Prodigiosin of Serratia marcescens ZPG19 Alters the Gut Microbiota Composition of Kunming Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of S. marcescens
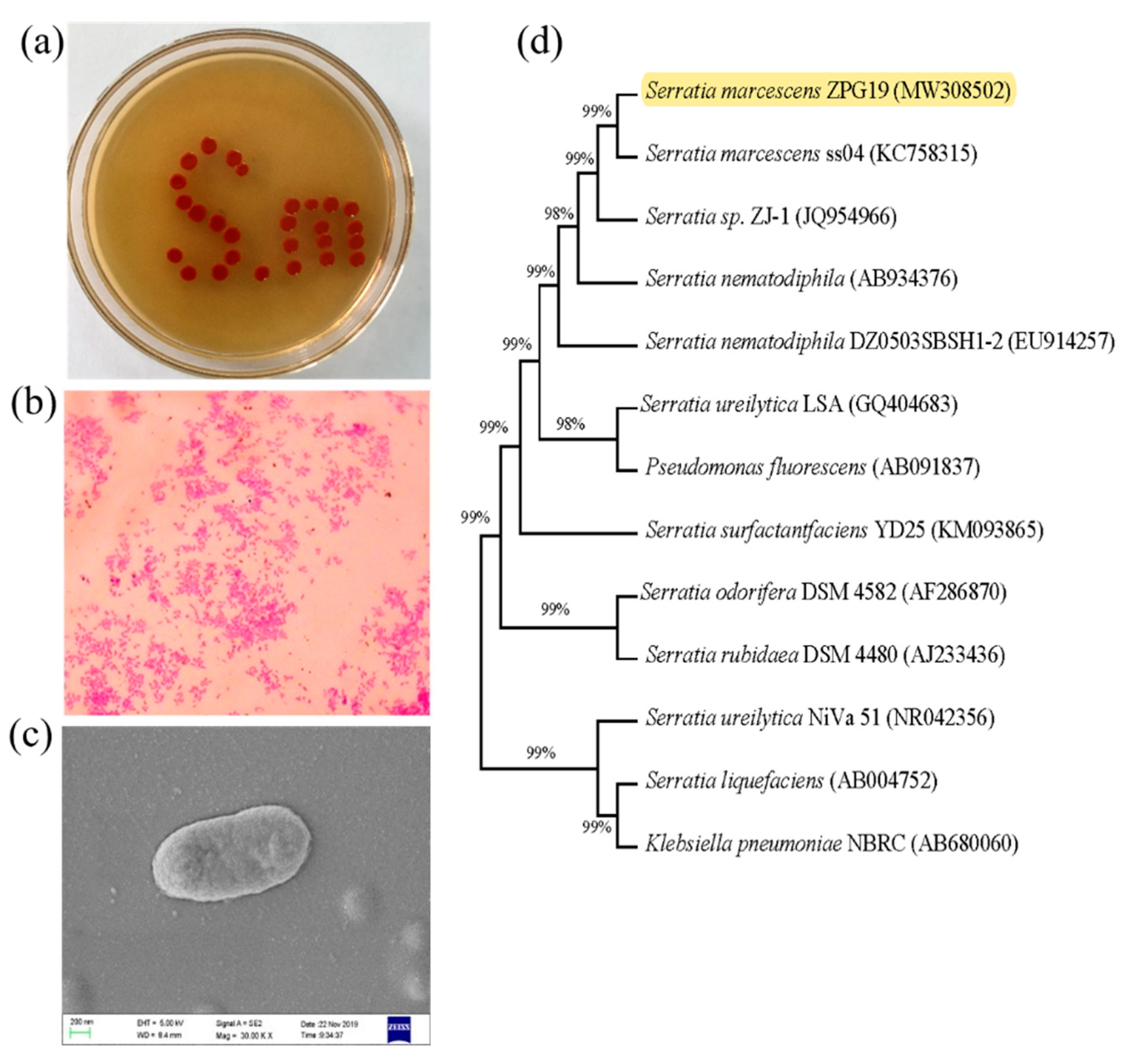
2.2. 16S rRNA Gene-based Phylogenetic Analysis
2.3. Extraction and Identification of the Red Pigment
2.4. Effect of Prodigiosin on Mice
2.5. Microbiota Analysis
2.6. Statistical Analysis
3. Results
3.1. Phenotypic and Phylogenetic Analysis and Identification of ZPG19
3.2. Identification of Metabolites of S. marcescens ZPG19
3.3. Effect of Prodigiosin on the Internal Organs of Mice
3.4. Effects of Prodigiosin on Mouse Gut Microbiota Diversity and Richness
3.5. Prodigiosin Alters the Gut Microbiota Composition
3.6. Effects of Prodigiosin on Intestinal Microbiota Functions in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Approval
References
- Zang, C.-Z.; Yeh, C.-W.; Chang, W.-F.; Lin, C.-C.; Kan, S.-C.; Shieh, C.-J.; Liu, Y.-C. Identification and enhanced production of prodigiosin isoform pigment from Serratia marcescens N10612. J. Taiwan Inst. Chem. Eng. 2014, 45, 1133–1139. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Chen, J.; Liu, X.; Xiang, T.; Zhang, L.; Wan, Y. The red pigment prodigiosin is not an essential virulence factor in entomopathogenic Serratia marcescens. J. Invertebr. Pathol. 2016, 136, 92–94. [Google Scholar] [CrossRef] [PubMed]
- El-Bialy, H.A.; El-Nour, S.A.A. Physical and chemical stress on Serratia marcescens and studies on prodigiosin pigment production. Ann. Microbiol. 2014, 65, 59–68. [Google Scholar] [CrossRef]
- Bennett, J.; Bentley, R. Seeing red: The story of prodigiosin. Adv. Clin. Chem. 2000, 47, 1–32. [Google Scholar] [CrossRef]
- Zarei, M.; Aminzadeh, S.; Zolgharnein, H.; Safahieh, A.; Ghoroghi, A.; Motallebi, A.; Daliri, M.; Lotfi, A.S. Serratia marcescens B4A chitinase product optimization using Taguchi approach. Iran J. Biotechnol. 2010, 8, 252–262. [Google Scholar]
- Sevcikova, B.; Kormanec, J. Differential production of two antibiotics of Streptomyces coelicolor A3(2), actinorhodin and undecylprodigiosin, upon salt stress conditions. Arch. Microbiol. 2004, 181, 384–389. [Google Scholar] [CrossRef]
- Rossa, C.; White, J.; Kuiper, A.; Postma, P.; Bibb, M.; de Mattos, M.T. Carbon Flux Distribution in Antibiotic-Producing Chemostat Cultures of Streptomyces lividans. Metab. Eng. 2002, 4, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Stankovic, N.; Senerovic, L.; Ilic-Tomic, T.; Vasiljevic, B.; Nikodinovic-Runic, J. Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. [Google Scholar] [CrossRef]
- Andreyeva, I.N.; Ogorodnikova, T.I. Pigmentation of Serratia marcescens and spectral properties of prodigiosin. Microbiology 2015, 84, 28–33. [Google Scholar] [CrossRef]
- Drink, E.; Dugourd, P.; Dumont, E.; Aronssohn, N.; Antoine, R.; Loison, C. Optical properties of prodigiosin and obatoclax: Action spectroscopy and theoretical calculations. Phys. Chem. Chem. Phys. 2015, 17, 25946–25955. [Google Scholar] [CrossRef] [PubMed]
- Williamson, N.R.; Fineran, P.C.; Gristwood, T.; Chawrai, S.R.; Leeper, F.J.; Salmond, G.P.C. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2007, 2, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, J.E.H.; Nitcheu, J.; Predicala, R.Z.; Mangalindan, G.C.; Nesslany, F.; Marzin, D.; Concepcion, G.P.; Diquet, B. Heptyl prodigiosin, a bacterial metabolite, is antimalarial in vivo and non-mutagenic in vitro. J. Nat. Toxins 2002, 11, 367–377. [Google Scholar] [PubMed]
- Nakashima, T.; Kurachi, M.; Kato, Y.; Yamaguchi, K.; Oda, T. Characterization of Bacterium Isolated from the Sediment at Coastal Area of Omura Bay in Japan and Several Biological Activities of Pigment Produced by This Isolate. Microbiol. Immunol. 2005, 49, 407–415. [Google Scholar] [CrossRef]
- Nakashima, T.; Kato, Y.; Yamaguchi, K.; Oda, T. Evaluation of the anti-Trichophyton activity of a prodigiosin analogue produced by gamma-proteobacterium, using stratum corneum epidermis of the Yucatan micropig. J. Infect. Chemother. 2005, 11, 123–128. [Google Scholar] [CrossRef]
- Azambuja, P.; Feder, D.; Garcia, E. Isolation of Serratia marcescens in the midgut of Rhodnius prolixus: Impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp. Parasitol. 2004, 107, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Genes, C.; Baquero, E.; Echeverri, F.; Maya, J.D.; Triana, O. Mitochondrial dysfunction in Trypanosoma cruzi: The role of Serratia marcescens prodigiosin in the alternative treatment of Chagas disease. Parasites Vectors 2011, 4, 66–68. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wei, H.Y.; Li, X.Q.; Li, Y.H.; Yin, L.H.; Pu, Y.P. Isolation and characterization of an algicidal bacterium indigenous to lake Taihu with a red pigment able to lyse microcystis aeruginosa. Biomed. Environ. Sci. 2013, 26, 148–154. [Google Scholar]
- Suryawanshi, R.K.; Patil, C.D.; Borase, H.P.; Narkhede, C.P.; Stevenson, A.; Hallsworth, J.E.; Patil, S.V. Towards an understanding of bacterial metabolites prodigiosin and violacein and their potential for use in commercial sunscreens. Int. J. Cosmet. Sci. 2014, 37, 98–107. [Google Scholar] [CrossRef]
- Teshima, S.; Nakanishi, H.; Kamata, K.; Kaibori, M.; Kwon, A.-H.; Kamiyama, Y.; Nishizawa, M.; Ito, S.; Okumura, T. Cycloprodigiosin up-regulates inducible nitric oxide synthase gene expression in hepatocytes stimulated by interleukin-1beta. Nitric Oxide 2004, 11, 9–16. [Google Scholar] [CrossRef]
- Ikeda, H.; Shikata, Y.; Watanapokasin, R.; Tashiro, E.; Imoto, M. Metacycloprodigiosin induced cell death selectively in β-catenin-mutated tumor cells. J. Antibiot. 2016, 70, 109–112. [Google Scholar] [CrossRef]
- Morita, N.; Umemoto, E.; Fujita, S.; Hayashi, A.; Kikuta, J.; Kimura, I.; Haneda, T.; Imai, T.; Inoue, A.; Mimuro, H.; et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nat. Cell Biol. 2019, 566, 110–114. [Google Scholar] [CrossRef]
- Li, S.; Chen, T.; Xu, F.; Dong, S.; Xu, H.; Xiong, Y.; Wei, H. The beneficial effect of exopolysaccharides from Bifidobacterium bifidum WBIN03 on microbial diversity in mouse intestine. J. Sci. Food Agric. 2013, 94, 256–264. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolbert, C.P.; Persing, D.H. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr. Opin. Microbiol. 1999, 2, 299–305. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Sun, S.-Q.; Wang, Y.-J.; Xu, W.; Zhu, C.-J.; Liu, X.-X. Optimizing Ultrasound-Assisted Extraction of Prodigiosin by Response Surface Methodology. Prep. Biochem. Biotechnol. 2015, 45, 101–108. [Google Scholar] [CrossRef]
- Alihosseini, F.; Ju, K.; Lango, J.; Hammock, B.D.; Sun, G. Antibacterial Colorants: Characterization of Prodiginines and Their Applications on Textile Materials. Biotechnol. Prog. 2008, 24, 742–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-C.; Wang, Y.-H.; Chern, C.-M.; Liou, K.-T.; Hou, Y.-C.; Peng, Y.-T.; Shen, Y.-C. Prodigiosin inhibits gp91phox and iNOS expression to protect mice against the oxidative/nitrosative brain injury induced by hypoxia–ischemia. Toxicol. Appl. Pharmacol. 2011, 257, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Magoč, M.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, H.H.; Rodgers, G.C.; Keith, D.D. Metacycloprodigiosin, a tripyrrole pigment from Streptomyces longisporus ruber. J. Am. Chem. Soc. 1969, 91, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Lazović, S.; Leskovac, A.; Petrović, S.; Senerovic, L.; Krivokapić, N.; Mitrović, T.; Božović, N.; Vasić, V.; Nikodinovic-Runic, J. Biological effects of bacterial pigment undecylprodigiosin on human blood cells treated with atmospheric gas plasma in vitro. Exp. Toxicol. Pathol. 2017, 69, 55–62. [Google Scholar] [CrossRef]
- Wilson, Q.N.; Wells, M.; Davis, A.T.; Sherrill, C.; Tsilimigras, M.C.B.; Jones, R.B.; Fodor, A.A.; Kavanagh, K. Greater Microbial Translocation and Vulnerability to Metabolic Disease in Healthy Aged Female Monkeys. Sci. Rep. 2018, 8, 11373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochhead, J.J.; Ronaldson, P.T.; Davis, T.P. Hypoxic Stress and Inflammatory Pain Disrupt Blood-Brain Barrier Tight Junctions: Implications for Drug Delivery to the Central Nervous System. AAPS J. 2017, 19, 910–920. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Hedin, L. Microbiota and the control of blood-tissue barriers. Tissue Barriers 2015, 3, e1039691. [Google Scholar] [CrossRef]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.V. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004, 12, 129–134. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Genet. 2012, 10, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-J.; Zhao, D.-D.; Liu, H.; Chen, H.-T.; Li, J.-J.; Mu, X.-Q.; Liu, Z.; Li, X.; Tang, L.; Zhao, Z.-Y.; et al. Cancer killers in the human gut microbiota: Diverse phylogeny and broad spectra. Oncotarget 2017, 8, 49574–49591. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, aad3311. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.M.; Tan, R.X. Interaction between gut microbiota and ethnomedicine constituents. Nat. Prod. Rep. 2019, 36, 788–809. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, C.; Bell, R.; Klag, K.A.; Lee, S.-H.; Soto, R.; Ghazaryan, A.; Buhrke, K.; Ekiz, H.A.; Ost, K.S.; Boudina, S.; et al. T cell–mediated regulation of the microbiota protects against obesity. Science 2019, 365, eaat9351. [Google Scholar] [CrossRef]
- Magae, J.; Miller, M.W.; Nagai, K.; Shearer, G.M. Effect of Metacycloprodigiosin, an Inhibitor of Killer T Cells, on Murine Skin and Heart Transplants. J. Antibiot. 1996, 49, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-H.; Kataoka, T.; Honjo, N.; Magae, J.; Nagai, K. In vivo rapid reduction of alloantigen-activated CD8+ mature cytotoxic T cells by inhibitors of acidification of intracellular organelles, prodigiosin 25-C and concanamycin B. Immunology 2000, 99, 243–248. [Google Scholar] [CrossRef]
- Lee, M.-H.; Yamashita, M.; Tsuji, R.F.; Yamasaki, M.; Kataoka, T.; Magae, J.; Nagai, K. Suppression of T Cell Stimulating Function of Allogeneic Antigen Presenting Cells by Prodigiosin 25-C. J. Antibiot. 1998, 51, 92–94. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.-E.; Yim, J.-H.; Lee, H.-K.; Moon, E.-Y.; Rhee, D.-K.; Pyo, S. Prodigiosin isolated from Hahella chejuensis suppresses lipopolysaccharide-induced NO production by inhibiting p38 MAPK, JNK and NF-κB activation in murine peritoneal macrophages. Int. Immunopharmacol. 2007, 7, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Lapenda, J.C.; Silva, P.A.; Vicalvi, M.C.; Sena, K.X.F.R.; Nascimento, S.C. Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 2014, 31, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.-S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nat. Cell Biol. 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Dabour, N.; Zihler, A.; Kheadr, E.; Lacroix, C.; Fliss, I. In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int. J. Food Microbiol. 2009, 133, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Umu, O.C.O.; Bauerl, C.; Oostindjer, M.; Pope, P.B.; Hernandez, P.E.; Perez-Martinez, G.; Diep, O.B. The Potential of Class II Bacteriocins to Modify Gut Microbiota to Improve Host Health. PLoS ONE 2016, 11, e0164036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Jones, S.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, E.J.C.; Citron, D.M.; Peraino, V.A.; Cross, S.A. Desulfovibrio desulfuricans Bacteremia and Review of Human Desulfovibrio Infections. J. Clin. Microbiol. 2003, 41, 2752–2754. [Google Scholar] [CrossRef] [Green Version]
- Urata, T.; Kikuchi, M.; Hino, T.; Yoda, Y.; Tamai, K.; Kodaira, Y.; Hitomi, S. Bacteremia caused by Desulfovibrio fairfieldensis. J. Infect. Chemother. 2008, 14, 368–370. [Google Scholar] [CrossRef]





| BW/g | C | PF |
|---|---|---|
| d1 | 44.498 ± 1.5 | 45.786 ± 2.0 |
| d6 | 44.704 ± 2.0 | 45.726 ± 1.2 |
| d12 | 45.242 ± 1.7 | 46.926 ± 1.5 |
| d18 | 46.126 ± 1.4 | 46.944 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tan, X.; Chen, Q.; Zhu, X.; Zhang, J.; Zhang, J.; Jia, B. Prodigiosin of Serratia marcescens ZPG19 Alters the Gut Microbiota Composition of Kunming Mice. Molecules 2021, 26, 2156. https://doi.org/10.3390/molecules26082156
Li X, Tan X, Chen Q, Zhu X, Zhang J, Zhang J, Jia B. Prodigiosin of Serratia marcescens ZPG19 Alters the Gut Microbiota Composition of Kunming Mice. Molecules. 2021; 26(8):2156. https://doi.org/10.3390/molecules26082156
Chicago/Turabian StyleLi, Xue, Xinfeng Tan, Qingshuang Chen, Xiaoling Zhu, Jing Zhang, Jie Zhang, and Baolei Jia. 2021. "Prodigiosin of Serratia marcescens ZPG19 Alters the Gut Microbiota Composition of Kunming Mice" Molecules 26, no. 8: 2156. https://doi.org/10.3390/molecules26082156







