Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Fingerprint Profile of Buddleja Globosa Methanol Extract
2.2. Isolation of Main Polyphenols from B. globosa by Centrifugal Partition Chromatography:
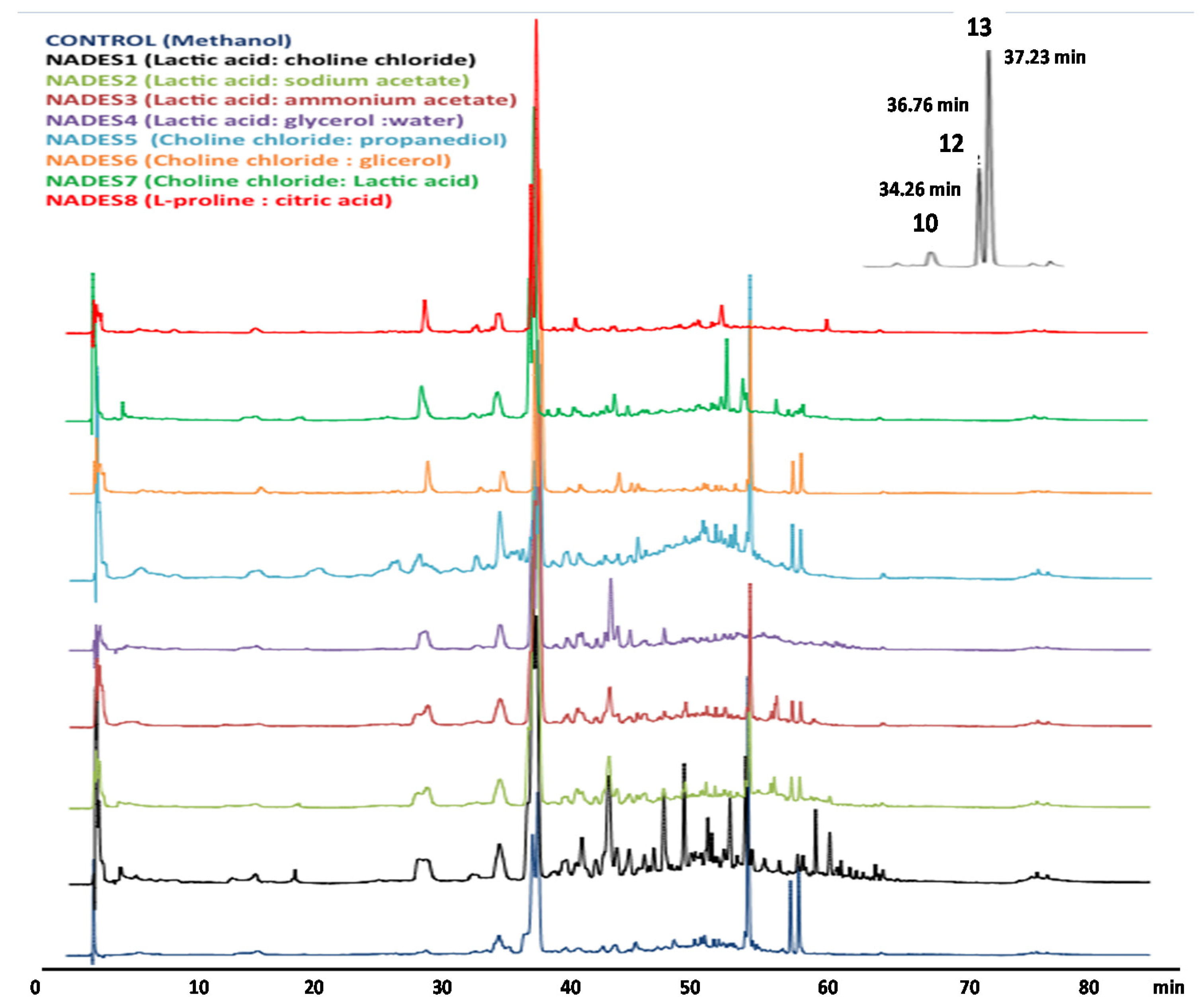
2.3. Comparison of the Extractability of Phenolic Compounds with NADES.
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Instruments and Chromatographic Conditions
3.3.1. Qualitative and Quantitative HPLC-DAD-IT-MS/MS Analysis
3.3.2. Q-TOF High-Resolution Mass Spectrometry Measurements
3.4. Centrifugal Partition Chromatography (CPC)
3.4.1. General Procedure
3.4.2. KD, Ni, and RS Calculations
3.5. Preparation of NADES
Extraction of Phenolic Compounds of B. globosa Leaves with Different NADES
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vogel, H.; Razmilic, I.; González, B. Matico (Buddleja globosa Hope): Evaluación de diferentes accesiones, número de cosechas, humedad del suelo y extracción de nutrientes. Agric. Técnica 2004, 64, 413–420. [Google Scholar] [CrossRef]
- Muñoz, O.; Montes, M.; Wilkomirsky, T. Plantas Medicinales de Uso en Chile: Química y Farmacología; Editorial Universitaria: Santiago, Chile, 2004; Volume 1. [Google Scholar]
- Montes, M.; Wilkomirsky, T. Medicina Tradicional Chilena; Editiorial de la Universidad de Concepción: Concepción, Chile, 1984; Volume 50. [Google Scholar]
- Houghton, P. Ethnopharmacology of some Buddleja species. J. Ethnopharmacol. 1984, 11, 293–308. [Google Scholar] [CrossRef]
- Mensah, A.Y.; Houghton, P.J.; Bloomfield, S.; Vlietinck, A.; Berghe, D.V. Known and novel terpenes from Buddleja globosa displaying selective antifungal activity against dermatophytes. J. Nat. Prod. 2000, 63, 1210–1213. [Google Scholar] [CrossRef]
- Houghton, P.; Hylands, P.; Mensah, A.; Hensel, A.; Deters, A. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J. Ethnopharmacol. 2005, 100, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Estomba, D.; Ladio, A.; Lozada, M. Medicinal wild plant knowledge and gathering patterns in a Mapuche community from North-western Patagonia. J. Ethnopharmacol. 2006, 103, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Mensah, A.; Sampson, J.; Houghton, P.; Hylands, P.; Westbrook, J.; Dunn, M.; Hughes, M.; Cherry, G. Effects of Buddleja globosa leaf and its constituents relevant to wound healing. J. Ethnopharmacol. 2001, 77, 219–226. [Google Scholar] [CrossRef]
- Backhouse, N.; Delporte, C.; Apablaza, C.; Farías, M.; Goïty, L.; Arrau, S.; Negrete, R.; Castro, C.; Miranda, H. Antinociceptive activity of Buddleja globosa (matico) in several models of pain. J. Ethnopharmacol. 2008, 119, 160–165. [Google Scholar] [CrossRef]
- Letelier, M.E.; Molina-Berríos, A.; Cortés-Troncoso, J.; Jara-Sandoval, J.; Holst, M.; Palma, K.; Montoya, M.; Miranda, D.; González-Lira, V. DPPH and oxygen free radicals as pro-oxidant of biomolecules. Toxicol. Vitr. 2008, 22, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, N.; Rosales, L.; Apablaza, C.; Goïty, L.; Erazo, S.; Negrete, R.; Theodoluz, C.; Rodriguez, J.; Delporte, C. Analgesic, anti-inflammatory and antioxidant properties of Buddleja globosa, Buddlejaceae. J. Ethnopharmacol. 2008, 116, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, B.; Assef, Y.; van Baren, C.; Di Leo Lira, P.; Retta, D.; Bandoni, A.L.; González, S.B. Actividad antioxidante en infusiones, tinturas y aceites esenciales de especies nativas de la Patagonia Argentina. Rev. Cuba. Plantas Med. 2016, 21, 51–62. [Google Scholar]
- Chen, X.-F.; Wu, H.-T.; Tan, G.-G.; Zhu, Z.-Y.; Chai, Y.-F. Liquid chromatography coupled with time-of-flight and ion trap mass spectrometry for qualitative analysis of herbal medicines. J. Pharm. Anal. 2011, 1, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Miron, A.; Ignatova, S.; Skalicka-Woźniak, K. An overview of the two-phase solvent systems used in the countercurrent separation of phenylethanoid glycosides and iridoids and their biological relevance. Phytochem. Rev. 2019, 18, 377–403. [Google Scholar] [CrossRef] [Green Version]
- Pardo, F.; Perich, F.; Villarroel, L.; Torres, R. Isolation of verbascoside, an antimicrobial constituent of Buddleja globosa leaves. J. Ethnopharmacol. 1993, 39, 221–222. [Google Scholar] [CrossRef]
- Choi, Y.H.; Van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Li, D.-D.; Zhang, Y.-H.; Zhang, W.; Zhao, P. Meta-Analysis of Randomized Controlled Trials on the Efficacy and Safety of Donepezil, Galantamine, Rivastigmine, and Memantine for the Treatment of Alzheimer’s Disease. Front. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.M.A.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [Green Version]
- Friščić, M.; Bucar, F.; Pilepić, K.H. LC-PDA-ESI-MSnanalysis of phenolic and iridoid compounds fromGlobulariaspp. J. Mass Spectrom. 2016, 51, 1211–1236. [Google Scholar] [CrossRef] [PubMed]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J. Food Drug Anal. 2019, 27, 692–702. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef]
- Houghton, P.J.; Mensah, A.Y. Biologically Active Compounds from Buddleja Species. In Phytochemicals in Human Health Protection, Nutrition, and Plant Defense; Springer: Boston, MA, USA, 1999; pp. 343–368. [Google Scholar]
- Pastene, E.; Speisky, H.; Troncoso, M.; Alarcón, J.; Figueroa, G. In Vitro Inhibitory Effect of Apple Peel Extract on the Growth ofHelicobacter pyloriand Respiratory Burst Induced on Human Neutrophils. J. Agric. Food Chem. 2009, 57, 7743–7749. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, C.; Chen, Y.; Liu, W.; Feng, F.; Xie, N. Comparative analysis of three Callicarpa herbs using high performance liquid chromatography with diode array detector and electrospray ionization-trap mass spectrometry method. J. Pharm. Biomed. Anal. 2013, 75, 239–247. [Google Scholar] [CrossRef]
- Ado, M.A.; Abas, F.; Leong, S.W.; Shaari, K.; Ismail, I.S.; Ghazali, H.M.; Lajis, N.H. Chemical constituents and biological activities of Callicarpa maingayi leaves. South Afr. J. Bot. 2016, 104, 98–104. [Google Scholar] [CrossRef]
- Guo, H.; Liu, A.-H.; Ye, M.; Yang, M.; Guo, D.-A. Characterization of phenolic compounds in the fruits ofForsythia suspensa by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tsao, R.; Liu, Z.; Liu, S.; Yang, R.; Young, J.C.; Zhu, H.; Deng, Z.; Xie, M.; Fu, Z. Isolation and purification of acteoside and isoacteoside from Plantago psyllium L. by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1063, 161–169. [Google Scholar] [CrossRef]
- Lee, W.-J.; Ou, H.-C.; Hsu, W.-C.; Chou, M.-M.; Tseng, J.-J.; Hsu, S.-L.; Tsai, K.-L.; Sheu, W.H.-H. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J. Vasc. Surg. 2010, 52, 1290–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crop. Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Mitreski, I.; Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S. Polyphenols in Representative Teucrium Species in the Flora of R. Macedonia: LC/DAD/ESI-MSn Profile and Content. Nat. Prod. Commun. 2014, 9, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, C.M.; Liu, Z.Q.; Wang, J. Isolation and purification of phenylethanoid glycosides from plant extract of Plantago asiatica by high performance centrifugal partition chromatography. Chin. Chem. Lett. 2008, 19, 1349–1352. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.-H.; Yue, H.-L.; Li, Y.-L.; Chen, T. Separation of Phenylpropanoid Glycosides from a Chinese Herb by HSCCC. J. Chromatogr. Sci. 2013, 52, 395–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.; Chi, X.; Zhou, G.; Zhao, X. Antioxidants from Pedicularis longiflora var. tubiformis (Klotzsch) P. C. Tsoong. Rec. Nat. Prod. 2018, 12, 332–339. [Google Scholar] [CrossRef]
- He, F.; Chen, L.; Liu, Q.; Wang, X.; Li, J.; Yu, J. Preparative Separation of Phenylethanoid and Secoiridoid Glycosides from Ligustri Lucidi Fructus by High-Speed Counter-Current Chromatography Coupled with Ultrahigh Pressure Extraction. Molecules 2018, 23, 3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roehrer, S.; Minceva, M. Evaluation of Inter-Apparatus Separation Method Transferability in Countercurrent Chromatography and Centrifugal Partition Chromatography. Separations 2019, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Frey, A.; Hopmann, E.; Minceva, M. Selection of Biphasic Liquid Systems in Liquid-Liquid Chromatography Using Predictive Thermodynamic Models. Chem. Eng. Technol. 2014, 37, 1663–1674. [Google Scholar] [CrossRef]
- Hopmann, E.; Frey, A.; Minceva, M. A priori selection of the mobile and stationary phase in centrifugal partition chromatography and counter-current chromatography. J. Chromatogr. A 2012, 1238, 68–76. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pérez-Navarro, J.; Pastene-Navarrete, E. Green Extraction of Alkaloids and Polyphenols from Peumus boldus Leaves with Natural Deep Eutectic Solvents and Profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep eutectic solvents: An overview on their interactions with water and biochemical compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar] [CrossRef]
- Shikov, A.N.; Kosman, V.M.; Flissyuk, E.V.; Smekhova, I.E.; Elameen, A.; Pozharitskaya, O.N. Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola Rosea L. Molecules 2020, 25, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef]
- Mulia, K.; Putri, S.; Krisanti, E. Nasruddin Natural deep eutectic solvents (NADES) as green solvents for carbon dioxide capture. AIP Conf. Pro. 2017, 1823, 020022. [Google Scholar] [CrossRef] [Green Version]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Mulia, K.; Fauzia, F.; Krisanti, E.A. Polyalcohols as Hydrogen-Bonding Donors in Choline Chloride-Based Deep Eutectic Solvents for Extraction of Xanthones from the Pericarp of Garcinia Mangostana L. Molecules 2019, 24, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Ahlgren, S.; Korthout, H.A.; Salomé-Abarca, L.F.; Bayona, L.M.; Verpoorte, R.; Choi, Y.H. Broad range chemical profiling of natural deep eutectic solvent extracts using a high performance thin layer chromatography–based method. J. Chromatogr. A 2018, 1532, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
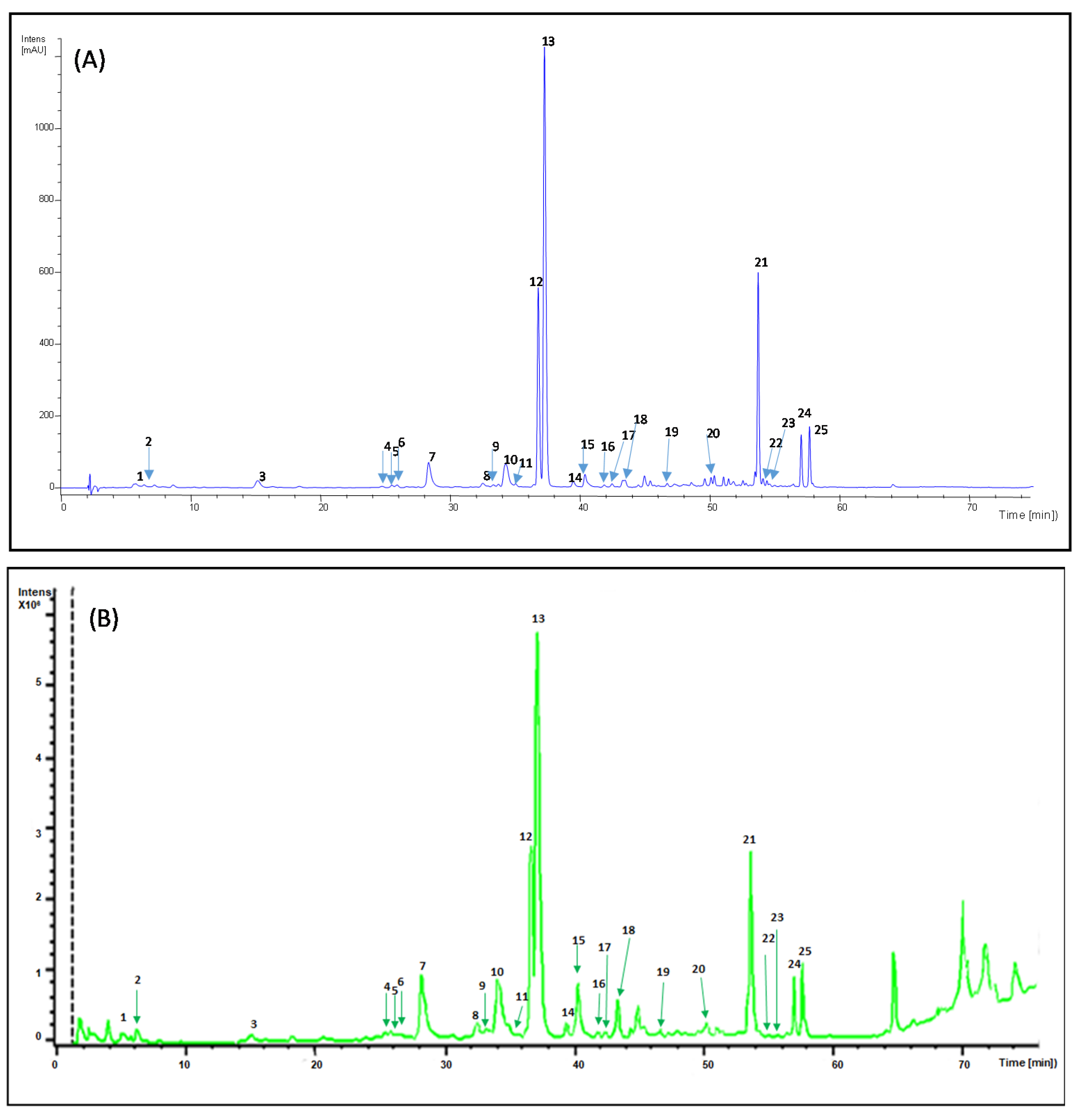
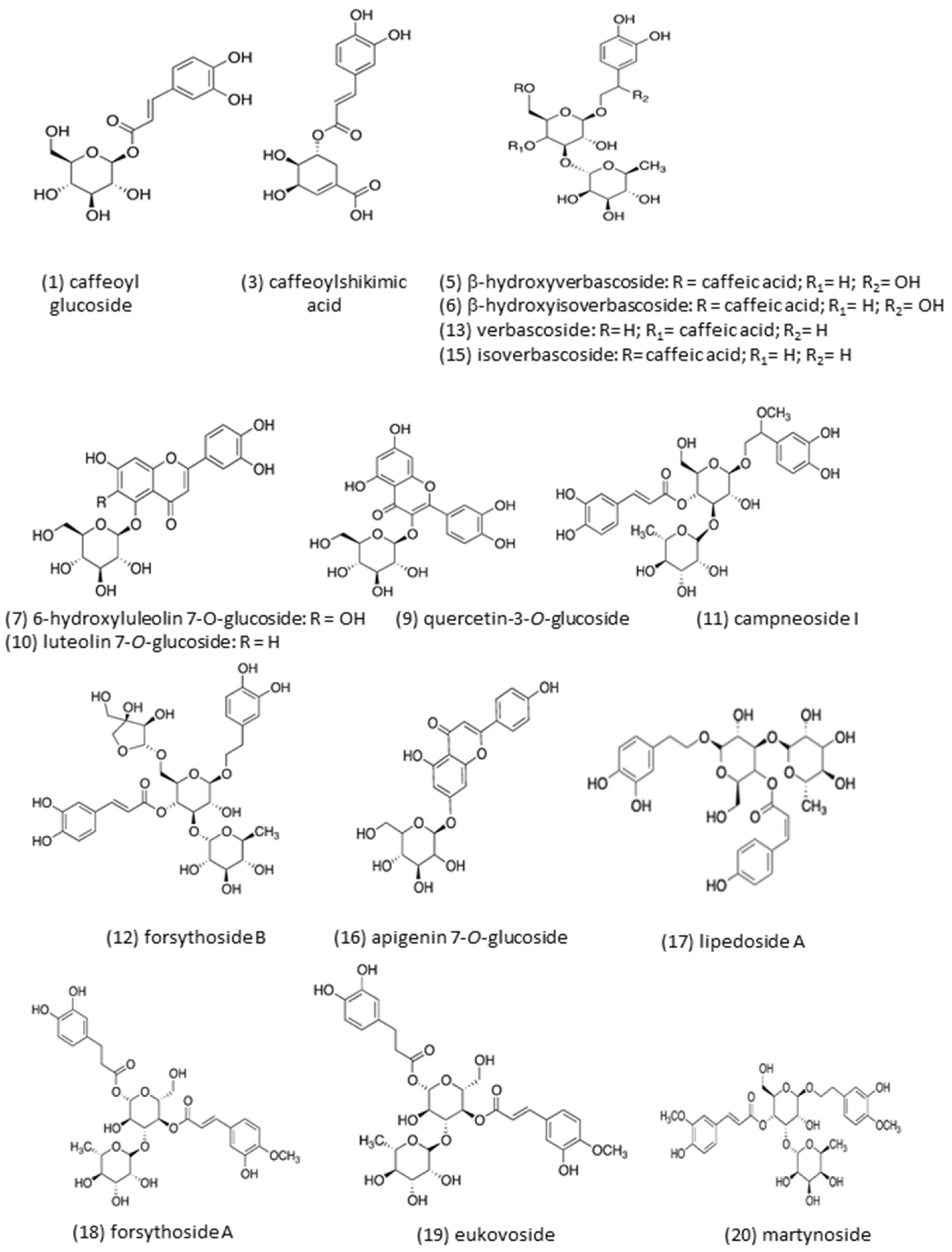
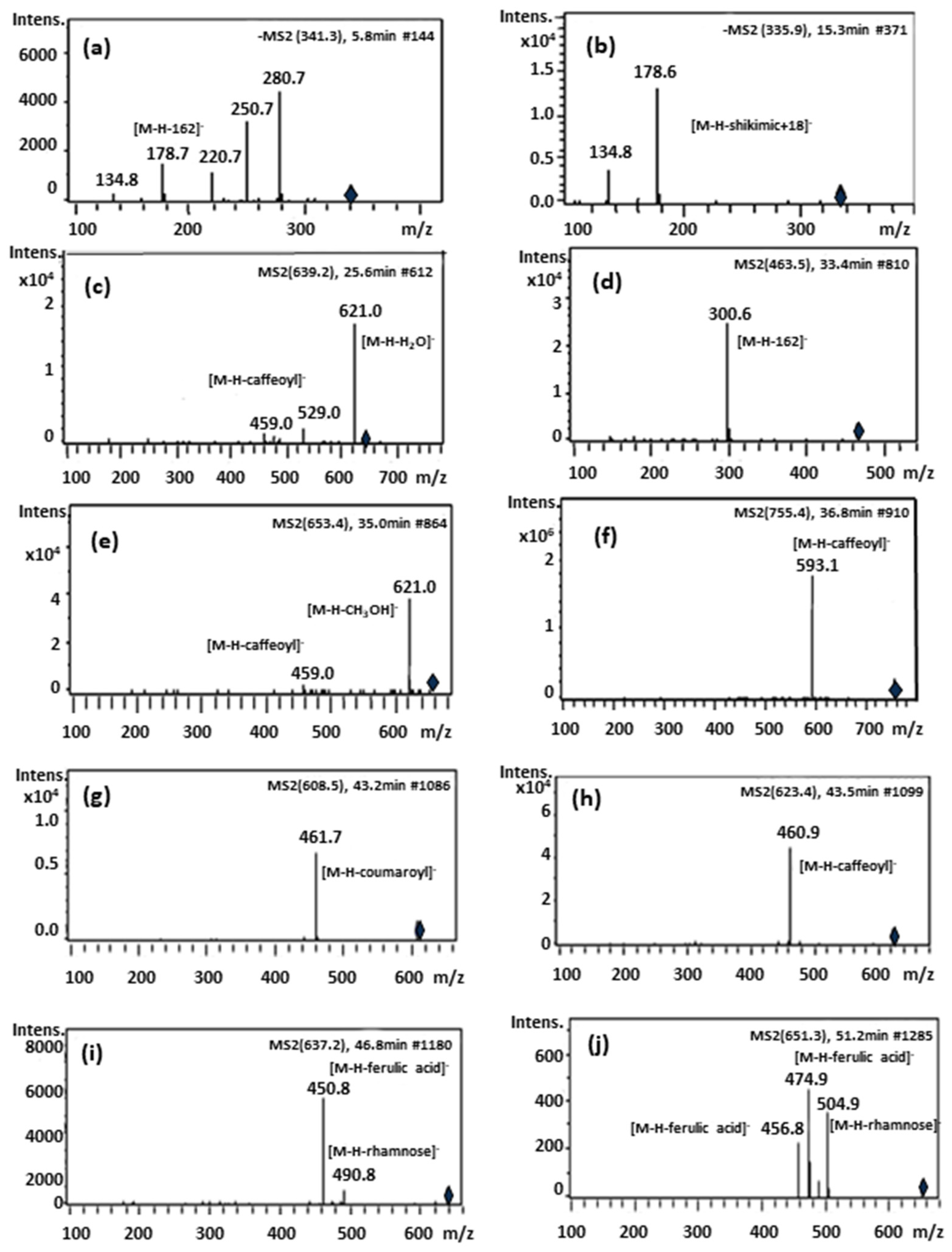



| Peak | Rt (min) | [M-H]− m/z | MS-MS Fragments | λ Max (nm) | Proposed Compound |
|---|---|---|---|---|---|
| 1 | 5.61 | 341.3 | 280.7, 250.7, 220.7, 178.7, 134. 7 | 228, 294 sh, 327 | Caffeoyl glucoside (isomer 1) |
| 2 | 7.19 | 341.0 | 280.7, 250.7, 220.6,178.6, 134.6 | 296 sh, 326 | Caffeoyl glucoside (isomer 2) |
| 3 | 15.14 | 335.6 | 178.6, 134.8 | 296 sh, 326 | Caffeoylshikimic acid |
| 4 | 24.72 | 537.3 | 518.9, 342.2, 294.7, 234.7, 178.7 | 294 sh, 321 | Unknown 1 |
| 5 | 25.49 | 639.2 | 621.0, 529.0, 459.0 | 296 sh, 327 | β -hydroxy-verbascoside |
| 6 | 25.89 | 639.3 | 621.0, 528.9, 459.1 | 296 sh, 329 | β -hydroxy-isoverbascoside |
| 7 | 28.32 | 463.2 | 300.6 | 255 o 258 sh, 281, 344 | 6-Hydroxyluteolin7-O-glucoside |
| 8 | 32.49 | 463.4 | 285.3 | 261 sh, 281 sh, 347 | Unknown 2 |
| 9 | 33.30 | 463.5 | 300.6 | 256, 260 sh, 283 sh, 351 | Quercetin-3-O-glucoside |
| 10 | 34.26 | 447.2 | 284.6 | 255 sh, 265, 282 sh, 346 | Luteolin 7-O-glucoside |
| 11 | 35.02 | 653.2 | 620.9, 459.0 | 296 sh, 332 | Campneoside I |
| 12 | 36.76 | 755.2 | 593.1 | 232, 294 sh, 331 | Forsythoside B |
| 13 | 37.23 | 623.3 | 460.9 | 296 sh, 330 | Verbascoside |
| 14 | 39.47 | 653.3 | 623.8, 490.8, 376.8, 308.7, 252.7 | 296 sh, 329 | Unknown 3 |
| 15 | 40.37 | 623.2 | 460.8 | 290 sh, 327 | Isoverbascoside |
| 16 | 40.61 | 431.2 | 268.7 | 266, 333 | Apigenin-7-O-glucoside |
| 17 | 41.85 | 607.5 | 460.9 | 296 sh, 326 | Lipedoside A |
| 18 | 43.45 | 623.4 | 460.9 | 290 sh, 326 | Forsythoside A |
| 19 | 46.70 | 637.3 | 490.8, 460.8, 314.8 | 290 sh, 327 | Eukovoside |
| 20 | 51.04 | 651.3 | 504.9, 474.9, 456.8, 372.8, 329.0, 250.6, | 286 sh, 329 | Martynoside |
| 21 | 53.73 | 763.4 | 667.8, 548.6, 488.9, 301.7 | 312 | Unknown 4 |
| 22 | 54.08 | 785.2 | 738.9, 678.9, 576.8, 546.9, 462.7 | 296 sh, 322 | Unknown 5 |
| 23 | 54.38 | 785.4 | 738.9, 678.9, 576.9, 547.0, 505.0, 462.9, | 296 sh, 324 | Unknown 6 |
| 24 | 57.03 | 755.5 | 709.0, 649.0, 546.9, 517.0, 433.0 | 298, 312 | Unknown 7 |
| 25 | 57.67 | 755.4 | 709.0, 649.0, 546.9, 517.0, 432.9 | 298 sh, 314 | Unknown 8 |
| Peak | Formula | Experimental (Observed) Mass | Mass (Monoisotopic Mass) Calculated | Error ppm | [M-H]− m/z | MS-MS Fragments | Proposed Compound |
|---|---|---|---|---|---|---|---|
| 1 | C15H18O9 | 342.09569 | 342.09508 | 1.78 | 341.08548 | 281.06520, 221.04528, 179.03544, 135.04563 | Caffeoyl glucoside (isomer 1) |
| 2 | C15H18O9 | 342.09544 | 342.09508 | 1.06 | 341.08499 | 281.06582, 179.03449, 135.04463 | Caffeoyl glucoside (isomer 2) |
| 3 | C16H16O8 | 336.2129 | 336. 2087 | 1.2 | 335.17819 | 179.03503, 135.04486 | Caffeoylshikimic acid |
| 4 | C25H30O13 | 538.1693 | 538.16864 | 1.22 | 537.1617 | 537.16155, 459.14979, 399.12976, 309.06201 | Unknown 1 |
| 5 | C29H36O16 | 640.20136 | 640.20034 | 1.6 | 639.19359 | 621.18255, 529.15682, 459.15179, 251.05644, | β -hydroxy-verbascoside |
| 6 | C29H36O16 | 640.20113 | 640.20034 | 1.25 | 639.19374 | 621.18123, 529.15366, 459.15003, 325.09311, 251.05530 | β -hydroxy-isoverbascoside |
| 7 | C21H20O12 | 464.09707 | 464.09548 | 3.43 | 463.08839 | 301.03568 | 6-Hydroxyluteolin 7-O-glucoside |
| 8 | C21H20O12 | 464.09547 | 464.09548 | 0.02 | 463.07781 | 282.06828 | Unknown 2 |
| 9 | C21H20O12 | 464.09569 | 464.09548 | 0.21 | 463.0885 | 300.02774 | Quercetin-3-O-glucoside |
| 10 | C21H20O11 | 448.10213 | 448.10056 | 3.5 | 447.0949 | 285.04091 | Luteolin 7-O-glucoside |
| 11 | C30H38O16 | 654.21747 | 654.21599 | 2.26 | 653.20999 | 621.07113, 459.12421 | Campneoside I |
| 12 | C34H44O19 | 756.2506 | 756.24768 | 3.86 | 755.24353 | 593.21042 | Forsythoside B |
| 13 | C29H36O15 | 624.20799 | 624.20542 | 4.12 | 623.20069 | 461.16809, 315.10869, 161.02544 | Verbascoside |
| 14 | C30H38O16 | 654.21716 | 654.21599 | 1.79 | 653.20931 | 377.12629, 249.07689, 163.04006 | Unknown 3 |
| 15 | C29H36O15 | 624.20749 | 624.20542 | 3.31 | 623.20047 | 461.16773, 315.10891, 161.02550 | Isoverbascoside |
| 16 | C21H20O10 | 432.10609 | 432.10565 | 1.03 | 431.09884 | 268.03810 | Apigenin-7-O-glucoside |
| 17 | C28H32O15 | 608.17317 | 608.17412 | 1.57 | 607.16510 | 461.07231 | Lipedoside A |
| 18 | C29H36O15 | 624.20729 | 624.20542 | 2.2 | 623.19644 | 461.16656 | Forsythoside A |
| 19 | C30H38O15 | 638.22184 | 638.22107 | 1.21 | 637.21464 | 461.16669, 315.10963, 175.04031 | Eukovoside |
| 20 | C31H40O15 | 652.23711 | 652.23672 | 0.59 | 651.23043 | 505.08127, 475.06172, 456.15642 | Martynoside |
| 21 | C35H40O19 | 764.25426 | 764.25314 | 1.36 | 763.18271 | 668.02938, 549.15312, 489.07663, 301.81022 | Unknown 4 |
| 22 | C35H46O20 | 786.25933 | 786.25824 | 1.38 | 785.25227 | 623.18693, 547.17931, 463.16063, 378.91956, 291.08289, 207.06629, | Unknown 5 |
| 23 | C35H46O20 | 786.25931 | 786.25824 | 1.35 | 785.25239 | 547.18298, 463.16087, 341.09857, 207.06621, 163.03947 | Unknown 6 |
| 24 | C34H44O19 | 756.25029 | 756.24768 | 3.46 | 755.2433 | 709.23749, 465.02019, 405.01723, 341.00981, 285.04084 | Unknown 7 |
| 25 | C34H44O19 | 756.25026 | 756.24768 | 3.42 | 755.24323 | 709.23553, 593.13026, 541.03515, 497.02419, | Unknown 8 |
| # | Solvent System | Ratio v/v | 12 a | 13 a | Ref. |
|---|---|---|---|---|---|
| 1 | Ethyl acetate-n-butanol-ethanol-water | 0.25: 0.75: 0.1: 1 | 0.51 | 3.02 | [32] |
| 2 | Ethyl acetate-n-butanol-ethanol-water | 0.5: 0.5: 0.1: 1 | 0.19 | 4.96 | [36] |
| 3 | Ethyl acetate-n-butanol-ethanol-water | 4: 0.6: 0.6: 5 | 0.08 | 1.93 | [36] |
| 4 | Ethyl acetate- n-butanol-water | 10: 6: 15 | 1.33 | 0.33 | [15] |
| 5 | Ethyl acetate- n-butanol-water | 2:1:3 | 1.94 | 0.24 | [37] |
| 6 | Ethyl acetate- n-butanol-water | 13: 3: 9 | 0.01 | 0.35 | [38] |
| 7 | Ethyl acetate-water | 1: 1 | 0.00 | 0.02 | [38] |
| 8 | Chloroform- n-butanol-methanol-water | 3: 2: 4: 5 | 37.42 | 3.51 | [39] |
| 9 | Chloroform- n-butanol-methanol-water | 4: 3: 4: 5 | 41.86 | 3.50 | [39] |
| Code | NADES Composition | Molar Ratio | Conditions |
|---|---|---|---|
| NADES1 | Lactic acid: choline chloride | 3:1 | 15 min; 50 °C; 700 rpm |
| NADES2 | Lactic acid: sodium acetate | 3:1 | 15 min; 50 °C; 700 rpm |
| NADES3 | Lactic acid: ammonium acetate | 3:1 | 15 min; 50 °C; 700 rpm |
| NADES4 | Lactic acid: glycerol: water | 3:1:3 | 40 min; 50 °C; 900 rpm |
| NADES5 | Choline chloride: 1,2- propanediol | 1:3 | 20 min; 60 °C; 1000 rpm |
| NADES6 | Choline chloride: glycerol | 1:2 | 60 min; 80 °C; 1000 rpm |
| NADES7 | Choline chloride: lactic acid | 1:1 | 60 min; 60 °C; 1000 rpm |
| NADES8 | L-Proline: Citric acid | 1:1 | 120 min; 80 °C; 1000 rpm |
Publisher’s Note:MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Vega, J.; Gómez-Alonso, S.; Pérez-Navarro, J.; Alarcón-Enos, J.; Pastene-Navarrete, E. Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography. Molecules 2021, 26, 2192. https://doi.org/10.3390/molecules26082192
Torres-Vega J, Gómez-Alonso S, Pérez-Navarro J, Alarcón-Enos J, Pastene-Navarrete E. Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography. Molecules. 2021; 26(8):2192. https://doi.org/10.3390/molecules26082192
Chicago/Turabian StyleTorres-Vega, Jeniffer, Sergio Gómez-Alonso, José Pérez-Navarro, Julio Alarcón-Enos, and Edgar Pastene-Navarrete. 2021. "Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography" Molecules 26, no. 8: 2192. https://doi.org/10.3390/molecules26082192
APA StyleTorres-Vega, J., Gómez-Alonso, S., Pérez-Navarro, J., Alarcón-Enos, J., & Pastene-Navarrete, E. (2021). Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography. Molecules, 26(8), 2192. https://doi.org/10.3390/molecules26082192






