Effect of Anode Material on Electrochemical Oxidation of Low Molecular Weight Alcohols—A Review
Abstract
1. Introduction
- Structure of anodic material that describes the influence of morphology and structure of anodic materials on its properties;
- Electrooxidation of alcohols is further divided into four subsections—methanol, ethanol, ethylene glycol and propanols. They contain a description of the sources of each fuel, mechanism of its oxidation, most popular materials used for its oxidation and direction of development necessary for the commercialization of fuel cells based on each alcohol;
- Comparison of discussed alcohols that shows advantages and disadvantages of each alcohol as a fuel.
2. Structure of Anodic Material
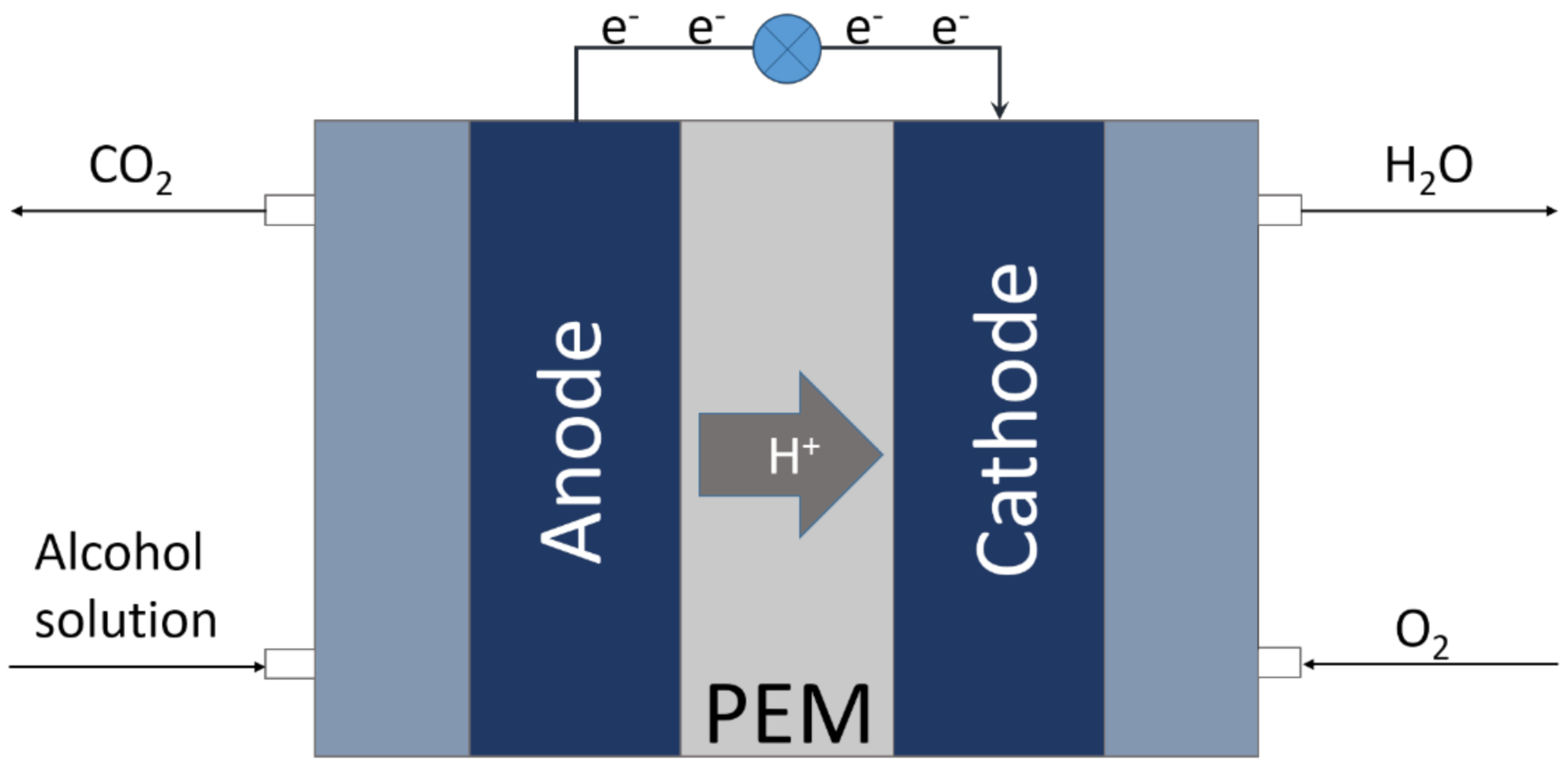
3. Electrooxidation of Alcohols
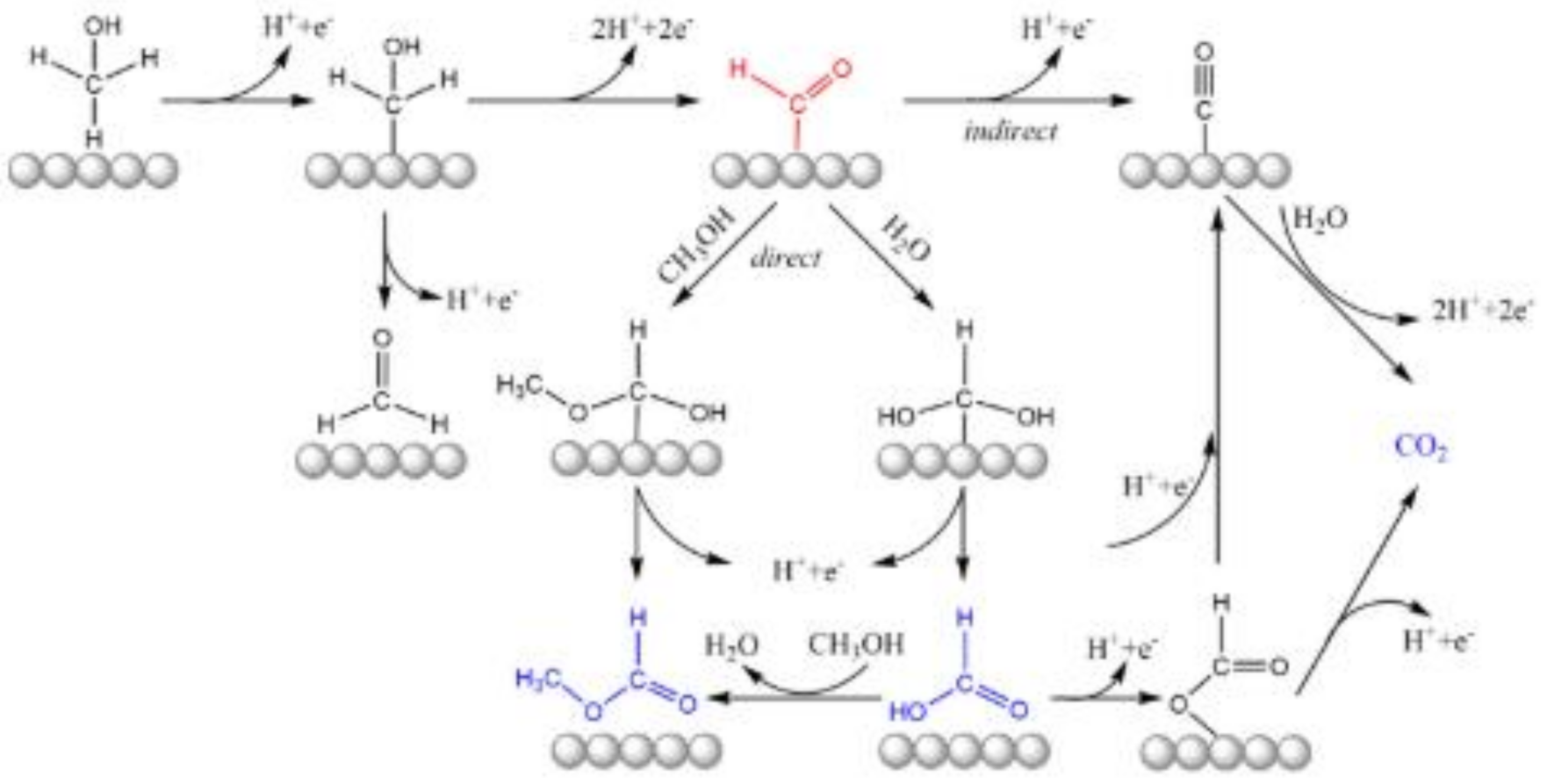
3.1. Methanol Oxidation
- Stepwise dehydrogenation to adsorbed CO and subsequent oxidation to CO2;
- Reaction along parallel, “direct” paths to CO2;
- Partial oxidation to formic acid and/or formaldehyde.
3.2. Ethanol Oxidation
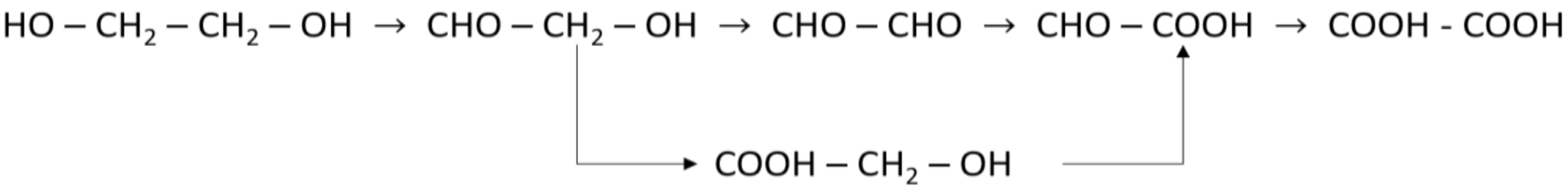
3.3. Ethylene Glycol Oxidation
3.4. Propanols
4. Comparisons of Alcohols Oxidation
5. Conclusions
- Methanol is considered the most likely fuel for industrial-scale fuel cells because it is the smallest alcohol, and its oxidation leads to carbon dioxide and water;
- It can be oxidized in both acidic and alkaline environments on platinum-based electrodes, mainly with the addition of ruthenium;
- The main problem with this kind of electrode material is that it can easily be poisoned with intermediate products and low reaction kinetics. If we also consider platinum shortages and their consequent high prices, it becomes clear that other electrocatalytic materials must be developed;
- Nickel- and cobalt-based materials have the greatest chance of replacing platinum-based electrodes because of their low price, high activity and immunity to poisoning with carbon oxide intermediates;
- Problems exist during methanol electrooxidation in addition to those associated with the electrode materials. Because of this particle’s small size, methanol can crossover the membrane, separating the anodic and cathodic parts of the fuel cell, which results in lower efficiency of the whole system.
- Ethanol, which has only one more carbon atom than methanol, is an obvious candidate for this role;
- Ethanol can also be oxidized in both acidic and alkaline environments, mainly on platinum catalysts, but these catalysts are doped with tin;
- The oxidation of ethanol is more complicated than that of methanol because it requires the breaking of strong, inter-carbon bonds—the same feature that gives ethanol its stability and makes it an interesting fuel is the main cause of problems during its oxidation. Additionally, in this case, catalyst poisoning can deactivate the electrodes;
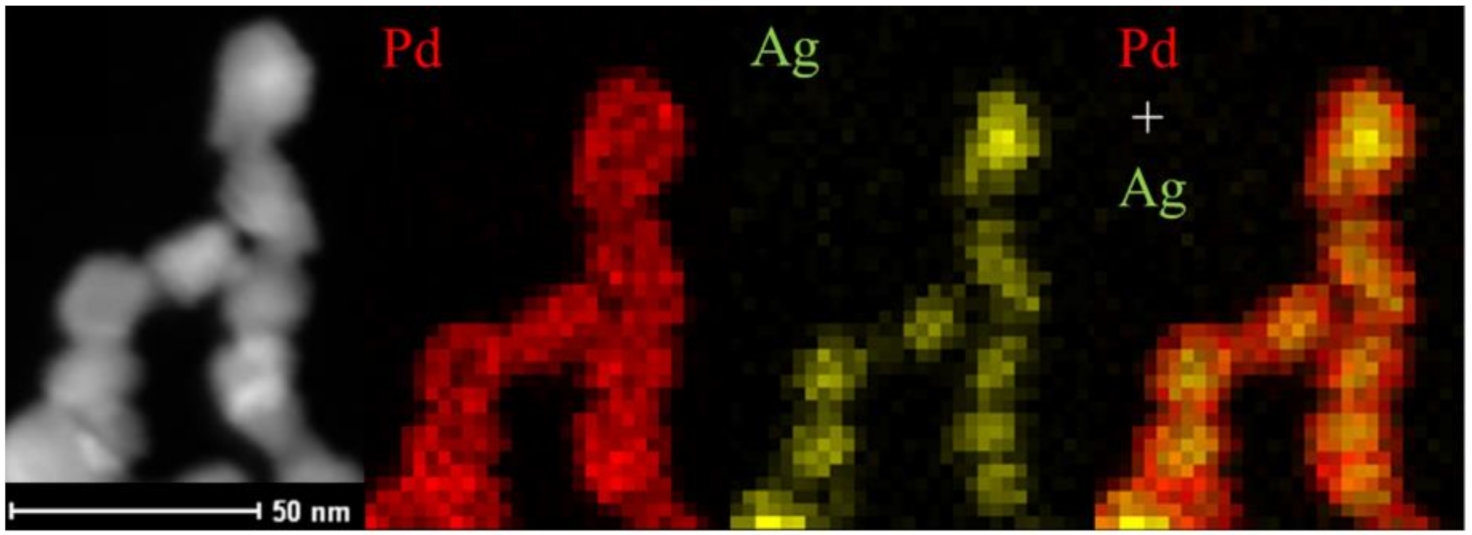
- Other materials have been developed—palladium-based electrodes doped with oxophilic elements, such as copper, silver or nickel, have yielded very interesting results;
- Because C–C bonds are so hard to break for larger alcohol molecules—such as ethylene glycol (the smallest diol) and isopropanol (the smallest secondary alcohols)—different approaches have been taken. The main goal is not their full oxidation to carbon dioxide but to valuable intermediates;
- The products of ethylene glycol oxidation, such as glycolates and formates, can be marketed as substrates for other processes;
- Isopropanol oxidation, which leads to the formation of acetone, can be coupled with its hydrogenation and thus can play the role of a liquid hydrogen carrier;
- For both alcohols mentioned in point 3, electricity production can take place without carbon dioxide emissions, and thus, it can be more environmentally friendly than previously described systems. Such reactions require selective catalysts that guarantee that only the desired products are obtained;
- For both, this effect is observed for palladium-based electrodes doped with oxophilic elements, such as gold, copper or nickel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| List of used symbols | |
| CNC | Carbon nanocages |
| CNT | Carbon nanotubes |
| COads | Adsorbed carbon oxide intermediates |
| DAFC | Direct alcohol fuel cell |
| DMFC | Direct methanol fuel cell |
| ECSA | Electrochemically active surface |
| EGr | Exfoliated graphite |
| EG | Ethylene glycol |
| EGOR | Ethylene glycol oxidation reaction |
| ESA | Electrode surface area |
| EOR | Ethanol oxidation reaction |
| GC | Glassy carbon |
| GNS | Graphene nanosheets |
| MOR | Methanol oxidation reaction |
| MWCNT | Multi-walled carbon nanotubes |
| OHads | Adsorbed hydroxide ions |
| OCP | open-circuit potential |
| PEM | Proton-exchange membrane |
| PEMFC | Proton-exchange membrane fuel cell |
| rGO | Reduced graphene oxide |
| RHE | Reversible hydrogen electrode |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
References
- Çelebi, Y.; Aydın, H. An overview on the light alcohol fuels in diesel engines. Fuel 2019, 236, 890–911. [Google Scholar] [CrossRef]
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2012; ISBN 9783527303854. [Google Scholar]
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Sheldon, D. Methanol production-A technical history. Johnson Matthey Technol. Rev. 2017, 61, 172–182. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Yoon, S.H.; Liu, P.H.; Chai, X.S. Methanol formation during alkaline wood pulping. TAPPI J. 2000, 83, 65. [Google Scholar]
- Sequeira, C.A.C.; Cardoso, D.S.P.; Martins, M.; Amaral, L. Novel materials for fuel cells operating on liquid fuels. AIMS Energy 2017, 5, 458–481. [Google Scholar] [CrossRef]
- Lamy, C.; Belgsir, E.M.; Léger, J.M. Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC). J. Appl. Electrochem. 2001, 31, 799–809. [Google Scholar] [CrossRef]
- Leo, T.J.; Raso, M.A.; Navarro, E.; Sánchez-De-La-Blanca, E. Comparative exergy analysis of direct alcohol fuel cells using fuel mixtures. J. Power Sources 2011, 196, 1178–1183. [Google Scholar] [CrossRef]
- Zhao, T.S.; Yang, W.W. Fuel Cells-Direct Alcohol Fuel Cells | Modeling. Encycl. Electrochem. Power Sources 2009, 436–445. [Google Scholar] [CrossRef]
- Mandikarappa Subramani, S.; Gantigiah, K. Deposition of cobalt nanoparticles on reduced graphene oxide and the electrocatalytic activity for methanol and ethanol oxidation. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Dong, S. PdM (M = Pt, Au) bimetallic alloy nanowires with enhanced electrocatalytic activity for electro-oxidation of small molecules. Adv. Mater. 2012, 24, 2326–2331. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, Z.; Xie, J. Core/shell Ni@Pd nanoparticles supported on MWCNTs at improved electrocatalytic performance for alcohol oxidation in alkaline media. Electrochim. Acta 2012, 77, 237–243. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.; Yang, J.; Chen, Y. Nanocatalysts for Electrocatalytic Oxidation of Ethanol. ChemSusChem 2019, 12, 2117–2132. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.H.; Eriksson, S.G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells-A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, L.; Shao, Q.; Huang, X. Structurally Ordered Pt3Sn Nanofibers with Highlighted Antipoisoning Property as Efficient Ethanol Oxidation Electrocatalysts. ACS Catal. 2020, 10, 3455–3461. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, S.M.; Nam, S.H.; Seo, M.H.; Choi, S.H.; Kim, W.B. Influence of Sn content on PtSn/C catalysts for electrooxidation of C1-C3 alcohols: Synthesis, characterization, and electrocatalytic activity. Appl. Catal. B Environ. 2008, 82, 89–102. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Motlak, M. CoxNiy-decorated graphene as novel, stable and super effective non-precious electro-catalyst for methanol oxidation. Appl. Catal. B Environ 2014, 154–155, 221–231. [Google Scholar] [CrossRef]
- Basumatary, P.; Konwar, D.; Yoon, Y.S. A novel Ni–Cu/ZnO@MWCNT anode employed in urea fuel cell to attain superior performances. Electrochim. Acta 2018, 261, 78–85. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, Y.; Chen, D.; Fan, Y.; Wang, X.; Wang, W.; Tian, J. One-pot synthesis of reduced graphene oxide supported PtCuy catalysts with enhanced electro-catalytic activity for the methanol oxidation reaction. Electrochim. Acta 2014, 136, 292–300. [Google Scholar] [CrossRef]
- Xiao, Y.; Fu, Z.; Zhan, G.; Pan, Z.; Xiao, C.; Wu, S.; Chen, C.; Hu, G.; Wei, Z. Increasing Pt methanol oxidation reaction activity and durability with a titanium molybdenum nitride catalyst support. J. Power Sources 2015, 273, 33–40. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, M.; Ma, L.; Liang, L.; Liu, C.; Liao, J.; Lu, T.; Xing, W. Recent advances in catalysts for direct methanol fuel cells. Energy Environ. Sci. 2011, 4, 2736–2753. [Google Scholar] [CrossRef]
- López-Coronel, A.; Torres-Pacheco, L.J.; Bañuelos, J.A.; Álvarez-López, A.; Guerra-Balcázar, M.; Álvarez-Contreras, L.; Arjona, N. Highly active PdNi bimetallic nanocubes electrocalysts for the ethylene glycol electro-oxidation in alkaline medium. Appl. Surf. Sci. 2020, 530, 147210. [Google Scholar] [CrossRef]
- Karuppasamy, L.; Lee, G.J.; Anandan, S.; Wu, J.J. Synthesis of shape-controlled Pd nanocrystals on carbon nanospheres and electrocatalytic oxidation performance for ethanol and ethylene glycol. Appl. Surf. Sci. 2020, 519, 146266. [Google Scholar] [CrossRef]
- Zhao, M.; Lyu, Z.; Xie, M.; Hood, Z.D.; Cao, Z.; Chi, M.; Xia, Y. Pd-Ru Alloy Nanocages with a Face-Centered Cubic Structure and Their Enhanced Activity toward the Oxidation of Ethylene Glycol and Glycerol. Small Methods 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Kehoe, D.K.; Romeral, L.; Lundy, R.; Morris, M.A.; Lyons, M.G.; Gun’ko, Y.K. One Dimensional AuAg Nanostructures as Anodic Catalysts in the Ethylene Glycol Oxidation. Nanomaterials 2020, 10. [Google Scholar] [CrossRef]
- You, H.; Gao, F.; Song, T.; Zhang, Y.; Wang, H.; Liu, X.; Yuan, M.; Wang, Y.; Du, Y. Tunable long-chains of core@shell PdAg@Pd as high-performance catalysts for ethanol oxidation. J. Colloid Interface Sci. 2020, 574, 182–189. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Liu, Y.; Jia, C.; Yang, P. Ternary PtNiCu self-assembled nanocubes for plasmon-enhanced electrocatalytic hydrogen evolution and methanol oxidation reaction in visible light. Electrochim. Acta 2020, 349, 136366. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Y.R.; Wang, A.J.; Feng, J.J. Simple wet-chemical synthesis of core-shell Au-Pd@Pd nanocrystals and their improved electrocatalytic activity for ethylene glycol oxidation reaction. Int. J. Hydrogen Energy 2016, 41, 2547–2553. [Google Scholar] [CrossRef]
- Zheng, J.N.; Li, S.S.; Ma, X.; Chen, F.Y.; Wang, A.J.; Chen, J.R.; Feng, J.J. Green synthesis of core-shell gold-palladium@palladium nanocrystals dispersed on graphene with enhanced catalytic activity toward oxygen reduction and methanol oxidation in alkaline media. J. Power Sources 2014, 262, 270–278. [Google Scholar] [CrossRef]
- Chen, S.S.; Yang, Z.Z.; Wang, A.J.; Fang, K.M.; Feng, J.J. Facile synthesis of bimetallic gold-palladium nanocrystals as effective and durable advanced catalysts for improved electrocatalytic performances of ethylene glycol and glycerol oxidation. J. Colloid Interface Sci. 2018, 509, 10–17. [Google Scholar] [CrossRef]
- Weng, X.; Liu, Q.; Feng, J.J.; Yuan, J.; Wang, A.J. Dendrite-like PtAg alloyed nanocrystals: Highly active and durable advanced electrocatalysts for oxygen reduction and ethylene glycol oxidation reactions. J. Colloid Interface Sci. 2017, 504, 680–687. [Google Scholar] [CrossRef]
- Cai, J.; Zeng, Y.; Guo, Y. Copper@palladium-copper core-shell nanospheres as a highly effective electrocatalyst for ethanol electro-oxidation in alkaline media. J. Power Sources 2014, 270, 257–261. [Google Scholar] [CrossRef]
- Fashedemi, O.O.; Miller, H.A.; Marchionni, A.; Vizza, F.; Ozoemena, K.I. Electro-oxidation of ethylene glycol and glycerol at palladium-decorated FeCo@Fe core-shell nanocatalysts for alkaline direct alcohol fuel cells: Functionalized MWCNT supports and impact on product selectivity. J. Mater. Chem. A 2015, 3, 7145–7156. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Lim, K.L.; Wong, W.Y.; Kamarudin, S.K.; Nazirah Kamarudin, N.H. Recent progress of anode catalysts and their support materials for methanol electrooxidation reaction. Int. J. Hydrogen Energy 2019, 44, 14744–14769. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, S. Investigation of methanol electrooxidation on Au/C catalyst in alkaline medium. Int. J. Hydrogen Energy 2011, 36, 13392–13397. [Google Scholar] [CrossRef]
- Sun, R.; Ren, F.; Wang, D.; Yao, Y.; Fei, Z.; Wang, H.; Liu, Z.; Xing, R.; Du, Y. Polydopamine functionalized multi-walled carbon nanotubes supported PdAu nanoparticles as advanced catalysts for ethylene glycol oxidation. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 578, 123566. [Google Scholar] [CrossRef]
- Roca-Ayats, M.; García, G.; Peña, M.A.; Martínez-Huerta, M.V. Titanium carbide and carbonitride electrocatalyst supports: Modifying Pt-Ti interface properties by electrochemical potential cycling. J. Mater. Chem. A 2014, 2, 18786–18790. [Google Scholar] [CrossRef]
- Roca-Ayats, M.; Yeung, K.L.; Hernández-Caricol, M.; Chen, W.Y.; Deng, R.; Fierro, J.L.G.; Lázaro, M.J.; Martínez-Huerta, M.V. Titanium carbonitride–graphene composites assembled with organic linkers as electrocatalytic supports for methanol oxidation reaction. Catal. Today 2019, 101–109. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Ding, K.; Wu, P.; Abbas, S.C.; Ghausi, M.A.; Zhang, T.; Wang, Y. Newly designed PdRuBi/N-Graphene catalysts with synergistic effects for enhanced ethylene glycol electro-oxidation. Electrochim. Acta 2016, 191, 940–945. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Hua, X.; Zhang, R. Methanol electrooxidation on glassy carbon electrode modified with bimetallic Ni(II)Co(II)salen complexes encapsulated in mesoporous zeolite A. Electrochim. Acta 2015, 163, 48–56. [Google Scholar] [CrossRef]
- Rostami, T.; Jafarian, M.; Miandari, S.; Mahjani, M.G.; Gobal, F. Synergistic effect of cobalt and copper on a nickel-based modified graphite electrode during methanol electro-oxidation in NaOH solution. Cuihua Xuebao/Chin. J. Catal 2015, 36, 1867–1874. [Google Scholar] [CrossRef]
- Gao, S.; Yang, X.; Liang, S.; Wang, Y.H.; Zang, H.Y.; Li, Y.G. One step synthesis of PtNi electrocatalyst for methanol oxidation. Inorg. Chem. Commun. 2019, 106, 104–110. [Google Scholar] [CrossRef]
- Tran, M.H.; Park, B.J.; Kim, B.H.; Yoon, H.H. Mesoporous silica template-derived nickel-cobalt bimetallic catalyst for urea oxidation and its application in a direct urea/H2O2 fuel cell. Int. J. Hydrogen Energy 2019, 45, 1784–1792. [Google Scholar] [CrossRef]
- Rutkowska, I.A.; Marks, D.; Perruchot, C.; Jouini, M.; Kulesza, P.J. Admixing palladium nanoparticles with tungsten oxide nanorods toward more efficient electrocatalytic oxidation of formic acid. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 439, 200–206. [Google Scholar] [CrossRef]
- Wang, F.; Xue, H.; Tian, Z.; Xing, W.; Feng, L. Fe2P as a novel efficient catalyst promoter in Pd/C system for formic acid electro-oxidation in fuel cells reaction. J. Power Sources 2018, 375, 37–42. [Google Scholar] [CrossRef]
- Guo, T.; Xu, X.J.; Wang, X.; Zhou, J.; Wang, H.; Shi, Z.; Huang, M. Enabling the full exposure of Fe2P@NixP heterostructures in tree-branch-like nanoarrays for promoted urea electrolysis at high current densities. Chem. Eng. J. 2020, 128067. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, T.; Yan, X.; Zeng, L.; Xu, J. Ordered Mesoporous Carbon/Titanium Carbide Composites as Support Materials for Platinum Catalysts. Energy Technol. 2016, 4, 1064–1070. [Google Scholar] [CrossRef]
- Abraham, B.G.; Bhaskaran, R.; Chetty, R. Electrodeposited Bimetallic (PtPd, PtRu, PtSn) Catalysts on Titanium Support for Methanol Oxidation in Direct Methanol Fuel Cells. J. Electrochem. Soc. 2020, 167, 024512. [Google Scholar] [CrossRef]
- Chetty, R.; Scott, K. Catalysed titanium mesh electrodes for ethylene glycol fuel cells. J. Appl. Electrochem. 2007, 37, 1077–1084. [Google Scholar] [CrossRef]
- Wu, J.; Xu, M.; Lei, S.; Jin, C. High electrocatalytic activity and stability of PtAg supported on rutile TiO2 for methanol oxidation. Int. J. Hydrogen Energy 2020, 45, 12815–12821. [Google Scholar] [CrossRef]
- Gao, F.; Xu, H.; Zhang, Y.; Wang, J.; Wang, C.; Du, Y. Facile construction of pompon-like PtAg alloy catalysts for enhanced ethylene glycol electrooxidation. Int. J. Hydrogen Energy 2018, 43, 9644–9651. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Tian, J.; Wang, F.; Zhan, L. Methanol electro-oxidation on Ni@Pd core-shell nanoparticles supported on multi-walled carbon nanotubes in alkaline media. Int. J. Hydrogen Energy 2010, 35, 3249–3257. [Google Scholar] [CrossRef]
- Li, Y.; Gao, W.; Ci, L.; Wang, C.; Ajayan, P.M. Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon N. Y. 2010, 48, 1124–1130. [Google Scholar] [CrossRef]
- Mpeta, L.S.; Gwebu, S.S.; Arotiba, O.A.; Maxakato, N.W. Methanol Oxidation in Alkaline Media with Pt-Au/fMWCNTs and Pt-Pd/fMWCNTs Electrocatalysts on an Exfoliated Graphite Electrode. Electrocatalysis 2019, 10, 672–679. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene-Metal particle nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Krawczyk, P.; Rozmanowski, T.; Frankowski, M. Methanol Electrooxidation at Electrodes Made of Exfoliated Graphite/Nickel/Palladium Composite. Catal. Lett. 2019, 149, 2307–2316. [Google Scholar] [CrossRef]
- Maumau, T.R.; Modibedi, R.M.; Mathe, M.K. Electro-oxidation of alcohols using carbon supported gold, palladium catalysts in alkaline media. Mater. Today Proc. 2018, 5, 10542–10550. [Google Scholar] [CrossRef]
- Mahoney, E.G.; Sheng, W.; Cheng, M.; Lee, K.X.; Yan, Y.; Chen, J.G. Analyzing the electrooxidation of ethylene glycol and glucose over platinum-modified gold electrocatalysts in alkaline electrolyte using in-situ infrared spectroscopy. J. Power Sources 2016, 305, 89–96. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Wang, Y.; Jing, W.; Niu, X.; Lei, Z. Achieving high electrocatalytic performance towards isopropanol electrooxidation based on a novel N-doping carbon anchored Pd3Fe alloy. Int. J. Hydrogen Energy 2018, 43, 15952–15961. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K.; Basri, S.; Zainoodin, A.M. The potential of novel carbon nanocages as a carbon support for an enhanced methanol electro-oxidation reaction in a direct methanol fuel cell. Int. J. Energy Res. 2020, 1–16. [Google Scholar] [CrossRef]
- Mari, E.; Tsai, P.C.; Eswaran, M.; Ponnusamy, V.K. Efficient electro-catalytic oxidation of ethylene glycol using flower-like graphitic carbon nitride/iron oxide/palladium nanocomposite for fuel cell application. Fuel 2020, 280, 118646. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K. State-of-the-art review of morphological advancements in graphitic carbon nitride (g-CN) for sustainable hydrogen production. Renew. Sustain. Energy Rev. 2021, 135, 110235. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Kung, H.H. Methanol Porduction and Use; Marcel Dekker, Inc.: New York, NY, USA, 1994; ISBN 0-8247-9223-8. [Google Scholar]
- Olah, G.A. Beyond oil and gas: The methanol economy. Angew. Chem.-Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Richard, A.R.; Fan, M. Rare earth elements: Properties and applications to methanol synthesis catalysis via hydrogenation of carbon oxides. J. Rare Earths 2018, 36, 1127–1135. [Google Scholar] [CrossRef]
- Adekoya, D.; Tahir, M.; Amin, N.A.S. Recent trends in photocatalytic materials for reduction of carbon dioxide to methanol. Renew. Sustain. Energy Rev. 2019, 116, 109389. [Google Scholar] [CrossRef]
- Leonzio, G. State of art and perspectives about the production of methanol, dimethyl ether and syngas by carbon dioxide hydrogenation. J. CO2 Util. 2018, 27, 326–354. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Ni, P.; Zhao, Y.; Li, M.; Li, L. Effects of cetane number improvers on the performance of diesel engine fuelled with methanol/biodiesel blend. Fuel 2014, 128, 180–187. [Google Scholar] [CrossRef]
- Ogunkunle, O.; Ahmed, N.A. A review of global current scenario of biodiesel adoption and combustion in vehicular diesel engines. Energy Rep. 2019, 5, 1560–1579. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Shamsul, N.S.; Ghani, J.A.; Chia, S.K.; Liew, H.S.; Samsudin, A.S. Production of methanol from biomass waste via pyrolysis. Bioresour. Technol. 2013, 129, 463–468. [Google Scholar] [CrossRef]
- Trop, P.; Anicic, B.; Goricanec, D. Production of methanol from a mixture of torrefied biomass and coal. Energy 2014, 77, 125–132. [Google Scholar] [CrossRef]
- Gurau, B.; Smotkin, E.S. Methanol crossover in direct methanol fuel cells: A link between power and energy density. J. Power Sources 2002, 112, 339–352. [Google Scholar] [CrossRef]
- Guaitolini, S.V.M.; Fardin, J.F. Fuel Cells: History (Short Remind), Principles of Operation, Main Features, and Applications. Adv. Renew. Energies Power Technol. 2018, 2, 123–150. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Fadzillah, D.M.; Kamarudin, S.K.; Zainoodin, M.A.; Masdar, M.S. Critical challenges in the system development of direct alcohol fuel cells as portable power supplies: An overview. Int. J. Hydrogen Energy 2019, 44, 3031–3054. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Guaitolini, S.V.M.; Yahyaoui, I.; Fardin, J.F.; Encarnacao, L.F.; Tadeo, F. A review of fuel cell and energy cogeneration technologies. In Proceedings of the 2018 9th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 20–22 March 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Trens, P.; Durand, R.; Coq, B.; Coutanceau, C.; Rousseau, S.; Lamy, C. Poisoning of Pt/C catalysts by CO and its consequences over the kinetics of hydrogen chemisorption. Appl. Catal. B Environ. 2009, 92, 280–284. [Google Scholar] [CrossRef]
- Lai, S.C.S.; Lebedeva, N.P.; Housmans, T.H.M.; Koper, M.T.M. Mechanisms of carbon monoxide and methanol oxidation at single-crystal electrodes. Top. Catal. 2007, 46, 320–333. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Thorat, G.M.; Seo, J.G. Electrochemical growth of Co(OH)2 nanoflakes on Ni foam for methanol electro-oxidation. New J. Chem. 2017, 41, 9546–9553. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Wieckowski, A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media. Phys. Chem. Chem. Phys. 2007, 9, 2654–2675. [Google Scholar] [CrossRef]
- Falbe, J.; Bahrmann, H.; Lipps, W.; Mayer, D.; Frey, G.D. Alcohols, Aliphatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Kaplan, D.; Alon, M.; Burstein, L.; Rosenberg, Y.; Peled, E. Study of core-shell platinum-based catalyst for methanol and ethylene glycol oxidation. J. Power Sources 2011, 196, 1078–1083. [Google Scholar] [CrossRef]
- Yongprapat, S.; Therdthianwong, A.; Therdthianwong, S. Au/C catalysts promoted with metal oxides for ethylene glycol electro-oxidation in alkaline solution. J. Electroanal. Chem. 2013, 697, 46–52. [Google Scholar] [CrossRef]
- Lamy, C.; Lima, A.; LeRhun, V.; Delime, F.; Coutanceau, C.; Léger, J.M. Recent advances in the development of direct alcohol fuel cells (DAFC). J. Power Sources 2002, 105, 283–296. [Google Scholar] [CrossRef]
- Demarconnay, L.; Brimaud, S.; Coutanceau, C.; Léger, J.M. Ethylene glycol electrooxidation in alkaline medium at multi-metallic Pt based catalysts. J. Electroanal. Chem. 2007, 601, 169–180. [Google Scholar] [CrossRef]
- Kosaric, N.; Ducnjak, Z.; Farkas, A.; Sahm, H.; Bringer-Meyer, S.; Goebel, O.; Mayer, D. Ethanol; Wiley-VCH Verlag GmbH &, Co. KGaA: Weinheim, Gemany, 2012; Volume 13, ISBN 9780471238966. [Google Scholar]
- Rebsdat, S.; Mayer, D. Ethylene Glycol in Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Gemany, 2012; ISBN 3527306730. [Google Scholar]
- Liu, Y.; Hu, P.; Wei, M.; Wang, C. Electrocatalytic Study of Ethylene Glycol Oxidation on Pt 3 Sn Alloy Nanoparticles. ChemElectroChem 2019, 6, 1004–1008. [Google Scholar] [CrossRef]
- Klabunde, J.; Bischoff, C.; Papa, A.J. Propanols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Gemany, 2018; pp. 1–14. [Google Scholar] [CrossRef]
- Jusys, Z.; Kaiser, J.; Behm, R.J. Methanol electrooxidation over Pt/C fuel cell. Catalysts: Dependence of product yields on catalyst loading. Langmuir 2003, 19, 6759–6769. [Google Scholar] [CrossRef]
- Zeng, R.; Yang, Y.; Shen, T.; Wang, H.; Xiong, Y.; Zhu, J.; Wang, D.; Abruña, H.D. Methanol Oxidation Using Ternary Ordered Intermetallic Electrocatalysts: A DEMS Study. ACS Catal. 2020, 10, 770–776. [Google Scholar] [CrossRef]
- Wasmus, S.; Wang, J.-T.; Savinell, R.F. Real-Time Mass Spectrometric Investigation of the Methanol Oxidation in a Direct Methanol Fuel Cell. J. Electrochem. Soc. 1995, 142, 3825–3833. [Google Scholar] [CrossRef]
- Iwasita, T. Electrocatalysis of methanol oxidation. Electrochim. Acta 2002, 47, 3663–3674. [Google Scholar] [CrossRef]
- Bonastre, A.M. Catalysts for Alcohol-Fuelled Direct Oxidation Fuel Cells. Platin. Met. Rev. 2013, 57, 297–301. [Google Scholar] [CrossRef]
- Yue, X.; Pu, Y.; Zhang, W.; Zhang, T.; Gao, W. Ultrafine Pt nanoparticles supported on double-shelled C/TiO2 hollow spheres material as highly efficient methanol oxidation catalysts. J. Energy Chem. 2020, 49, 275–282. [Google Scholar] [CrossRef]
- Nassr, A.B.A.A.; Sinev, I.; Grünert, W.; Bron, M. PtNi supported on oxygen functionalized carbon nanotubes: In depth structural characterization and activity for methanol electrooxidation. Appl. Catal. B Environ. 2013, 142–143, 849–860. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, C.; Li, H.; Wang, Z.; Zhang, J.; Wang, X.; Chai, Z. Pt/N-rGO/Nb4N5 Electrocatalyst with Multilayered Structure and Ternary Synergy for Promoting Alcohol Oxidation. J. Alloys Compd. 2020, 845, 156117. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P. Superior catalytic performance of NiCo2O4 nanorods loaded rGO towards methanol electro-oxidation and hydrogen evolution reaction. J. Mol. Liq. 2019, 291, 111306. [Google Scholar] [CrossRef]
- Holade, Y.; Tuleushova, N.; Tingry, S.; Servat, K.; Napporn, T.W.; Guesmi, H.; Cornu, D.; Kokoh, K.B. Recent advances in the electrooxidation of biomass-based organic molecules for energy, chemicals and hydrogen production. Catal. Sci. Technol. 2020, 10, 3071–3112. [Google Scholar] [CrossRef]
- Sieben, J.M.; Duarte, M.M.E. Methanol, ethanol and ethylene glycol electro-oxidation at Pt and Pt-Ru catalysts electrodeposited over oxidized carbon nanotubes. Int. J. Hydrogen Energy 2012, 37, 9941–9947. [Google Scholar] [CrossRef]
- Beden, B.; Kadirgan, F.; Lamy, C.; Leger, J.M. Electrocatalytic oxidation of methanol on platinum-based binary electrodes. J. Electroanal. Chem. 1981, 127, 75–85. [Google Scholar] [CrossRef]
- Hoster, H.; Iwasita, T.; Baumgärtner, H.; Vielstich, W. Pt-Ru model catalysts for anodic methanol oxidation: Influence of structure and composition on the reactivity. Phys. Chem. Chem. Phys. 2001, 3, 337–346. [Google Scholar] [CrossRef]
- Yang, J.-H.; Song, X.; Zhao, X.; Wang, Y.; Yang, Y.; Gao, L. Nickel phosphate materials regulated by doping cobalt for urea and methanol electro-oxidation. Int. J. Hydrogen Energy 2019, 11, 16305–16314. [Google Scholar] [CrossRef]
- Ullah, N.; Xie, M.; Oluigbo, C.J.; Xu, Y.; Xie, J.; Rasheed, H.U.; Zhang, M. Nickel and cobalt in situ grown in 3-dimensional hierarchical porous graphene for effective methanol electro-oxidation reaction. J. Electroanal. Chem. 2019, 838, 7–15. [Google Scholar] [CrossRef]
- Kaplan, D.; Burstein, L.; Rosenberg, Y.; Peled, E. Comparison of methanol and ethylene glycol oxidation by alloy and Core-Shell platinum based catalysts. J. Power Sources 2011, 196, 8286–8292. [Google Scholar] [CrossRef]
- Dinh, H.N.; Ren, X.; Garzon, F.H.; Zelenay, P.; Gottesfeld, S. Electrocatalysis in direct methanol fuel cells: In-situ probing of PtRu anode catalyst surfaces. J. Electroanal. Chem. 2000, 491, 222–233. [Google Scholar] [CrossRef]
- Bauer, J.C.; Chen, X.; Liu, Q.; Phan, T.H.; Schaak, R.E. Converting nanocrystalline metals into alloys and intermetallic compounds for applications in catalysis. J. Mater. Chem. 2008, 18, 275–282. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Holden, R.; Zhang, L.; Zhang, J.; Botton, G.A.; Couillard, M.; Nazar, L.F. Nanocrystalline intermetallics on mesoporous carbon for direct formic acid fuel cell anodes. Nat. Chem. 2010, 2, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Chen, H.; Zhao, M.; Marshall, D.; Yu, Y.; Abruña, H.; Disalvo, F.J. Synthesis of structurally ordered Pt3Ti and Pt3V nanoparticles as methanol oxidation catalysts. J. Am. Chem. Soc. 2014, 136, 10206–10209. [Google Scholar] [CrossRef]
- Maksimuk, S.; Yang, S.; Peng, Z.; Yang, H. Synthesis and characterization of ordered intermetallic PtPb nanorods. J. Am. Chem. Soc. 2007, 129, 8684–8685. [Google Scholar] [CrossRef]
- Wang, W.; Chai, D.; Yang, Y.; Liu, Y.; Kang, Y.; Lei, Z. Fe-Co hybrid oxides promoted Pd electrocatalysts with enhanced catalytic performance for ethylene glycol oxidation. Int. J. Hydrogen Energy 2015, 40, 10041–10048. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Pieta, I.S.; Rutkowska, I.A.; Wadas, A.; Marks, D.; Klak, K.; Stobinski, L.; Cox, J.A. Electrocatalytic oxidation of small organic molecules in acid medium: Enhancement of activity of noble metal nanoparticles and their alloys by supporting or modifying them with metal oxides. Electrochim. Acta 2013, 110, 474–483. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.; Sun, X.; Asiri, A.M. Hierarchical nickel oxide nanosheet@nanowire arrays on nickel foam: An efficient 3D electrode for methanol electro-oxidation. Catal. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Ferdowsi, G.S.; Seyedsadjadi, S.A.; Ghaffarinejad, A. Ni nanoparticle modified graphite electrode for methanol electrocatalytic oxidation in alkaline media. J. Nanostruct. Chem. 2015, 5, 17–23. [Google Scholar] [CrossRef]
- Rahim, M.A.A.; Hameed, R.M.A.; Khalil, M.W. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Gu, Z.; Hohn, K.L. Catalytic Oxidation of Methanol on Nanoscale Copper Oxide and Nickel Oxide. Ind. Eng. Chem. Res. 2004, 43, 30–35. [Google Scholar] [CrossRef]
- Chen, G.; Pan, Y.; Lu, T.; Wang, N.; Li, X. Highly catalytical performance of nanoporous copper for electro-oxidation of methanol in alkaline media. Mater. Chem. Phys. 2018, 218, 108–115. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wojtaszek-Gurdak, A.; Yang, C.M.; Ziolek, M. Enhancement of selectivity in methanol oxidation over copper containing SBA-15 by doping with boron species. Catal. Today 2019, 122–131. [Google Scholar] [CrossRef]
- Dong, H.; Tang, P.; Wang, X.; Li, K.; Wang, Y.; Wang, D.; Liu, H.; Yang, S.-T.; Wu, C. Pt/NiO Microsphere Composite as Efficient Multifunctional Catalysts for Nonaqueous Lithium–Oxygen Batteries and Alkaline Fuel Cells: The Synergistic Effect of Pt and Ni. ACS Appl. Mater. Interfaces 2019, 11, 39789–39797. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, M.; Sathish, A.; Ramachandran, T. Nickel boride and cobalt boride coated stainless steel gauze for enhanced electrochemical oxidation of methanol. Ionics 2020, 26, 1875–1884. [Google Scholar] [CrossRef]
- El-Shafei, A.A. Electrocatalytic oxidation of methanol at a nickel hydroxide/glassy carbon modified electrode in alkaline medium. J. Electroanal. Chem. 1999, 471, 89–95. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Elzatahry, A.A.; Abdullah, A.M.; Vinu, A.; Iwai, H.; Al-Deyab, S.S. High electrocatalytic performance of nitrogen-doped carbon nanofiber-supported nickel oxide nanocomposite for methanol oxidation in alkaline medium. Appl. Surf. Sci. 2017, 401, 306–313. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Z.J. Composition dependence of methanol oxidation activity in nickel-cobalt hydroxides and oxides: An optimization toward highly active electrodes. Electrochim. Acta 2015, 165, 56–66. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Maciej, A.; Simka, W. Electrocatalytic properties of Co decorated graphene and graphene oxide for small organic molecules oxidation. Int. J. Hydrogen Energy 2019, 45, 1769–1783. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Dong, S.; Wang, C.; Feng, A.; Fan, X.; Li, Y. Reduced graphene oxide supported Pd-Cu-Co trimetallic catalyst: Synthesis, characterization and methanol electrooxidation properties. J. Energy Chem. 2018, 29, 72–78. [Google Scholar] [CrossRef]
- Hassan, H.B.; Hamid, Z.A. Electrodeposited Ni-Cr2O3 nanocomposite anodes for ethanol electrooxidation. Int. J. Hydrogen Energy 2011, 36, 5117–5127. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.; Xu, C.; Jiang, S.P.; Tong, Y. Electro-oxidation of methanol, 1-propanol and 2-propanol on Pt and Pd in alkaline medium. J. Power Sources 2008, 177, 67–70. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Porous NiCo2O4 nanostructures as bi-functional electrocatalysts for CH3OH oxidation reaction and H2O 2 reduction reaction. Electrochim. Acta 2013, 113, 290–301. [Google Scholar] [CrossRef]
- El Boraei, N.F.; Ibrahim, M.A.M. Black binary nickel cobalt oxide nano-powder prepared by cathodic electrodeposition; characterization and its efficient application on removing the Remazol Red textile dye from aqueous solution. Mater. Chem. Phys. 2019, 238, 121894. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Charles, E.A. Some effects of cobalt hydroxide upon the electrochemical behaviour of nickel hydroxide electrodes. J. Power Sources 1989, 25, 89–97. [Google Scholar] [CrossRef]
- Rahmani, K.; Habibi, B. NiCo alloy nanoparticles electrodeposited on an electrochemically reduced nitrogen-doped graphene oxide/carbon-ceramic electrode: A low cost electrocatalyst towards methanol and ethanol oxidation. RSC Adv. 2019, 9, 34050–34064. [Google Scholar] [CrossRef]
- Wang, J.; Wasmus, S.; Savinell, R.F. Evaluation of Ethanol, 1-Propanol, and 2-Propanol in a Direct Oxidation Polymer-Electrolyte Fuel Cell. J. Electrochem. Soc. 1995, 142, 4218–4224. [Google Scholar] [CrossRef]
- Kamarudin, M.Z.F.; Kamarudin, S.K.; Masdar, M.S.; Daud, W.R.W. Review: Direct ethanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 9438–9453. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Huang, R.; Liu, J.; Luo, C.X.; Wang, C.Y.; Song, Q.T.; Xiao, C.; Yin, S.H.; Xu, B.B.; Sun, S.G. PdPt concave nanocubes directly electrodeposited on carbon paper as high active and durable catalysts for formic acid and ethanol oxidation. Electrochim. Acta 2020, 354, 136654. [Google Scholar] [CrossRef]
- Altarawneh, R.M.; Brueckner, T.M.; Chen, B.; Pickup, P.G. Product distributions and efficiencies for ethanol oxidation at PtNi octahedra. J. Power Sources 2018, 400, 369–376. [Google Scholar] [CrossRef]
- Coutanceau, C.; Brimaud, S.; Lamy, C.; Léger, J.M.; Dubau, L.; Rousseau, S.; Vigier, F. Review of different methods for developing nanoelectrocatalysts for the oxidation of organic compounds. Electrochim. Acta 2008, 53, 6865–6880. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.; Lei, Z.; Zeng, T.; Tan, Y.; Cheng, N.; Sun, X. High-Performance Alcohol Electrooxidation on Pt3Sn-SnO2 Nanocatalysts through Transformation of Pt-Sn Nanoparticles. J. Mater. Chem. A 2020, 8, 592–598. [Google Scholar] [CrossRef]
- Li, J.; Jilani, S.Z.; Lin, H.; Liu, X.; Wei, K.; Jia, Y.; Zhang, P.; Chi, M.; Tong, Y.J.; Xi, Z.; et al. Ternary CoPtAu Nanoparticles as a General Catalyst for Highly Efficient Electro-Oxidation of Liquid Fuels. Angew. Chem.-Int. Ed. 2019, 58, 11527–11533. [Google Scholar] [CrossRef]
- Wongyao, N.; Therdthianwong, A.; Therdthianwong, S. Performance of direct alcohol fuel cells fed with mixed methanol/ethanol solutions. Energy Convers. Manag. 2011, 52, 2676–2681. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, G.; Zhou, Z.; Zhou, W.; Xin, Q. Preparation and characterization of PtSn/C anode electrocatalysts for direct ethanol fuel cell. Catal. Today 2004, 93–95, 665–670. [Google Scholar] [CrossRef]
- Sieben, J.M.; Duarte, M.M.E. Nanostructured Pt and Pt-Sn catalysts supported on oxidized carbon nanotubes for ethanol and ethylene glycol electro-oxidation. Int. J. Hydrogen Energy 2011, 36, 3313–3321. [Google Scholar] [CrossRef]
- Lycke, D.R.; Gyenge, E.L. Electrochemically assisted organosol method for Pt-Sn nanoparticle synthesis and in situ deposition on graphite felt support: Extended reaction zone anodes for direct ethanol fuel cells. Electrochim. Acta 2007, 52, 4287–4298. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Y.; Wang, X.; Zhang, G.; Cui, P. Boron as a superior activator for Pt anode catalyst in direct alcohol fuel cell. J. Power Sources 2019, 431, 125–134. [Google Scholar] [CrossRef]
- Rizo, R.; Arán-Ais, R.M.; Padgett, E.; Muller, D.A.; Lázaro, M.J.; Solla-Gullón, J.; Feliu, J.M.; Pastor, E.; Abruña, H.D. Pt-Richcore/Sn-Richsubsurface/Ptskin Nanocubes As Highly Active and Stable Electrocatalysts for the Ethanol Oxidation Reaction. J. Am. Chem. Soc. 2018, 140, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Gao, F.; Jin, L.; Zhang, Y.; Wang, C.; Li, S.; Chen, C.; Du, Y. From bimetallic PdCu nanowires to ternary PdCu-SnO2 nanowires: Interface control for efficient ethanol electrooxidation. J. Colloid Interface Sci. 2020, 560, 802–810. [Google Scholar] [CrossRef]
- Lv, H.; Wang, Y.; Xu, D.; Liu, B. Engineering porous architectures in multicomponent PdCuBP mesoporous nanospheres for electrocatalytic ethanol oxidation. Nano Res. 2021, 12. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, H.; Sun, F.; Fu, C.; Zeng, F.; Li, T.; Kuang, Y. Facile self-assembly synthesis of PdPt bimetallic nanotubes with good performance for ethanol oxidation in an alkaline medium. Chem.-A Eur. J. 2013, 19, 13720–13725. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, N.; Banis, M.N.; Xiao, B.; Riese, A.; Sun, X. Comparative study to understand the intrinsic properties of Pt and Pd catalysts for methanol and ethanol oxidation in alkaline media. Electrochim. Acta 2015, 185, 267–275. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; Mohamed, A.T.; Alashraf, A.; Matalqeh, M.; El-Shafei, A.; Al-Qaradawi, S.Y.; Aljaber, A.S. Highly porous PtPd nanoclusters synthesized via selective chemical etching as efficient catalyst for ethanol electro-oxidation. Appl. Surf. Sci. 2020, 508, 145222. [Google Scholar] [CrossRef]
- Nguyen, M.T.X.; Nguyen, M.K.; Pham, P.T.T.; Huynh, H.K.P.; Nguyen, S.T. Pd coated one-dimensional Ag nanostructures: Controllable architecture and their electrocatalytic performance for ethanol oxidation in alkaline media. Int. J. Hydrogen Energy 2021, 46, 3909–3921. [Google Scholar] [CrossRef]
- Pan, Z.F.; Chen, R.; An, L.; Li, Y.S. Alkaline anion exchange membrane fuel cells for cogeneration of electricity and valuable chemicals. J. Power Sources 2017, 365, 430–445. [Google Scholar] [CrossRef]
- Kottayintavida, R.; Gopalan, N.K. Nickel phosphate modified carbon supported Pd catalyst for enhanced alcohol electro oxidation. Int. J. Hydrogen Energy 2020, 45, 11116–11126. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Henriksson, G.; Cornell, A. Insights on the ethanol oxidation reaction at electrodeposited PdNi catalysts under conditions of increased mass transport. Int. J. Hydrogen Energy 2021, 46, 1615–1626. [Google Scholar] [CrossRef]
- Roy Chowdhury, S.; Banik, M.S.; Mahajan, A.; Kumar Bhattacharya, S. Anode Catalytic Activity of Palladium-Nickel Alloy Nanoparticles for Ethanol Oxidation in Alkali. ChemistrySelect 2020, 5, 9848–9856. [Google Scholar] [CrossRef]
- Almeida, C.V.S.; Tremiliosi-Filho, G.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Improved ethanol electro-oxidation at Ni@Pd/C and Ni@PdRh/C core–shell catalysts. J. Catal. 2020, 391, 175–189. [Google Scholar] [CrossRef]
- Shi, W.; Ding, R.; Li, X.; Xu, Q.; Liu, E. Enhanced performance and electrocatalytic kinetics of Ni-Mo/graphene nanocatalysts towards alkaline urea oxidation reaction. Electrochim. Acta 2017, 242, 247–259. [Google Scholar] [CrossRef]
- Acta launches catalyst, supplies electrodes to ethanol FCV. Fuel Cells Bull. 2007, 2007, 10. [CrossRef]
- Feng, Y.; Yin, W.; Li, Z.; Huang, C.; Wang, Y. Ethylene glycol, 2-propanol electrooxidation in alkaline medium on the ordered intermetallic PtPb surface. Electrochim. Acta 2010, 55, 6991–6999. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Recent achievements in direct ethylene glycol fuel cells (DEGFC). Appl. Catal. B Environ. 2010, 97, 1–12. [Google Scholar] [CrossRef]
- Miyazaki, K.; Matsumiya, T.; Abe, T.; Kurata, H.; Fukutsuka, T.; Kojima, K.; Ogumi, Z. Electrochemical oxidation of ethylene glycol on Pt-based catalysts in alkaline solutions and quantitative analysis of intermediate products. Electrochim. Acta 2011, 56, 7610–7614. [Google Scholar] [CrossRef]
- An, L.; Zhao, T.S.; Shen, S.Y.; Wu, Q.X.; Chen, R. Performance of a direct ethylene glycol fuel cell with an anion-exchange membrane. Int. J. Hydrogen Energy 2010, 35, 4329–4335. [Google Scholar] [CrossRef]
- Jin, C.; Song, Y.; Chen, Z. A comparative study of the electrocatalytic oxidation of ethylene glycol on PtAu nanocomposite catalysts in alkaline, neutral and acidic media. Electrochim. Acta 2009, 54, 4136–4140. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, S.; Yang, X.; Pi, L.; Cui, Z. Electro-oxidation of ethylene glycol on nanoporous Ti-Cu amorphous alloy. Electrochim. Acta 2011, 56, 10253–10258. [Google Scholar] [CrossRef]
- Shao, F.Q.; Lin, X.X.; Feng, J.J.; Yuan, J.; Chen, J.R.; Wang, A.J. Simple fabrication of core-shell AuPt@Pt nanocrystals supported on reduced graphene oxide for ethylene glycol oxidation and hydrogen evolution reactions. Electrochim. Acta 2016, 219, 321–329. [Google Scholar] [CrossRef]
- Neto, A.O.; Vasconcelos, T.R.R.; Da Silva, R.W.R.V.; Linardi, M.; Spinacé, E.V. Electro-oxidation of ethylene glycol on PtRu/C and PtSn/C electrocatalysts prepared by alcohol-reduction process. J. Appl. Electrochem. 2005, 35, 193–198. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, C.; Gao, H.; Fu, N.; Zhu, M. Highly efficient ethylene glycol electrocatalytic oxidation based on bimetallic PtNi on 2D molybdenum disulfide/reduced graphene oxide nanosheets. J. Colloid Interface Sci. 2019, 547, 102–110. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, Y.; Xiao, M.; Yu, G. Precise size and dominant-facet control of ultra-small Pt nanoparticles for efficient ethylene glycol, methanol and ethanol oxidation electrocatalysts. Int. J. Hydrogen Energy 2020, 45, 4341–4354. [Google Scholar] [CrossRef]
- Baronia, R.; Goel, J.; Baijnath; Kataria, V.; Basu, S.; Singhal, S.K. Electro-oxidation of ethylene glycol on Pt–Co metal synergy for direct ethylene glycol fuel cells: Reduced graphene oxide imparting a notable surface of action. Int. J. Hydrogen Energy 2019, 44, 10023–10032. [Google Scholar] [CrossRef]
- Al-Akraa, I.M.; Asal, Y.M.; Mohammad, A.M. Facile synthesis of a tailored-designed AU/PT nanoanode for enhanced formic acid, methanol, and ethylene glycol electrooxidation. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Maxakato, N.W.; Arendse, C.J.; Ozoemena, K.I. Insights into the electro-oxidation of ethylene glycol at Pt/Ru nanocatalysts supported on MWCNTs: Adsorption-controlled electrode kinetics. Electrochem. commun. 2009, 11, 534–537. [Google Scholar] [CrossRef]
- Chatterjee, M.; Chatterjee, A.; Ghosh, S.; Basumallick, I. Electro-oxidation of ethanol and ethylene glycol on carbon-supported nano-Pt and -PtRu catalyst in acid solution. Electrochim. Acta 2009, 54, 7299–7304. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Xing, L.; Duan, W.; Zhang, X.; Cao, Y.; Xia, H. Facet-Dependent Long-Term Stability of Gold Aerogels toward Ethylene Glycol Oxidation Reaction. ACS Appl. Mater. Interfaces 2020, 12, 39033–39042. [Google Scholar] [CrossRef]
- Etesami, M.; Mohamed, N. Electrooxidation of ethylene glycol using gold nanoparticles electrodeposited on pencil graphite in alkaline medium. Sci. China Chem. 2012, 55, 247–255. [Google Scholar] [CrossRef]
- Bock, C.; Paquet, C.; Couillard, M.; Botton, G.A.; MacDougall, B.R. Size-selected synthesis of PtRu nano-catalysts: Reaction and size control mechanism. J. Am. Chem. Soc. 2004, 126, 8028–8037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Yi, Q.F.; Chu, H.; Nie, H.D. Catalytic activity of Pd-Ag nanoparticles supported on carbon nanotubes for the electro-oxidation of ethanol and propanol. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol 2017, 45, 475–483. [Google Scholar] [CrossRef]
- Xu, Y.; Han, L. Comprehensive understanding of electro-oxidation of ethylene glycol. Int. J. Hydrogen Energy 2014, 39, 7278–7290. [Google Scholar] [CrossRef]
- El-Shafei, A.A.; El-Maksoud, S.A.A.; Fouda, A.S. Noble-metal-modified glassy carbon electrodes for ethylene glycol oxidation in alkaline medium. J. Electroanal. Chem. 1995, 395, 181–187. [Google Scholar] [CrossRef]
- Song, P.; Liu, L.; Feng, J.J.; Yuan, J.; Wang, A.J.; Xu, Q.Q. Poly(ionic liquid) assisted synthesis of hierarchical gold-platinum alloy nanodendrites with high electrocatalytic properties for ethylene glycol oxidation and oxygen reduction reactions. Int. J. Hydrogen Energy 2016, 41, 14058–14067. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Zhang, L.; Yuan, P.X.; Feng, J.J.; Yuan, J.; Zhang, Q.L.; Wang, A.J. Hollow Ag44Pt56 nanotube bundles with high electrocatalytic performances for hydrogen evolution and ethylene glycol oxidation reactions. J. Colloid Interface Sci. 2018, 532, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, W.; Liu, Y.; Wang, F.; Chai, D.; Lei, Z. Pd nanoparticles supported on phenanthroline modified carbon as high active electrocatalyst for ethylene glycol oxidation. Electrochim. Acta 2015, 154, 1–8. [Google Scholar] [CrossRef]
- Guo, X.; Shang, H.; Guo, J.; Xu, H.; Du, Y. Ultrafine two-dimensional alloyed PdCu nanosheets-constructed three-dimensional nanoflowers enable efficient ethylene glycol electrooxidation. Appl. Surf. Sci. 2019, 481, 1532–1537. [Google Scholar] [CrossRef]
- Wang, W.; Jing, W.; Liu, Y.; Wang, Y.; Zhao, J.; Lei, Z. Supporting Pd nanoparticles on riboflavin-derived carbon: An efficient electrocatalyst for ethylene glycol oxidation. Ionics (Kiel) 2018, 24, 1745–1754. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Wang, F.; Liu, Y.; Chai, D.; Lei, Z. Partially oxidized NiFe alloy: An effective promoter to enhance Pd electrocatalytic performance for ethylene glycol oxidation. Int. J. Hydrogen Energy 2015, 40, 12262–12267. [Google Scholar] [CrossRef]
- Marchionni, A.; Bevilacqua, M.; Bianchini, C.; Chen, Y.X.; Filippi, J.; Fornasiero, P.; Lavacchi, A.; Miller, H.; Wang, L.; Vizza, F. Electrooxidation of ethylene glycol and glycerol on Pd-(Ni-Zn)/C anodes in direct alcohol fuel cells. ChemSusChem 2013, 6, 518–528. [Google Scholar] [CrossRef]
- Mangeli, A.; Mostafavi, A.; Shamspur, T.; Fathirad, F. Binary nanostructured catalysts to facilitate electricity generation from ethylene glycol electrooxidation. Inorg. Chem. Commun. 2020, 118, 108038. [Google Scholar] [CrossRef]
- Ulas, B.; Caglar, A.; Kivrak, A.; Kivrak, H. Atomic molar ratio optimization of carbon nanotube supported PdAuCo catalysts for ethylene glycol and methanol electrooxidation in alkaline media. Chem. Pap. 2019, 73, 425–434. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Liu, L.; Wang, A.J.; Yuan, J.; Feng, J.J.; Xu, Q.Q. Simple wet-chemical strategy for large-scaled synthesis of snowflake-like PdAu alloy nanostructures as effective electrocatalysts of ethanol and ethylene glycol oxidation. Int. J. Hydrogen Energy 2017, 42, 2034–2044. [Google Scholar] [CrossRef]
- Łuczak, T.; Bułat, K. Gold Surface Functionalization with S-containing Organic Compounds and Gold Nanoparticles for Ethylene Glycol Electrooxidation. Electroanalysis 2016, 28, 711–717. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Liu, Y.; Wang, F.; Zhang, Z.; Lei, Z. Carbon supported heterostructured Pd-Ag nanoparticle: Highly active electrocatalyst for ethylene glycol oxidation. Int. J. Hydrogen Energy 2015, 40, 2225–2230. [Google Scholar] [CrossRef]
- Chai, D.; Zhang, X.; Yan, S.; Li, G. Ni2P as an electron donor stabilizing Pt for highly efficient isopropanol fuel cell. Int. J. Hydrogen Energy 2020, 45, 6573–6582. [Google Scholar] [CrossRef]
- Xu, C.; Tian, Z.; Chen, Z.; Jiang, S.P. Pd/C promoted by Au for 2-propanol electrooxidation in alkaline media. Electrochem. Commun. 2008, 10, 246–249. [Google Scholar] [CrossRef]
- Chelaghmia, M.L.; Nacef, M.; Fisli, H.; Affoune, A.M.; Pontié, M.; Makhlouf, A.; Derabla, T.; Khelifi, O.; Aissat, F. Electrocatalytic performance of Pt-Ni nanoparticles supported on an activated graphite electrode for ethanol and 2-propanol oxidation. RSC Adv. 2020, 10, 36941–36948. [Google Scholar] [CrossRef]
- Xu, H.; Yan, B.; Zhang, K.; Wang, C.; Zhong, J.; Li, S.; Du, Y.; Yang, P. PVP-stabilized PdAu nanowire networks prepared in different solvents endowed with high electrocatalytic activities for the oxidation of ethylene glycol and isopropanol. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 522, 335–345. [Google Scholar] [CrossRef]
- Hauenstein, P.; Seeberger, D.; Wasserscheid, P.; Thiele, S. High performance direct organic fuel cell using the acetone/isopropanol liquid organic hydrogen carrier system. Electrochem. Commun. 2020, 118, 106786. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Cao, D.; Wang, G.; Gao, Y. Effects of acetone on electrooxidation of 2-propanol in alkaline medium on the Pd/Ni-foam electrode. J. Power Sources 2011, 196, 3124–3128. [Google Scholar] [CrossRef]
- Khanipour, P.; Speck, F.D.; Mangoufis-Giasin, I.; Mayrhofer, K.J.J.; Cherevko, S.; Katsounaros, I. Electrochemical Oxidation of Isopropanol on Platinum-Ruthenium Nanoparticles Studied with Real-Time Product and Dissolution Analytics. ACS Appl. Mater. Interfaces 2020, 12, 33670–33678. [Google Scholar] [CrossRef]
- Qi, Z.; Kaufman, A. Performance of 2-propanol in direct-oxidation fuel cells. J. Power Sources 2002, 112, 121–129. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Liu, R.; Wu, H.; Wang, G.; Cao, D. Poisoning of acetone to Pt and Au electrodes for electrooxidation of 2-propanol in alkaline medium. Electrochim. Acta 2012, 76, 174–178. [Google Scholar] [CrossRef]
- Ye, J.; Liu, J.; Xu, C.; Jiang, S.P.; Tong, Y. Electrooxidation of 2-propanol on Pt, Pd and Au in alkaline medium. Electrochem. Commun. 2007, 9, 2760–2763. [Google Scholar] [CrossRef]
- Serov, A.; Martinez, U.; Falase, A.; Atanassov, P. Highly active Pd-Cu catalysts for electrooxidation of 2-propanol. Electrochem. Commun. 2012, 22, 193–196. [Google Scholar] [CrossRef]
- Xing, S.; Liu, Z.; Xue, Q.; Yin, S.; Li, F.; Cai, W.; Li, S.; Chen, P.; Jin, P.; Yao, H.; et al. Rh nanoroses for isopropanol oxidation reaction. Appl. Catal. B Environ. 2019, 259, 118082. [Google Scholar] [CrossRef]
- Garlisi, C.; Scandura, G.; Szlachetko, J.; Ahmadi, S.; Sa, J.; Palmisano, G. E-beam evaporated TiO2 and Cu-TiO2 on glass: Performance in the discoloration of methylene blue and 2-propanol oxidation. Appl. Catal. A Gen. 2016, 526, 191–199. [Google Scholar] [CrossRef]


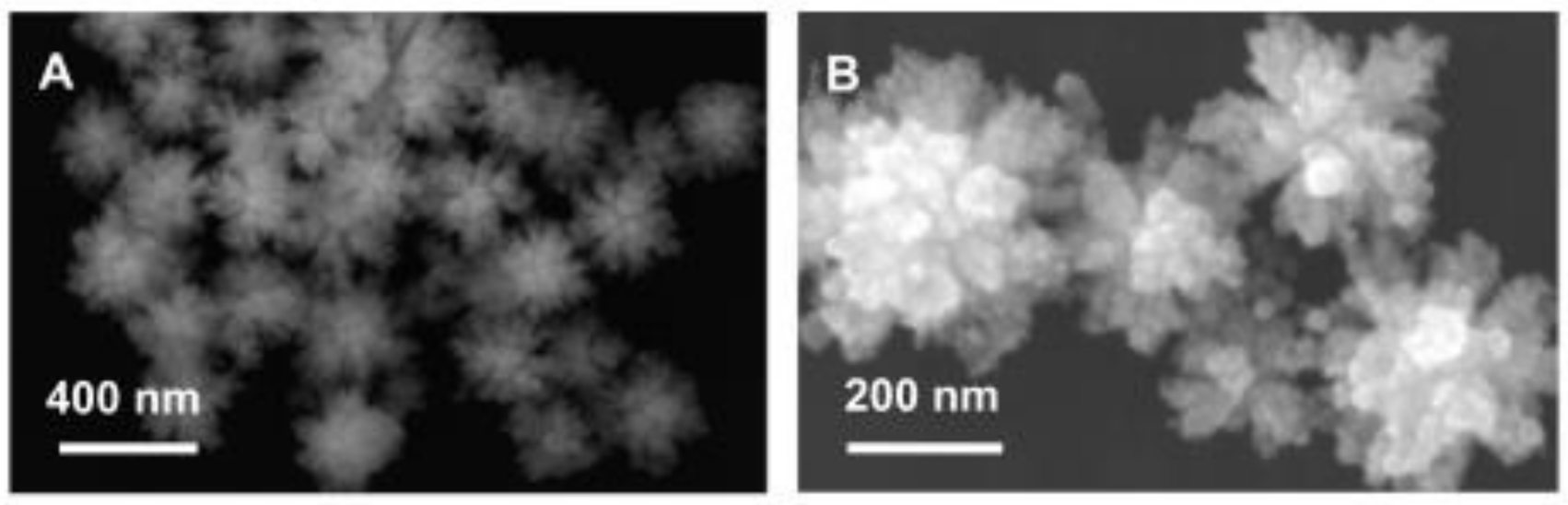
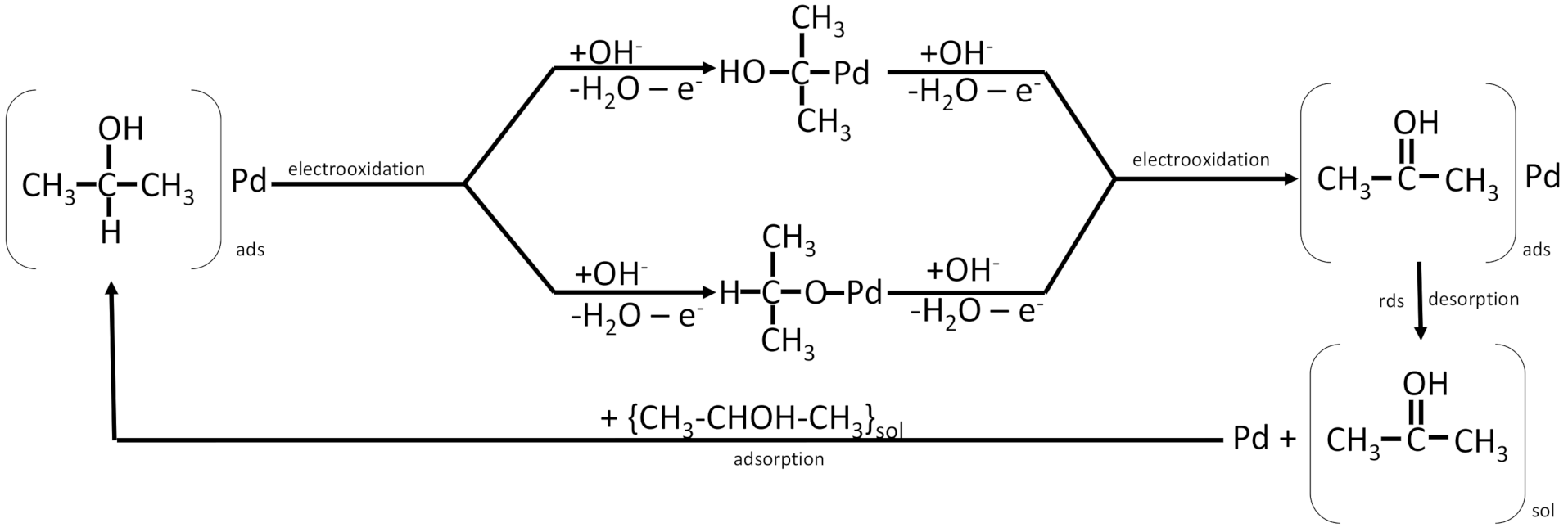
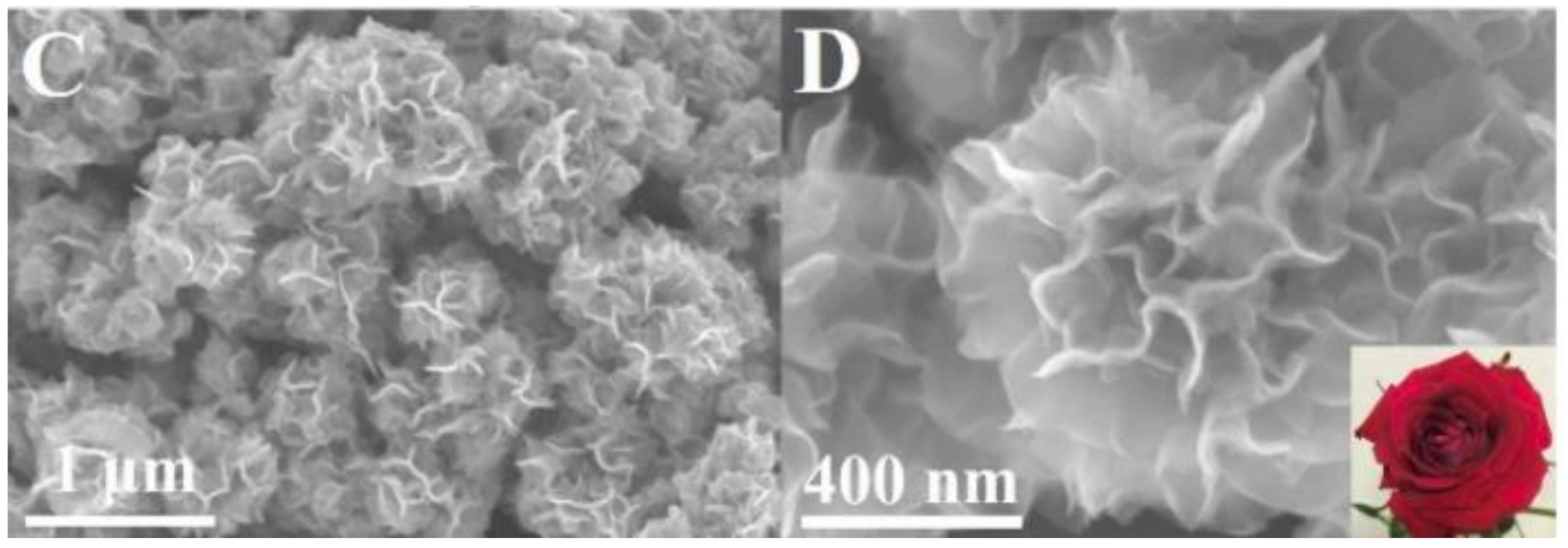
| Alcohol | Density (kg m−3) * | Boiling Point (°C) ** | Energetic Density (MJ L−1) | Heat of Combustion (MJ kg−1) | Theoretical Energetic Density (kWh kg−1) | E° Cell (V) |
|---|---|---|---|---|---|---|
| Methanol | 786.68 [2] | 64.70 [84,85] | 17.85 based on [2] | 22.69 [2] | 6.1 [86,87,88] | 1.213 [87] |
| Ethanol | 789.30 [84] | 78.32 [84] | 23.49 based on [84,89] | 29.76 based on [89] | 8.00 [23,86,87] | 1.145 [87] |
| Ethylene glycol | 1113.50 [90] | 197.60 [85,90] | 21.23 based on [90] | 19.07 [90] | 5.2 [23,86,88,91] | 1.22 [91] 1.029 [87] |
| Propanol | 803.60 [92] | 97.22 [92] | 27.00 based on [92] | 33.60 [92] | 5.58 [87] | 1.067 [87] |
| Gasoline | 10–11 [87] |
| Property | Methanol | Ethanol | Propanol | Isopropanol |
|---|---|---|---|---|
| Oxidation OCP vs. RHE, V | 0.1 | 0.11 | 0.12 | 0.18 |
| % CO2 in product stream (for stoichiometric water content) | 87 | 27.4 | 19.5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wala, M.; Simka, W. Effect of Anode Material on Electrochemical Oxidation of Low Molecular Weight Alcohols—A Review. Molecules 2021, 26, 2144. https://doi.org/10.3390/molecules26082144
Wala M, Simka W. Effect of Anode Material on Electrochemical Oxidation of Low Molecular Weight Alcohols—A Review. Molecules. 2021; 26(8):2144. https://doi.org/10.3390/molecules26082144
Chicago/Turabian StyleWala, Marta, and Wojciech Simka. 2021. "Effect of Anode Material on Electrochemical Oxidation of Low Molecular Weight Alcohols—A Review" Molecules 26, no. 8: 2144. https://doi.org/10.3390/molecules26082144
APA StyleWala, M., & Simka, W. (2021). Effect of Anode Material on Electrochemical Oxidation of Low Molecular Weight Alcohols—A Review. Molecules, 26(8), 2144. https://doi.org/10.3390/molecules26082144







