]
- World Health Organization. UNICEF/WHO/The World Bank Group Joint Child Malnutrition estimates: Levels and trends in Child Malnutrition: Key Findings of the 2020 Edition; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/jme-2020-edition (accessed on 22 March 2021).
- Shinn, D.H. The Environmental Imact of China’s Investment in Africa. Cornell Int. Law J. 2016, 49, 25. [Google Scholar]
- Mertens, E.; Peñalvo, J.L. The burden of malnutrition and fatal COVID-19: A global burden of disease analysis. Front. Nutr. 2021, 7, 351. [Google Scholar] [CrossRef]
- De Onis, M.; Blössner, M. The World Health Organization Global Database on Child Growth and Malnutrition: Methodology and applications. Int. J. Epidemiol. 2003, 32, 518–526. [Google Scholar] [CrossRef] [Green Version]
- WFP. Global Report on Food Crises. 2020. Available online: https://www.wfp.org/publications/2020-global-report-food-crises (accessed on 22 March 2021).
- Available online: https://www.globalhungerindex.org/results.html (accessed on 22 March 2021).
- FAO. 2020. Available online: http://www.fao.org/3/ca9692en/online/ca9692en.html#chapter-1_1 (accessed on 22 March 2021).
- African Economic Research Consortium. Agriculture and Food Policies for Better Nutrition Outcomes in Africa. 2021. Available online: http://publication.aercafricalibrary.org/xmlui/bitstream/handle/123456789/1414/SPS%20XXII%20February%2019%202021.pdf?sequence=1 (accessed on 22 March 2021).
- Calcaterra, V.; Cena, H.; Verduci, E.; Bosetti, A.; Pelizzo, G.; Zuccotti, G.V. Nutritional Surveillance for the Best Start in Life, Promoting Health for Neonates, Infants and Children. Nutrients 2020, 12, 3386. [Google Scholar] [CrossRef]
- Headey, D.; Heidkamp, R.; Osendarp, S.; Ruel, M.; Scott, N.; Black, R.; Shekar, M.; Bouis, H.; Flory, A.; Haddad, L.; et al. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet 2020, 396, 519–521. [Google Scholar] [CrossRef]
- Ravaoarisoa, L.; Raherimandimby, H.; Rakotonirina, J.; Rakotomanga, J.D.D.M.; Dramaix, M.W.; Donnen, P. Mothers’ dietary practices in the Amoron’i Mania region Madagascar. Pan Afr. Med. J. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Global Nutrition Report. Country Nutrition Profile. 2020. Available online: https://globalnutritionreport.org/resources/nutrition-profiles/africa/eastern-africa/madagascar/ (accessed on 22 March 2021).
- Rakotosamimanana, V.R.; Arvisenet, G.; Valentin, D. Studying the nutritional beliefs and food practices of Malagasy school children parents. A contribution to the understanding of malnutrition in Madagascar. Appetite 2014, 81, 67–75. [Google Scholar] [CrossRef]
- Randrianarison, N.; Nischalke, S.; Andriamazaoro, H. The role of biodiversity and natural resource management in food security in south-eastern Madagascar. In International Symposium on Survey of Uses of Plant Genetic Resources to the Benefit of Local Populations 1267; ISHS: Leuven, Belgium, 2017; pp. 267–274. [Google Scholar]
- Ravaoarisoa, L.; Rakotonirina, J.; Andriamiandrisoa, D.; Humblet, P.; Rakotomanga, J.D.D.M. Women’s dietary habits during pregnancy and breastfeeding in Amoron’i Mania region, Madagascar: A qualitative study. Pan Afr. Med. J. 2018, 29, 194. [Google Scholar] [PubMed]
- WHO. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 22 March 2021).
- Reuter, K.E.; Randell, H.; Wills, A.R.; Sewall, B.J. The consumption of wild meat in Madagascar: Drivers, popularity and food security. Environ. Conserv. 2016, 43, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Klug, T. Understanding the Impacts of Traditional Cooking Practices in Rural Madagascar and a Way Forward with Improved Cookstoves. Ph.D. Thesis, Sanford School of Public Policy, Durham, NC, USA, 2018. [Google Scholar]
- FIXING FOOD. Barilla Center and The Economist. 2018. Available online: https://foodsustainability.eiu.com/whitepaper-2018/ (accessed on 22 March 2021).
- European Commission. Novel Food. Available online: https://ec.europa.eu/food/safety/novel_food_en (accessed on 22 March 2021).
- Clugston, G.A.; Smith, T.E. Global nutrition problems and novel foods. Asia Pac. J. Clin. Nutr. 2002, 11, S100–S111. [Google Scholar] [CrossRef] [Green Version]
- Vergara-Jimenez, M.; AlMatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padayachee, B.; Baijnath, H. An overview of the medicinal importance of Moringaceae. J. Med. Plants Res. 2012, 6, 5831–5839. [Google Scholar]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, J.L.; Anderson, B.G.; Casamatta, D.A. Potential uses of Moringa oleifera and an examination of antibiotic efficacy conferred by M. oleifera seed and leaf extracts using crude extraction techniques available to underserved indigenous populations. Int. J. Phytother. Res. 2013, 3, 61–71. [Google Scholar]
- Marciales, K.P.M.; Soto, J.A.; Castrillo, J.S.; Moncada, J.G.O.; Arias, J.C.G.; Rave, L.J.G. Effect of the addition of Moringa oleifera to fruit drinks on clinical parameters associated with iron deficiency anaemia in schoolchildren. Arch. Latinoam. Nutr. 2019, 69. Available online: https://www.alanrevista.org/ediciones/2019/1/art-2/ (accessed on 22 March 2021).
- Shija, A.E.; Rumisha, S.F.; Oriyo, N.M.; Kilima, S.P.; Massaga, J.J. Effect of Moringa Oleifera leaf powder sup-plementation on reducing anemia in children below two years in Kisarawe District, Tanzania. Food Sci. Nutr. 2019, 7, 2584–2594. [Google Scholar] [CrossRef] [Green Version]
- Boateng, L.; Quarpong, W.; Ohemeng, A.; Asante, M.; Steiner-Asiedu, M. Effect of complementary foods fortified with Moringa oleifera leaf powder on hemoglobin concentration and growth of infants in the Eastern Region of Ghana. Food Sci. Nutr. 2018, 7, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Glover-Amengor, M.; Aryeetey, R.; Afari, E.; Nyarko, A. Micronutrient composition and acceptability of Moringa oleifera leaf-fortified dishes by children in Ada-East district, Ghana. Food Sci. Nutr. 2017, 5, 317–323. [Google Scholar] [CrossRef]
- Nur, R.; Demak, I.P.K.; Radhiah, S.; Rusydi, M.; Mantao, E.; Larasati, R.D. The effect of moringa leaf extracton in-creasing hemoglobin and bodyweight in post-disaster pregnant women. Enferm. Clin. 2020, 30, 79–82. [Google Scholar] [CrossRef]
- Idohou-Dossou, N.; Diouf, A.; Gueye, A.; Guiro, A.; Wade, S. Impact of daily consumption of Moringa (Moringa oleifera) dry leaf powder on iron status of Senegalese lactating women. Afr. J. Food Agric. Nutr. Dev. 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Suzana, D.; Suyatna, F.D.; Azizahwati; Andrajati, R.; Sari, S.P.; Mun’Im, A. Effect of Moringa oleifera Leaves Extract Against Hematology and Blood Biochemical Value of Patients with Iron Deficiency Anemia. J. Young Pharm. 2017, 9, s79–s84. [Google Scholar] [CrossRef] [Green Version]
- Anisa, N.; Wahyuni, S.; Rahayu, S.; Choirunnisa, A.; Martanti, L.E. Effect of Moringa Leaves and Vitamin C Capsule Combinations in Increaseing Hemoglobin Levels of Young Women with Anemia. In Proceedings of the International Conference on Applied Science and Health, Nakhon Pathom, Thailand, 23–24 July 2019; pp. 565–570. [Google Scholar]
- Ahmad, J.; Khan, I.; Blundell, R. Moringa oleifera and glycemic control: A review of current evidence and possible mechanisms. Phytother. Res. 2019, 33, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Sánchez, K.; Garay-Jaramillo, E.; González-Reyes, R.E. Effects of Moringa oleifera on Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.P.; Singh, P.; Singh, S. Processing of Moringa oleifera leaves for human consumption. Bull. Environ. Pharmacol. Life Sci. 2012, 2, 28–31. [Google Scholar]
- Gallaher, D.D.; Gallaher, C.M.; Natukunda, S.; Schoenfuss, T.C.; Mupere, E.; Cusick, S.E. Iron bioavailability from Moringa oleifera leaves is very low. FASEB J. 2017, 31, 13. [Google Scholar]
- Ijarotimi, O.S.; Adeoti, O.A.; Ariyo, O. Comparative study on nutrient composition, phytochemical, and functional characteristics of raw, germinated, and fermented Moringa oleifera seed flour. Food Sci. Nutr. 2013, 1, 452–463. [Google Scholar] [CrossRef]
- Yang, R.Y.; Chang, L.C.; Hsu, J.C.; Weng, B.B.; Palada, M.C.; Chadha, M.L.; Levasseur, V. Nutritional and functional properties of Moringa leaves–From germplasm, to plant, to food, to health. In Moringa Leaves: Strategies, Standards and Markets for a Better Impact on Nutrition in Africa; MoringaNews; CDE; CTA; GFU: Paris, France, 2006. [Google Scholar]
- Sallau, A.B.; Mada, S.B.; Ibrahim, S.; Ibrahim, U. Effect of boiling, simmering, and blanching on the antinutritional content of Moringa oleifera leaves. Int. J. Food Nutr. Saf. 2012, 2, 1–6. [Google Scholar]
- Rakotosamimanana, V.R.; Valentin, D.; Arvisenet, G. How to use local resources to fight malnutrition in Madagascar? A study combining a survey and a consumer test. Appetite 2015, 95, 533–543. [Google Scholar] [CrossRef]
- Ali, M.A.; Yusof, Y.A.; Chin, N.L.; Ibrahim, M.N. Processing of Moringa leaves as natural source of nutrients by optimization of drying and grinding mechanism. J. Food Process. Eng. 2017, 40, e12583. [Google Scholar] [CrossRef]
- Cena, H.; Oggioni, C. Tasters, Supertasters, Genes and Environment: How Dietary Choices Influence Our Health. In Human Nutrition from the Gastroenterologist’s Perspective; Springer: Berlin/Heidelberg, Germany, 2016; pp. 123–138. [Google Scholar]
- Magalhães, E.R.B.; De Menezes, N.N.F.; Silva, F.L.; Garrido, J.W.A.; Sousa, M.A.D.S.B.; Dos Santos, E.S. Effect of oil extraction on the composition, structure, and coagulant effect of Moringa oleifera seeds. J. Clean. Prod. 2021, 279, 123902. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Technical Report on the Notification of Leaf Powder of Moringa Stenopetala as a Traditional Food from a Third Country Pursuant to Article 14 of Regulation (EU) 2015/2283; EFSA: Parma, Italy, 2019; Volume 16, p. 1672E. [CrossRef] [Green Version]
- Pal, P.; Roy, S. Edible Insects: Future of Human Food—A Review. Int. Lett. Nat. Sci. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Costa-Neto, E.; Dunkel, F. Insects as food: History, culture, and modern use around the world. In Insects as Sustainable Food Ingredients; Academic Press: Cambridge, MA, USA, 2016; pp. 29–60. [Google Scholar]
- Kelemu, S.; Niassy, S.; Torto, B.; Fiaboe, K.K.M.; Affognon, H.; Tonnang, H.E.Z.; Maniania, N.; Ekesi, S. African edible insects for food and feed: Inventory, diversity, commonalities and contribution to food security. J. Insects Food Feed. 2015, 1, 103–119. [Google Scholar] [CrossRef] [Green Version]
- Nesic, K.; Zagon, J. Insects—A promising feed and food protein source? Sci. J. Meat Technol. 2019, 60, 56–67. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Blank, S.; Verhoeckx, K.; Mueller, R.S.; Janda, J.; Marti, E.; Seida, A.A.; Rhyner, C.; DeBoer, D.J.; Jensen-Jarolim, E. EAACI position paper: Comparing insect hypersensitivity induced by bite, sting, inhalation or ingestion in human beings and animals. Allergy 2019, 74, 874–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunkel, F.; Payne, C. Introduction to edible insects. In Insects as Sustainable Food Ingredients; Academic Press: Cambridge, MA, USA, 2016; pp. 1–27. [Google Scholar]
- Govorushko, S. Economic and ecological importance of termites: A global review. Èntomol. Sci. 2019, 22, 21–35. [Google Scholar] [CrossRef]
- Barré, A.; Caze-Subra, S.; Gironde, C.; Bienvenu, F.; Bienvenu, J.; Rouge, P. Entomophagie et risque allergique. Rev. Franç. d’Allergol. 2014, 54, 315–321. [Google Scholar] [CrossRef]
- Van Mele, P. A historical review of research on the weaver ant Oecophylla in biological control. Agric. For. Èntomol. 2007, 10, 13–22. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed pro-duction. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Verkerk, M.; Tramper, J.; van Trijp, J.; Martens, D. Insect cells for human food. Biotechnol. Adv. 2007, 25, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M. Insects as feed and the Sustainable Development Goals. J. Insects Food Feed. 2018, 4, 147–156. [Google Scholar] [CrossRef]
- Vantomme, P.; Göhler, D.; N’Deckere-Ziangba, F. Contribution of forest insects to food security and forest con-servation: The example of caterpillars in Central Africa. ODI Wildl. Policy Brief. 2004, 3. Available online: https://cdn.odi.org/media/documents/3306.pdf (accessed on 22 March 2021).
- Akhtar, Y.; Isman, M. Insects as an alternative protein source. In Proteins in Food Processing; Woodhead Publishing: Cambridge, UK, 2018; pp. 263–288. [Google Scholar]
- Van Huis, A. Nutrition and health of edible insects. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Anankware, P.J.; Fening, K.O.; Osekre, E.; Obeng-Ofori, D. Insects as food and feed: A review. Int. J. Agric. Res. Rev. 2015, 3, 143–151. [Google Scholar]
- Lee, K.P.; Simpson, S.J.; Wilson, K. Dietary protein-quality influences melanization and immune function in an insect. Funct. Ecol. 2008, 22, 1052–1061. [Google Scholar] [CrossRef]
- Ramos-Rostro, B.; Ramos-Elorduy Blásquez, J.; Pino-Moreno, J.M.; Viesca-González, F.C.; Martínez-Maya, J.J.; Sierra-Gómez Pedroso, L.D.C.; Quintero-Salazar, B. Calidad sanitaria de alimentos elaborados con gusano rojo de agave (Comadia redtembacheri H.) en San Juan Teotihuacán, Estado de México, México. Agrociencia 2016, 50, 391–402. [Google Scholar]
- Randrianandrasana, M.; Berenbaum, M.R. Edible Non-Crustacean Arthropods in Rural Communities of Madagascar. J. Ethnobiol. 2015, 35, 354–383. [Google Scholar] [CrossRef] [Green Version]
- Dürr, J.; Andriamazaoro, H.; Nischalke, S.; Preteseille, N.; Rabenjanahary, A.; Randrianarison, N.; Ratompoarison, C.; Razafindrakotomamonjy, A.; Straub, P.; Wagler, I. “It is edible, so we eat it”: Insect supply and consumption in the central highlands of Madagascar. Int. J. Trop. Insect Sci. 2020, 40, 167–179. [Google Scholar] [CrossRef]
- Van Itterbeeck, J.; Andrianavalona, I.N.R.; Rajemison, F.I.; Rakotondrasoa, J.F.; Ralantoarinaivo, V.R.; Hugel, S.; Fisher, B.L. Diversity and Use of Edible Grasshoppers, Locusts, Crickets, and Katydids (Orthoptera) in Madagascar. Foods 2019, 8, 666. [Google Scholar] [CrossRef] [Green Version]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1700030. [Google Scholar] [CrossRef] [PubMed]
- Pambo, K.O.; Okello, J.J.; Mbeche, R.M.; Kinyuru, J.N.; Alemu, M.H. The role of product information on consumer sensory evaluation, expectations, experiences and emotions of cricket-flour-containing buns. Food Res. Int. 2018, 106, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.; Richardson, C.D.; McSweeney, M.B. Consumer attitudes toward entomophagy before and after evalating cricket (Acheta domesticus)-based protein powders. J. Food Sci. 2020, 85, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Aquilanti, L. Bread eriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Pauter, P.; Różańska, M.; Wiza, P.; Dworczak, S.; Grobelna, N.; Sarbak, P.; Kowalczewski, P.Ł. Effects of the re-placement of wheat flour with cricket powder on the characteristics of muffins. Acta Sci. Pol. Technol. Aliment. 2018, 17. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, E.M.; Chang, Y.J.; Ahn, M.Y.; Lee, Y.H.; Park, J.J.; Lim, J.H. Determination of the shelf life of cricket powder and effects of storage on its quality characteristics. Korean J. Food Preserv. 2016, 23, 211–217. [Google Scholar] [CrossRef]
- Mishyna, M.; Glumac, M. So different, yet so alike Pancrustacea: Health benefits of insects and shrimps. J. Funct. Foods 2021, 76, 104316. [Google Scholar] [CrossRef]
- Kewuyemi, Y.O.; Kesa, H.; Chinma, C.E.; Adebo, O.A. Fermented Edible Insects for Promoting Food Security in Africa. Insects 2020, 11, 283. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Binder, R.; Moens, Y.; Polesny, F.; Monsó, S. Edible insects—Defining knowledge gaps in biological and ethical considerations of entomophagy. Crit. Rev. Food Sci. Nutr. 2018, 59, 2760–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlongwane, Z.T.; Slotow, R.; Munyai, T.C. Nutritional Composition of Edible Insects Consumed in Africa: A Systematic Review. Nutrients 2020, 12, 2786. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, M.V.; Kalmpourtzidou, A.; Lambiase, S.; De Giuseppe, R.; Cena, H. Novel Foods and Sustainability as Means to Counteract Malnutrition in Madagascar. Molecules 2021, 26, 2142. https://doi.org/10.3390/molecules26082142
Conti MV, Kalmpourtzidou A, Lambiase S, De Giuseppe R, Cena H. Novel Foods and Sustainability as Means to Counteract Malnutrition in Madagascar. Molecules. 2021; 26(8):2142. https://doi.org/10.3390/molecules26082142
Chicago/Turabian StyleConti, Maria Vittoria, Aliki Kalmpourtzidou, Simonetta Lambiase, Rachele De Giuseppe, and Hellas Cena. 2021. "Novel Foods and Sustainability as Means to Counteract Malnutrition in Madagascar" Molecules 26, no. 8: 2142. https://doi.org/10.3390/molecules26082142
APA StyleConti, M. V., Kalmpourtzidou, A., Lambiase, S., De Giuseppe, R., & Cena, H. (2021). Novel Foods and Sustainability as Means to Counteract Malnutrition in Madagascar. Molecules, 26(8), 2142. https://doi.org/10.3390/molecules26082142







