Acanthopanax henryi: Review of Botany, Phytochemistry and Pharmacology
Abstract
1. Introduction
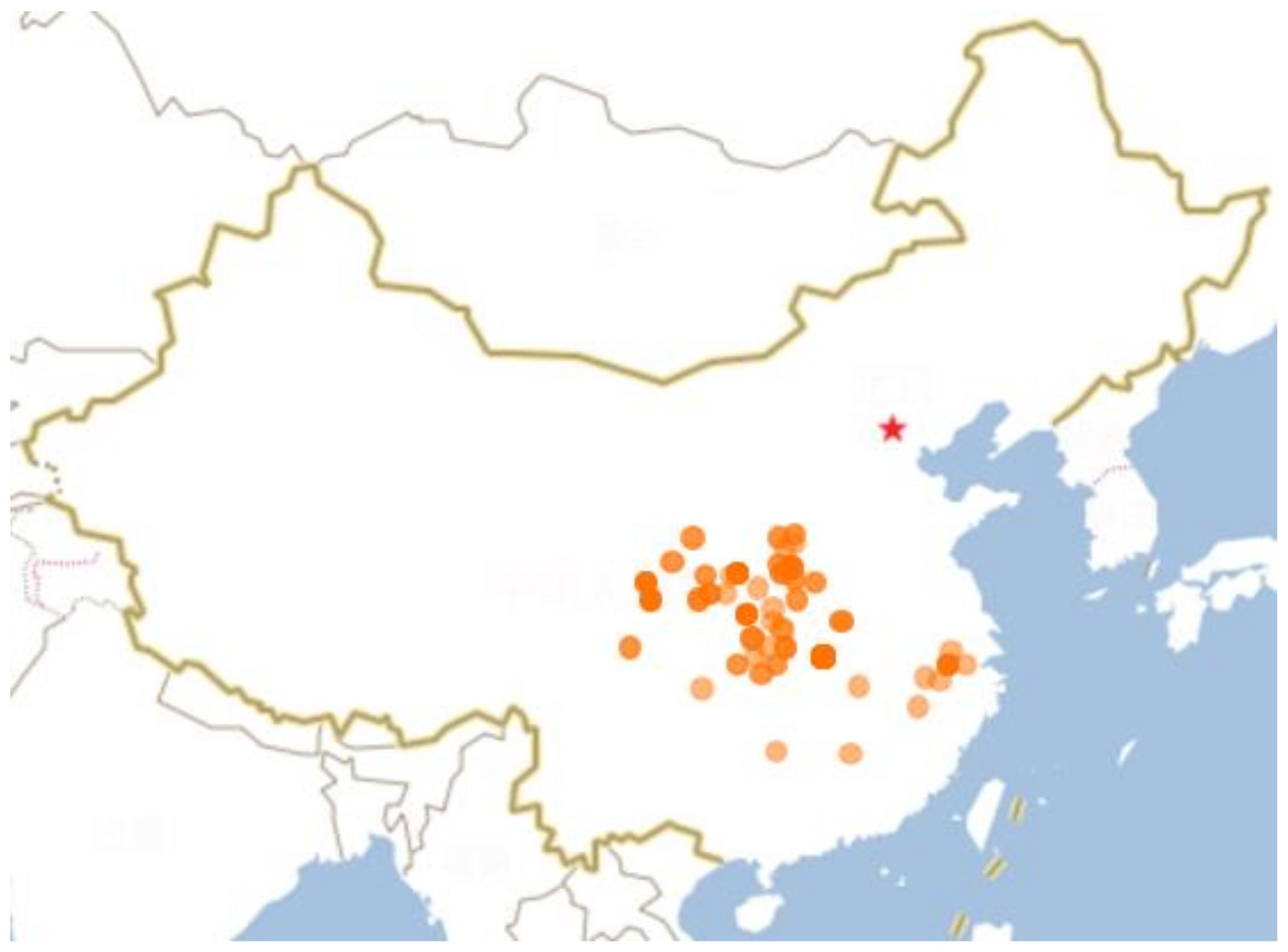
2. Botany
3. Chemical Constituents
3.1. Monoterpenoids
3.2. Diterpenoids
3.3. Triterpenoid Saponins
3.4. Phenylpropanoids
3.5. Caffeoyl Quinic Acids
3.6. Flavonoids
3.7. Lignans
3.8. Steroids
3.9. Fatty Acids
3.10. Other Compounds
4. Pharmacology Research
4.1. Anti-Neuroinflammatory Activity
4.2. Anti-Adipogenic Effects
4.3. Anti-Inflammatory Activity
4.4. Antimicrobial Activity
4.5. Anticancer/Antitumor Activities
4.6. Anti-Oxidant, Anti-AChE, and Anti-BuChE Activities
4.7. Anti-Hyaluronidase Activity
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Flora of China Editorial of Committee of Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1978; Volume 54, p. 102. [Google Scholar]
- Zhang, B.B.; Li, X.J.; Gao, D.L.; Xiao, S.; Lu, M.X.; Lu, M.F.; Liu, X.Q. Advances in Chemodiversity from Acanthopanax Miq. J. Tradit. Chin. Med. Univ. Hunan 2019, 39, 556–560. [Google Scholar]
- Liu, X.Q. Studies on the Active Constutuents of Acanthopanax gracilistylus W.W. Smith; Kyung Hee University: Seoul, Korea, 2003. [Google Scholar]
- Sithisarn, P.; Jarikasem, S. Antioxidant activity of Acanthopanax trifoliatus. Med. Princ. Pract. 2009, 18, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Perry, L. Medicinal Plants of East & Southeast Asia Attributed Properties and Uses; The MTT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Park, S.Y.; Chang, S.Y.; Oh, O.J.; Yook, C.S.; Nohara, T. Nor-oleanene type triterpene glycosides from the leaves of Acanthopanax japonicus. Phytochemistry 2002, 59, 379–384. [Google Scholar] [CrossRef]
- Zhang, X.D. Studies on the Active Constituents from Leaves of Acanthopanax henryi (Oliv.) Harms; Kyung Hee University: Seoul, Korea, 2013. [Google Scholar]
- Hunan Food and Drug Administration. Provincial Standard for Traditional Chinese Medicinal Materials in Hunan; Hunan Science and Technology Press: Changsha, China, 2009. [Google Scholar]
- Editorial Committee of Chinese Materia Medica of State Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999. [Google Scholar]
- Li, S.Z. Compendium of Materia Medica; People’s Medical Publishing House: Beijing, China, 1975. [Google Scholar]
- Li, X.J.; Kim, K.W.; Kim, D.C.; Oh, H.; Liu, X.Q.; Kim, Y.C. Three novel monoterpenoid glycosides from fruits of Eleutherococcus henryi. Nat. Prod. Res. 2019, 30, 1–8. [Google Scholar]
- Li, X.J.; Kim, K.W.; Zhang, X.D.; Oh, H.; Kim, Y.C.; Liu, X.Q. Study on chemical constituents and their anti-inflammatory activity from ethyl acetate extract of fruits of Acanthopanax henryi (Oliv.) Harms. Nat. Prod. Res. Dev. 2020, 32, 427–434. [Google Scholar]
- Tang, S.Q.; Luo, J.; Huang, H.; Oh, H.; Kim, Y.C.; Liu, X.Q.; Li, X.J. Study on anti-inflammatory active constituents from n-butanol extract of Acanthopanax henryi. Nat. Prod. Res. Dev. 2021, 1–15. Available online: http://kns.cnki.net/kcms/detail/51.1335.Q.20210208.1641.012.html (accessed on 9 February 2021).
- Li, Z.; Kim, J.H.; Liu, X.Q.; Lee, K.T.; Yook, C.S. Anticancer effects in vitro and chemical compositions of extracts of Acanthopanax henryi. J. Acup. Herbs 2014, 1, 49–54. [Google Scholar]
- Liu, H.Y.; Kim, J.W.; Liu, X.Q.; Yook, C.S. Study on chemical constituents from root barks of Acanthopanax henryi. J. Tradit. Chin. Med. Univ. Hunan 2012, 32, 34–37. [Google Scholar]
- Li, Z.; Li, X.J.; Kwon, O.K.; Wang, X.; Zou, Q.P.; Liu, X.Q.; Lee, H.K. Chemical constituents from leaves of Acanthopanax henryi (II). Nat. Prod. Sci. 2015, 21, 196–204. [Google Scholar]
- Wang, X.; Li, X.J.; Li, Z.; Zhang, X.D.; Yook, C.S.; Liu, X.Q. Chemotaxonomic significance of triterpenoid saponins and other secondary metabolites from Acanthopanax henryi. Nat. Prod. Res. Dev. 2016, 28, 1903. [Google Scholar]
- Zhang, X.D.; Li, Z.; Liu, G.Z.; Wang, X.; Kwon, O.K.; Lee, H.K.; Whang, W.K.; Liu, X.Q. Quantitative determination of 15 bioactive triterpenoid saponins in different parts of Acanthopanax henryi by HPLC with charged aerosol detection and confirmation by LC-ESI-TOF-MS. J. Sep. Sci. 2016, 39, 2252–2262. [Google Scholar]
- Li, X.J.; Kim, K.W.; Oh, H.; Liu, X.Q.; Kim, Y.C. Chemical constituents and an antineuroinflammatory lignan, savinin from the roots of Acanthopanax henryi. Evid. Based Complement. Altern. Med. 2019, 21, 1856294. [Google Scholar]
- Li, X.J.; Kim, K.W.; Oh, H.; Kim, Y.C.; Liu, X.Q. Chemical constituents from stems of Acanthopanax henryi. Chin. Tradit. Herb. Drugs 2019, 50, 1055–1060. [Google Scholar]
- Li, X.J.; Oh, H.; Kim, Y.C.; Liu, X.Q. Chemical constituents from the flowers of Acanthopanax henryi and their anti-inflammatory activities. Chin. Tradit. Pat. Med. 2019, 41, 1856–1862. [Google Scholar]
- Zhang, X.D.; Liu, X.Q.; Kim, Y.H.; Whang, W.K. Chemical constituents and their acetyl cholinesterase inhibitory and antioxidant activities from leaves of Acanthopanax henryi: Potential complementary source against Alzheimer’s disease. Arch. Pharm. Res. 2014, 37, 606–616. [Google Scholar]
- Li, Z.; Zou, Q.P.; Li, X.J.; Ye, H.X.; Huang, W.C.; Xie, X.; Liu, X.Q. Study on chemical constituents from the leaves of Acanthopanax henryi (Oliv.) Harms. J. Tradit. Chin. Med. Univ. Hunan 2014, 34, 24–27. [Google Scholar]
- Zhang, H.C.; Jia, Z.J. Study on the chemical constituents of Acanthopanax henryi (Oliv.) Harms. J. Lanzhou Univ. Nat. Sci. 1993, 29, 76–79. [Google Scholar]
- Feng, S.; Liu, X.Q.; Zhang, W.L.; Gao, J.M.; Lee, G.H. Determination of eleutherosides B and E from different parts of Acanthopanax henryi by RP-HPLC. Chin. Tradit. Herb. Drugs 2011, 6, 111–113. [Google Scholar]
- Li, Z. Study on chemical constituents from leaves of Acanthopanax henryi. Hunan Univ. Chin. Med. 2015, 21, 196–204. [Google Scholar] [CrossRef][Green Version]
- Huang, L.; Zhao, H.; Huang, B.; Zheng, C.; Peng, W.; Qin, L. Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Pharmazie 2011, 66, 83–97. [Google Scholar]
- Majdalawieh, A.F.; Massri, M.; Nasrallah, G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). Eur. J. Pharmacol. 2017, 815, 512–521. [Google Scholar]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of sesamin alleviates stress-Induced behavioral and psychological disorders via reshaping the gut microbiota structure. J. Agric. Food Chem. 2019, 67, 12441–12451. [Google Scholar]
- Han, Y.H.; Li, Z.; Um, J.Y.; Liu, X.Q.; Hong, S.H. Anti-adipogenic effect of Glycoside St-E2 and Glycoside St-C1 isolated from the leaves of Acanthopanax henryi (Oliv.) Harms in 3T3-L1 cells. Biosci. Biotecnolh. Biochem. 2016, 80, 2391–2400. [Google Scholar]
- Kang, D.H.; Kang, O.H.; Li, Z.; Mun, S.H.; Seo, Y.S.; Kong, R.; Zhou, T.; Liu, X.Q.; Kwon, D.Y. Anti-inflammatory effects of Ciwujianoside C3, extracted from the leaves of Acanthopanax henryi (Oliv.) Harms, on LPS-stimulated RAW 264.7 cells. Mol. Med. Rep. 2016, 14, 3749–3758. [Google Scholar]
- Seo, Y.S.; Lee, S.J.; Li, Z.; Kang, O.H.; Kong, R.; Kim, S.A.; Zhou, T.; Song, Y.S.; Liu, X.Q.; Kwon, D.Y. Araliasaponin II isolated from leaves of Acanthopanax henryi (Oliv.) Harms inhibits inflammation by modulating the expression of inflammatory markers in murine macrophages. Mol. Med. Rep. 2017, 16, 857–864. [Google Scholar]
- Kim, J.H.; Liu, X.Q.; Dai, L.; Yook, C.S.; Lee, K.T. Cytotoxicity and anti-inflammatory effects of root bark extracts of Acanthopanax henryi. Chin. J. Nat. Med. 2014, 12, 121–125. [Google Scholar]
- Zhou, T.; Li, Z.; Kang, O.H.; Mun, S.H.; Seo, Y.S.; Kong, R.; Shin, D.W.; Liu, X.Q.; Kwon, D.Y. Antimicrobial activity and synergism of ursolic acid 3-O-α-L-arabinopyranoside with oxacillin against methicillin-resistant Staphylococcus aureu. Int. J. Mol. Med. 2017, 40, 1285–1293. [Google Scholar]
- Li, Q.Q.; Luo, J.; Liu, X.Q.; Kwon, D.Y.; Kang, O.H. Eleutheroside K isolated from Acanthopanax henryi (Oliv.) Harms suppresses methicillin resistance of Staphylococcus aureus. Lett. Appl. Microbiol. 2020, 2020, 32955753. [Google Scholar]
- Adamczyk, K.; Olech, M.; Abramek, J.; Pietrzak, W.; Kuźniewski, R.; Bogucka-Kocka, A.; Nowak, R.; Ptaszyńska, A.A.; Rapacka-Gackowska, A.; Skalski, T.; et al. Eleutherococcus species cultivated in Europe: A new source of compounds with antiacetylcholinesterase, antihyaluronidase, anti-DPPH, and cytotoxic activities. Oxid. Med. Cell Longev. 2019, 2019, 8673521. [Google Scholar]
- Załuski, D.; Kuźniewski, R. In vitro anti-AChE, anti-BuChE, and antioxidant activity of 12 extracts of Eleutherococcus species. Oxid. Med. Cell Longev. 2016, 2016, 4135135. [Google Scholar]
- Załuski, D.; Janeczko, Z. Variation in phytochemicals and bioactivity of the fruits of Eleutherococcus species cultivated in Poland. Nat. Prod. Res. 2015, 29, 2207–2211. [Google Scholar]
- Yang, J.B.; Cai, W.; Li, M.H.; Li, N.X.; Ma, S.C.; Cheng, X.L.; Wei, F. Progress in Chemical and Pharmacological Research of Acanthopanax gracilistylus. Mod. Chin. Med. 2020, 22, 652–662. [Google Scholar]








| Classification | NO. | Chemical Component | Chemical Formula | Part of Plant | Ref. |
|---|---|---|---|---|---|
| Monoterpenoids | 1 | Eleuhenryiside A (new) | C18H30O8 | Fruit | [11] |
| 2 | Eleuhenryiside B (new) | C18H30O8 | Fruit | [11] | |
| 3 | Eleuhenryiside C (new) | C16H28O7 | Fruit | [11] | |
| 4 | 3,4-dihydroxy-p-menth-1-ene | C10H18O2 | Fruit | [12] | |
| 5 | (4R)-p-Menth-1-en-4,7-diol | C10H18O2 | Fruit | [12] | |
| 6 | (2E,6S)-1-hydroxy-2,6-dimethyl-2,7-octadien-6-yl-β-d-glucopyranoside | C16H28O7 | Fruit | [13] | |
| 7 | (2Z,6R)-6-hydroxy-2,6-dimethyl-2,7-octadien-1-yl-β-d-glucopyranoside | C16H28O7 | Fruit | [13] | |
| 8 | (2Z,6R)-1-hydroxy-2,6-dimethyl-2,7-octadien-6-yl-β-d-glucopyranoside | C16H28O7 | Fruit | [13] | |
| 9 | (2E,6R)-2,6-dimethyl-2,7-octadiene-1,6-diol | C10H18O2 | Fruit | [12] | |
| 10 | (2E,6R)-6-hydroxy-2,6-dimethyl-2,7-octadien-1-yl-β-d-glucopyranoside | C16H28O7 | Fruit | [13] | |
| 11 | (2E,6R)-1-hydroxy-2,6-dimethyl-2,7-octadien-6-yl-β-d-glucopyranoside | C16H28O7 | Fruit | [13] | |
| 12 | (+)-(3S,4S,6R)-3,6-dihydroxy-1-menthene | C10H18O2 | Fruit | [12] | |
| 13 | (−)-(3S,4S,6R)-3,6-dihydroxy-1-menthene 6-O-β-d-glucopyranoside | C16H28O7 | Fruit | [13] | |
| 14 | (−)-(3S,4S,6R)-3,6-dihydroxy-1-menthene 3-O-β-d-glucopyranoside | C16H28O7 | Fruit | [13] | |
| Diterpenoids | 15 | Acanthoic acid | C20H30O2 | Root | [14] |
| 16 | Kaurenoic acid | C20H30O2 | Root | [14] | |
| 17 | Pimaric acid | C20H30O2 | Root | [15] | |
| Triterpenoid saponins | 18 | Ursolic acid 3-O-α-L-arabinopyranoside | C35H56O7 | Leaf | [16,17,18] |
| 19 | Echinocystic acid 3-O-α-L-arabinopyranoside | C35H56O8 | Leaf | [16,17,18] | |
| 20 | Eleutheroside K | C41H66O11 | Leaf | [16,17,18] | |
| 21 | Prosapogenin CP2b | C40H64O11 | Leaf | [16,17,18] | |
| 22 | Tauroside D | C41H66O12 | Leaf | [16,17,18] | |
| 23 | Guaianin N (Glycoside St-C1) | C41H66O12 | Leaf | [16,17,18] | |
| 24 | Matesaponin J2 | C41H66O12 | Leaf | [16,17,18] | |
| 25 | Echinocystic acid 3-O-β-d-glucopyranosyl-(1→3) -O-α-L-arabinopyranoside | C41H66O13 | Leaf | [16,17,18] | |
| 26 | Hemslonin A | C42H68O13 | Leaf | [16,17,18] | |
| 27 | Cussonoside B | C48H78O17 | Leaf | [16,17,18] | |
| 28 | Oleanolic acid 3-O-[β-d-glucopyranosyl-(1→3)]-β-d-galactopyranosyl -(1→2)-O-α-L-arabinopyranoside (Glycoside St-E2) | C47H76O17 | Leaf | [16,17,18] | |
| 29 | Ciwujianoside C3 | C53H86O21 | Leaf | [16,17,18] | |
| 30 | Ursolic acid 3-O-α-L-arabinopyranosyl-28-O-α-L-rhamnopyranosyl-(1→4)-O-β-d -glucopyranosyl-(1→6)-O-β-d-glucopyranoside | C53H86O21 | Leaf | [16,17,18] | |
| 31 | Oleanolic acid 3-O-β-d-glucuronopyranoside | C36H56O9 | Leaf, fruit | [11,16,17,18] | |
| 32 | Araliasaponin II | C53H86O22 | Leaf | [16,17,18] | |
| 33 | Begoniifolide A | C59H96O26 | Leaf | [16,17,18] | |
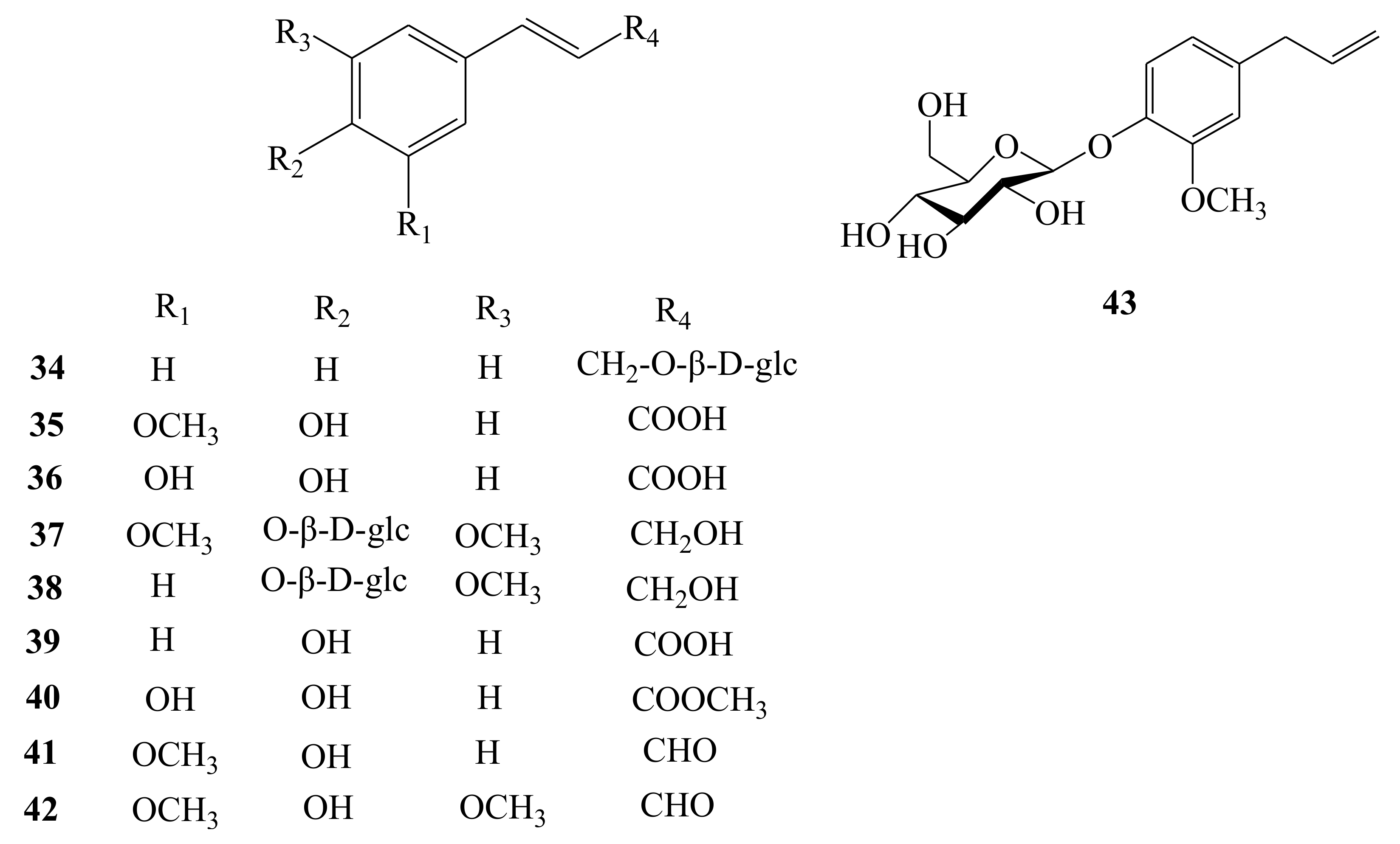
| Phenylpropanoids | 34 | Rosin | C15H20O6 | Fruit | [12] |
| 35 | Ferulic acid | C10H10O4 | Root | [19] | |
| 36 | Caffeic acid | C9H8O4 | Root, stem | [19,20] | |
| 37 | Syringin | C17H24O9 | Root | [19] | |
| 38 | Trans-coniferin | C16H22O8 | Root | [19] | |
| 39 | Trans-p-hydroxycinnamic acid | C9H8O3 | Stem | [20] | |
| 40 | (E)-caffeic acid methyl ester | C10H10O4 | Stem | [20] | |
| 41 | Trans-coniferyl aldehyde | C10H10O3 | Stem | [20] | |
| 42 | Trans-sinapaldehyde | C11H12O4 | Stem | [20] | |
| 43 | Eugenol glucoside | C16H22O7 | Flower | [21] | |
| Caffeoyl quinic acids | 44 | 1,3-di-O-caffeoyl quinic acid | C25H24O12 | Fruit, root, stem, flower | [13,19,20,21] |
| 45 | 1,4-di-O-caffeoyl quinic acid | C25H24O12 | Fruit, root, stem, flower | [13,19,20,21] | |
| 46 | 1,5-di-O-caffeoyl quinic acid | C25H24O12 | Fruit, root, stem, flower, leaf | [13,19,20,21,22] | |
| 47 | 3,4-di-O-caffeoyl quinic acid | C25H24O12 | Fruit, flower, leaf | [13,21,22] | |
| 48 | 3,5-di-O-caffeoyl quinic acid | C25H24O12 | Fruit, flower, leaf | [13,21,22] | |
| 49 | 4,5-di-O-caffeoyl quinic acid | C25H24O12 | Fruit, flower, leaf | [13,21,22] | |
| 50 | Methyl chlorogenate | C17H20O9 | Fruit | [13] | |
| 51 | 3-O-caffeoyl quinic acid | C16H18O9 | Root, stem | [19,20] | |
| 52 | 4-O-caffeoyl quinic acid | C16H18O9 | Leaf | [22] | |
| 53 | 5-O-caffeoyl quinic acid | C16H18O9 | Root, stem, leaf | [19,20,22] | |
| 54 | 3,5-dicaffeoylquinic acid methyl ester | C26H26O12 | Flower | [21] | |
| 55 | 3,4-dicaffeoylquinic acid methyl ester | C26H26O12 | Flower | [21] | |
| 56 | 1,3-dicaffeoylquinic acid methyl ester | C26H26O12 | Flower | [21] | |
| Flavonoids | 57 | Quercetin-3-O-β-d-glucopyranoside | C21H20O12 | Fruit, flower, leaf | [13,21,22,23] |
| 58 | Quercetin-3-O-β-d-galactopyranoside | C21H20O12 | Fruit | [13] | |
| 59 | Rutin | C27H30O16 | Fruit, flower, leaf | [13,21,22,23] | |
| 60 | Kaempferol-3-O-β-d-glucoside | C21H20O11 | Fruit, flower | [12,21] | |
| 61 | Kaempferol-3-rutinoside | C27H30O15 | Fruit, flower, leaf | [12,21,22] | |
| 62 | Kaempferol-3-O-α-L-rhamnoside | C21H20O10 | Flower | [21] | |
| 63 | Kaempferol | C15H10O6 | Flower, leaf | [21,23] | |
| 64 | Quercetin | C15H10O7 | Leaf | [22,23] | |
| 65 | Quercetin-3,7-di-β-O-glucopyranoside | C28H34O17 | Leaf | [22] | |
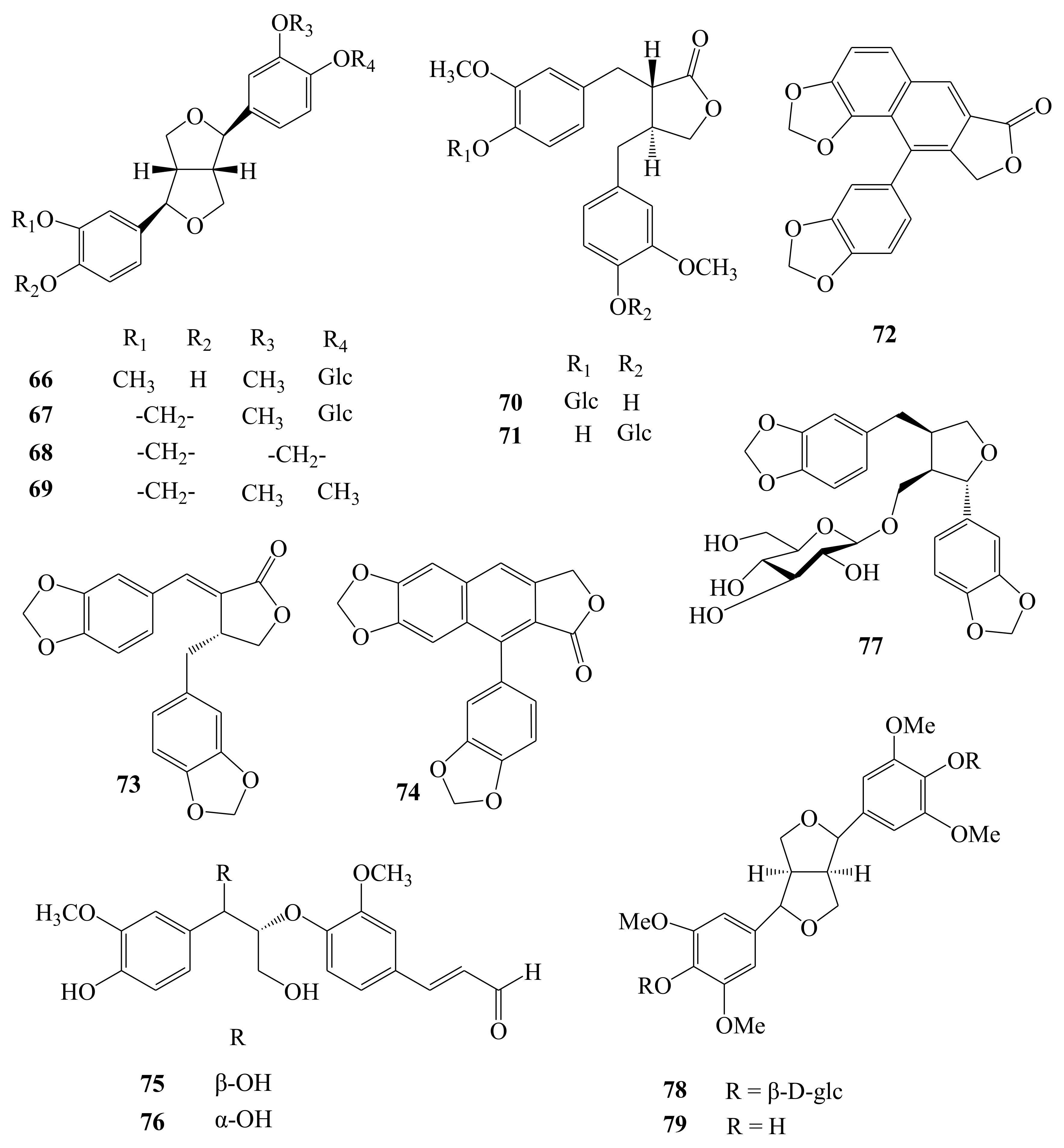
| Lignans | 66 | (−)-Pinoresinol 4-O-β-d-glucopyranoside | C26H32O11 | Fruit | [12] |
| 67 | (+)-Simplexoside | C26H30O11 | Fruit | [12] | |
| 68 | (−)-Sesamin | C20H18O6 | Fruit, root, stem | [12,19,20] | |
| 69 | (−)-Kobusin | C21H22O6 | Fruit | [11] | |
| 70 | Styraxlignolide E | C26H32O11 | Fruit | [12] | |
| 71 | Styraxlignolide D | C26H32O11 | Fruit | [12] | |
| 72 | Helioxanthin | C20H12O6 | Root | [19] | |
| 73 | Savinin | C20H16O6 | Root | [19] | |
| 74 | Taiwanin C | C20H12O6 | Root | [19] | |
| 75 | (+)-threo-(7R,8R)-guaiacylglycerol-β-coniferyl aldehyde ether | C20H22O7 | Root | [19] | |
| 76 | (+)-erythro-(7S,8R)-guaiacylglycerol-β-coniferyl aldehyde ether | C20H22O7 | Root | [19] | |
| 77 | Dihydrosesamin-9-O-β-d-glucopyranoside | C26H30O11 | Flower | [21] | |
| 78 | Syringaresinol diglucoside (Eleutheroside E) | C34H46O18 | Root | [15,24,25] | |
| 79 | Syringaresinol | C22H26O8 | Root | [24] | |
| Steroids | 80 | Stigmasterol | C29H48O | Root, stem, leaf | [15,19,20,23] |
| 81 | β-sitosterol | C29H50O | Root, stem | [15,19,20,24] | |
| 82 | Daucosterol | C35H60O6 | Root, leaf | [14,23] | |
| 83 | Stigmasterol-3-O-β-d-glucopyranoside | C35H58O6 | Leaf | [26] | |
| Fatty acids | 84 | Behenic acid | C22H44O2 | Root | [19] |
| 85 | Undecanedioic acid, monomethyl ester | C14H26O4 | Stem | [20] | |
| 86 | Octacosanic acid | C28H56O2 | Root | [24] | |
| 87 | Fumaric acid | C4H4O4 | Leaf | [16] | |
| 88 | Melissic acid | C30H60O2 | Leaf | [23] | |
| 89 | Lacceroic acid | C32H64O2 | Leaf | [23] | |
| 90 | Palmitic acid | C16H32O2 | Leaf | [23] | |
| 91 | Gheddic acid | C34H68O2 | Leaf | [23] | |
| Other compounds | 92 | 5-hydroxymethyl-2-furaldehyde | C6H6O3 | Fruit | [12] |
| 93 | 5-hydroxymaltol | C6H6O4 | Fruit | [12] | |
| 94 | Protocatechuic acid | C7H6O4 | Fruit | [12] | |
| 95 | 6-methoxy-7-hydroxycoumarin | C10H8O4 | Fruit, root, stem | [12,19,20] | |
| 96 | Phenylmethyl-β-d-glucopyranoside-6′-O-acetate | C15H20O7 | Fruit | [12] | |
| 97 | Adenosine | C10H13N5O4 | Root | [19] | |
| 98 | p-hydroxybenzoic acid | C7H6O3 | Stem | [20] | |
| 99 | Syringaldehyde | C9H10O4 | Stem | [20] | |
| 100 | Vanillin | C8H8O3 | Stem | [20] | |
| 101 | 1-O-β-d-glucopyranosyl-(2S,3S,4R,8E/Z)-2-(2′-hydrooxypalmitoyla mino)-8-octadecene-1,3,4-triol | C40H77NO10 | Leaf | [16] | |
| 102 | Glyceroyl-1,6,8-trihydroxy-3-methyl-9,10-dioxo-2-anthracene carboxylate | C19H16O9 | Leaf | [16] |
| Biological Activity | In Vitro Studies | Ref. | |
|---|---|---|---|
| Cell/Bacteria Model | Effects | ||
| Anti-neuroinflammatory | LPS-stimulated BV2 microglia | ↓ NO, PGE2, IL-1β, TNF-α production; ↓ iNOS, COX-2 expression, ↓ p38 MAPK phosphorylation | [11,12,19,20,21] |
| Anti-adipogenic | 3T3-L1 cells | ↑ AMPK-↓ PPARγ-↓ C/EBPα mechanism | [30] |
| Anti-inflammatory | LPS-stimulated RAW264.7 macrophages | ↓ NO, PGE2, IL-6, IL-1β, TNF-α production; ↓ iNOS, COX-2 expression, ↓ TLR4-NF-κB, MAPKs phosphorylation; ↓ NF-κB/p65 translocation | [11,20,21,31,32,33] |
| Antimicrobial | MRSA | MIC, time-kill growth curves, OD600, damage to the cell wall, broken cell membranes and cell lysis, ↓ PBP2a expression | [34,35] |
| Anticancer | HL-60, HT-29, A549 cells | ↓ Cell viability | [14,36] |
| Anti-oxidant | - | ↑ DPPH, O2(−), ABTS scavenging activity | [22,36,37,38] |
| Anti-AChE | - | ↑ AChE inhibitory activity | [22,36,37] |
| Anti-BuChE | - | ↑ BuChE inhibitory activity | [37] |
| Anti-hyaluronidase | - | ↑ hyaluronidase inhibitory activity | [36,38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-J.; Tang, S.-Q.; Huang, H.; Luo, J.; Zhang, X.-D.; Yook, C.-S.; Whang, W.-K.; Kim, Y.-C.; Liu, X.-Q. Acanthopanax henryi: Review of Botany, Phytochemistry and Pharmacology. Molecules 2021, 26, 2215. https://doi.org/10.3390/molecules26082215
Li X-J, Tang S-Q, Huang H, Luo J, Zhang X-D, Yook C-S, Whang W-K, Kim Y-C, Liu X-Q. Acanthopanax henryi: Review of Botany, Phytochemistry and Pharmacology. Molecules. 2021; 26(8):2215. https://doi.org/10.3390/molecules26082215
Chicago/Turabian StyleLi, Xiao-Jun, Si-Qi Tang, Hao Huang, Jiao Luo, Xiao-Dan Zhang, Chang-Soo Yook, Wan-Kyunn Whang, Youn-Chul Kim, and Xiang-Qian Liu. 2021. "Acanthopanax henryi: Review of Botany, Phytochemistry and Pharmacology" Molecules 26, no. 8: 2215. https://doi.org/10.3390/molecules26082215
APA StyleLi, X.-J., Tang, S.-Q., Huang, H., Luo, J., Zhang, X.-D., Yook, C.-S., Whang, W.-K., Kim, Y.-C., & Liu, X.-Q. (2021). Acanthopanax henryi: Review of Botany, Phytochemistry and Pharmacology. Molecules, 26(8), 2215. https://doi.org/10.3390/molecules26082215






