Design of Extractants for F-Block Elements in a Series of (2-(Diphenylphosphoryl)methoxyphenyl)diphenylphosphine Oxide Derivatives: Synthesis, Quantum-Chemical, and Extraction Studies
Abstract
:1. Introduction
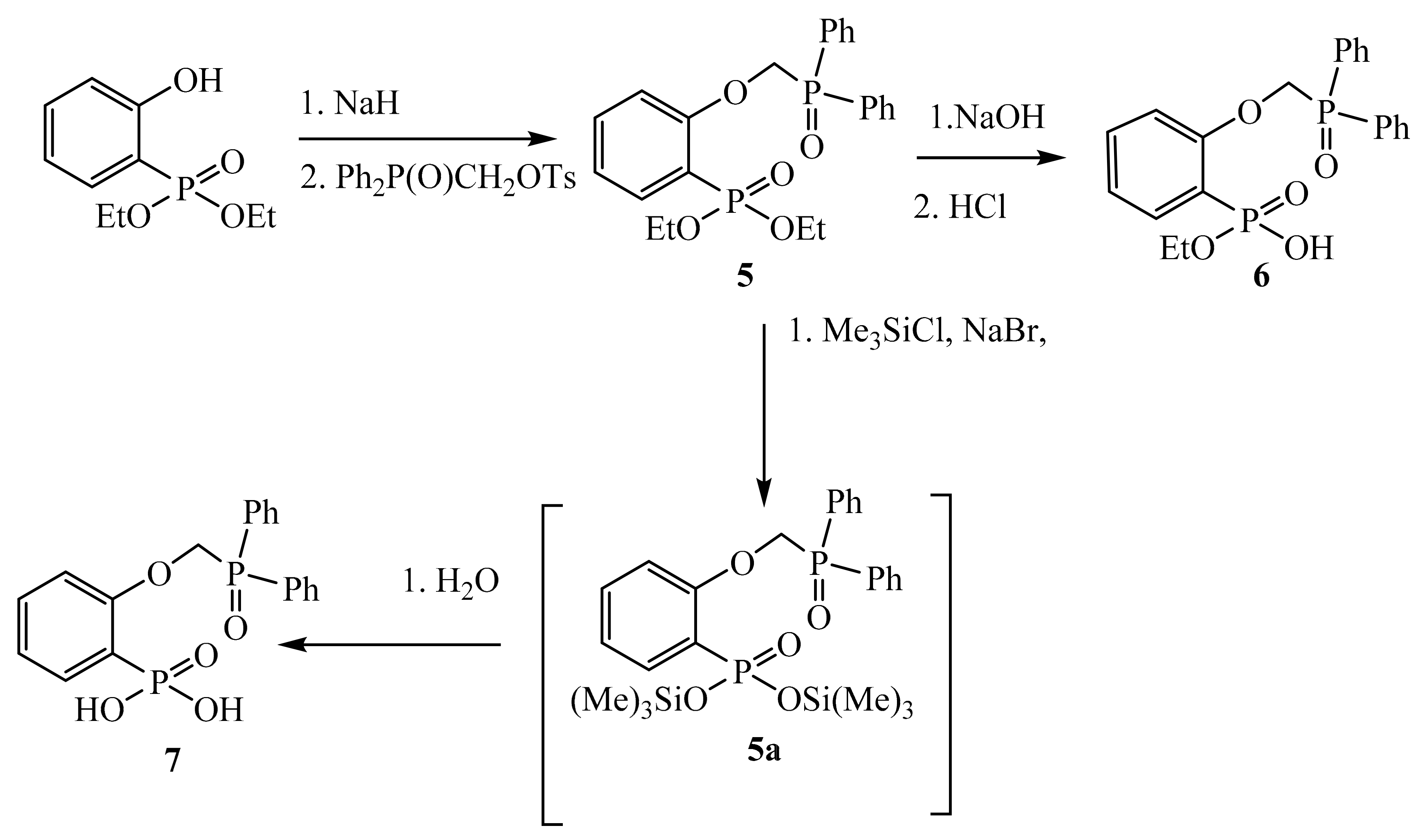
2. Experimental
2.1. X-ray Diffraction Analysis
2.2. Study of Metal Extraction
3. Results and Discussion
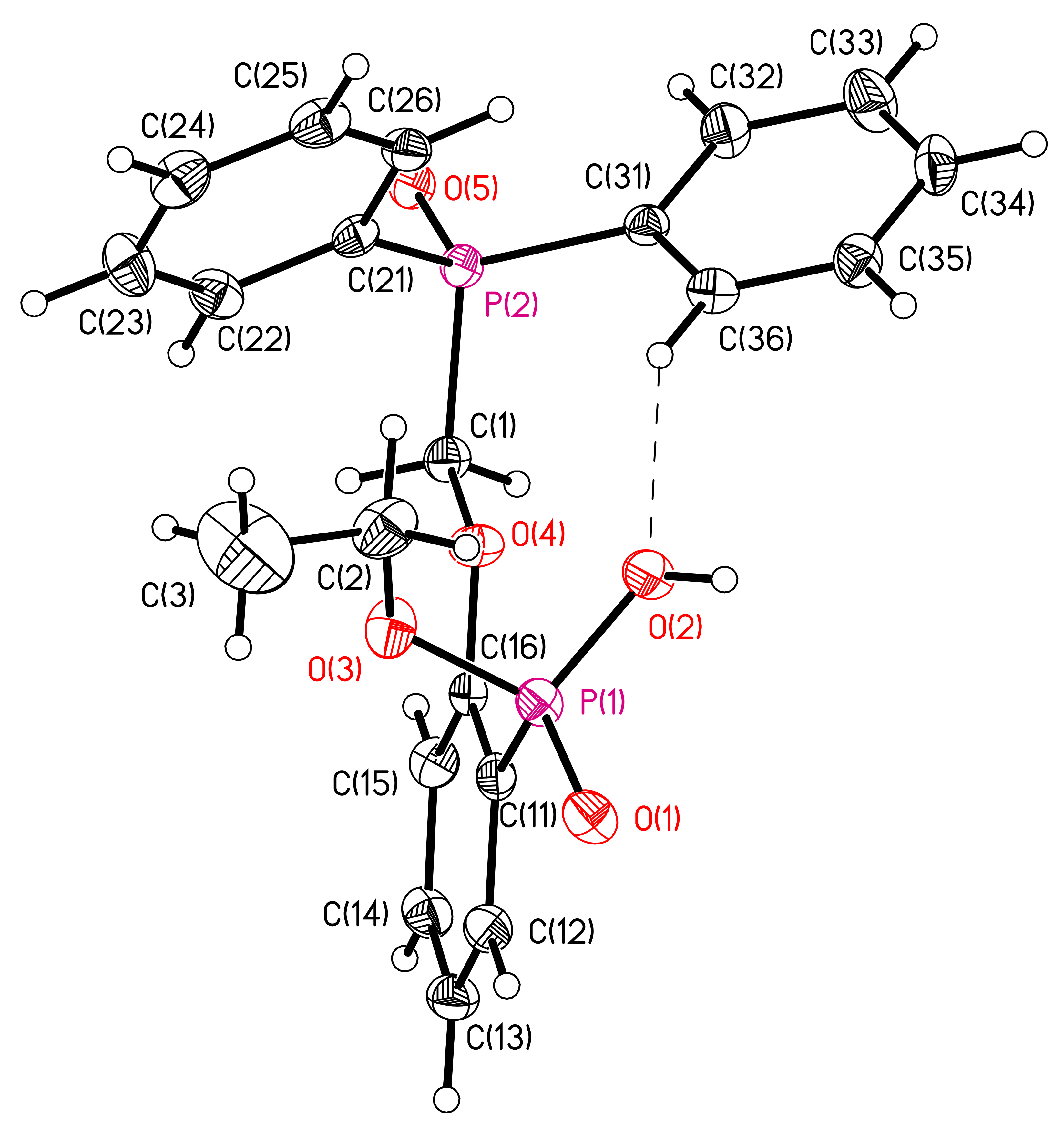
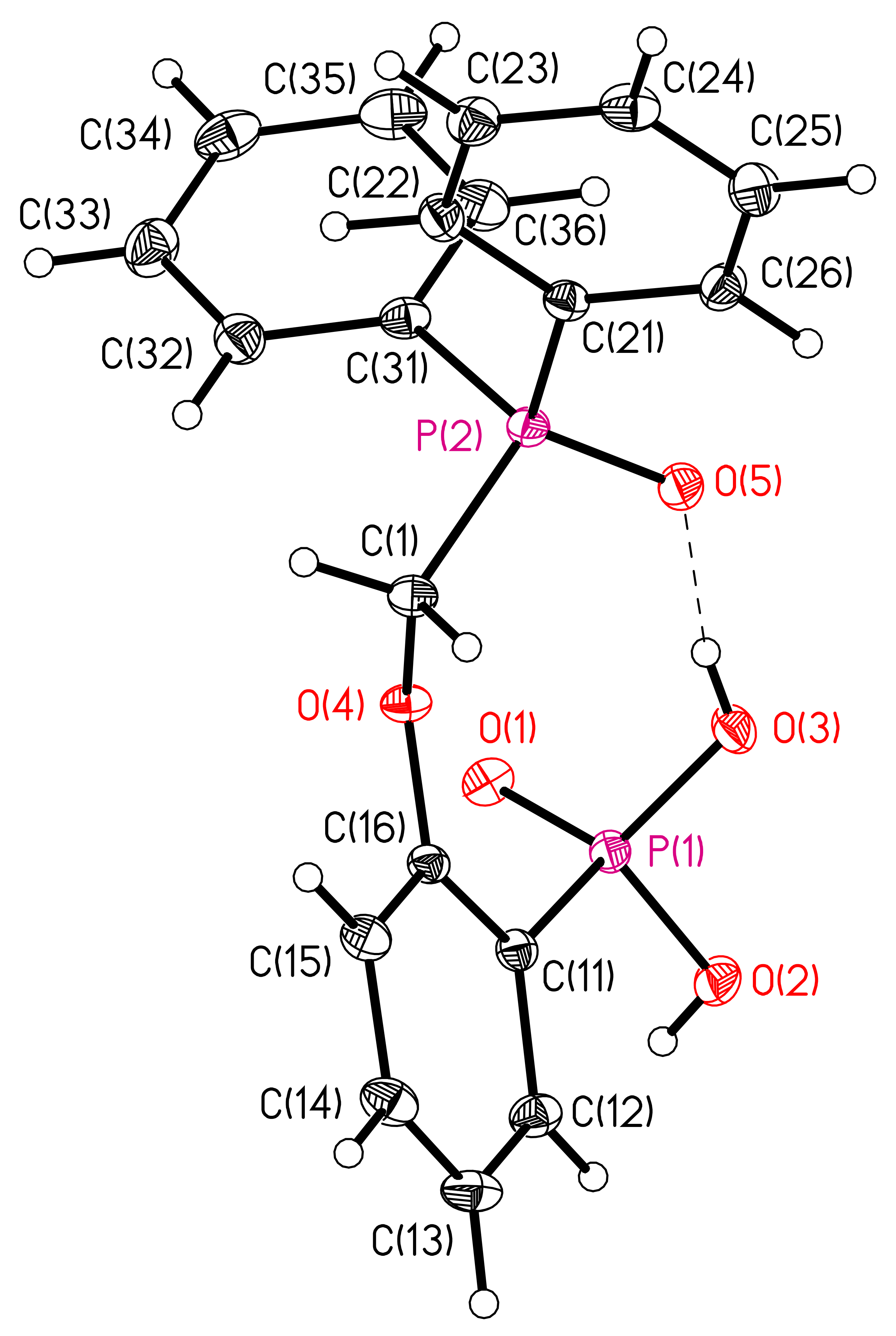
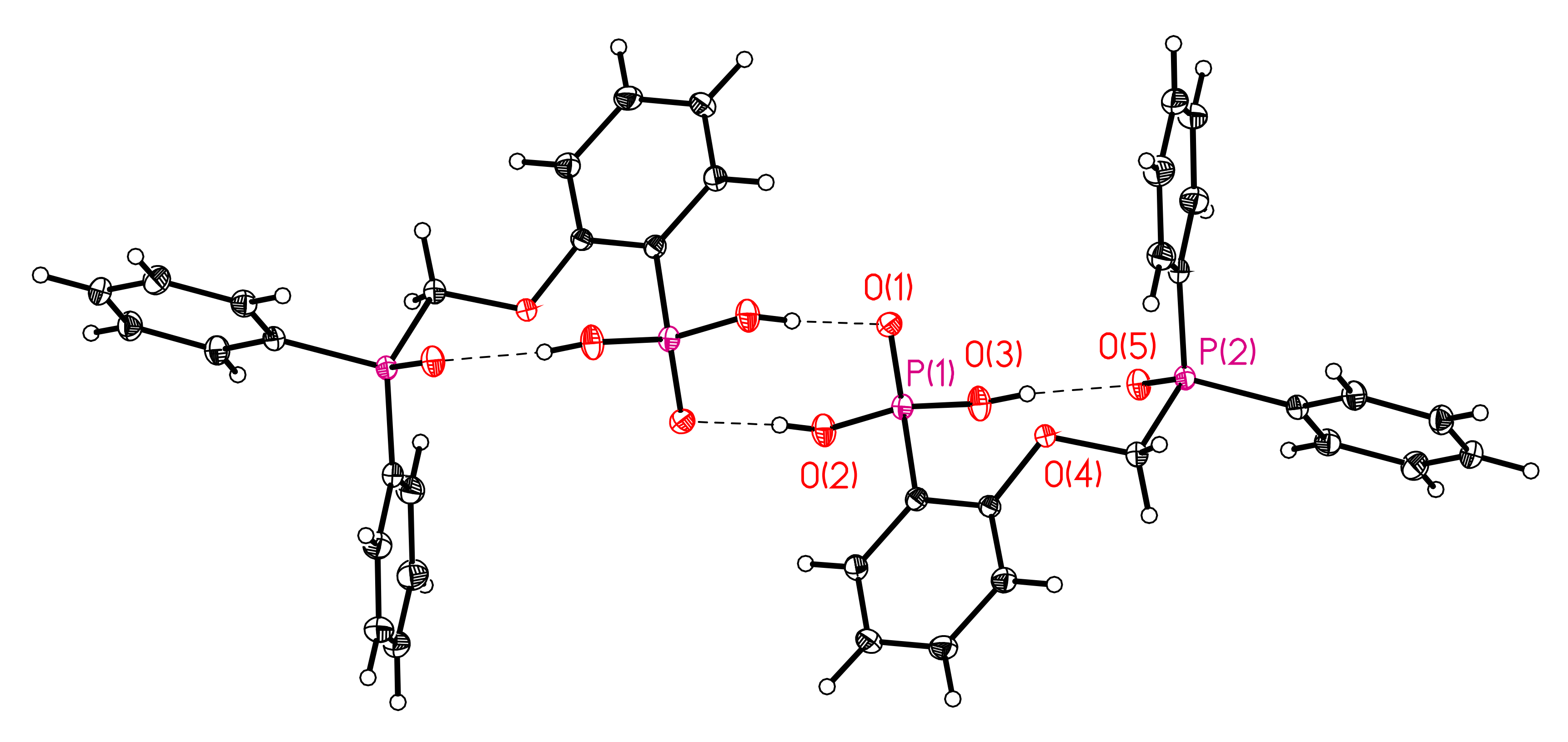
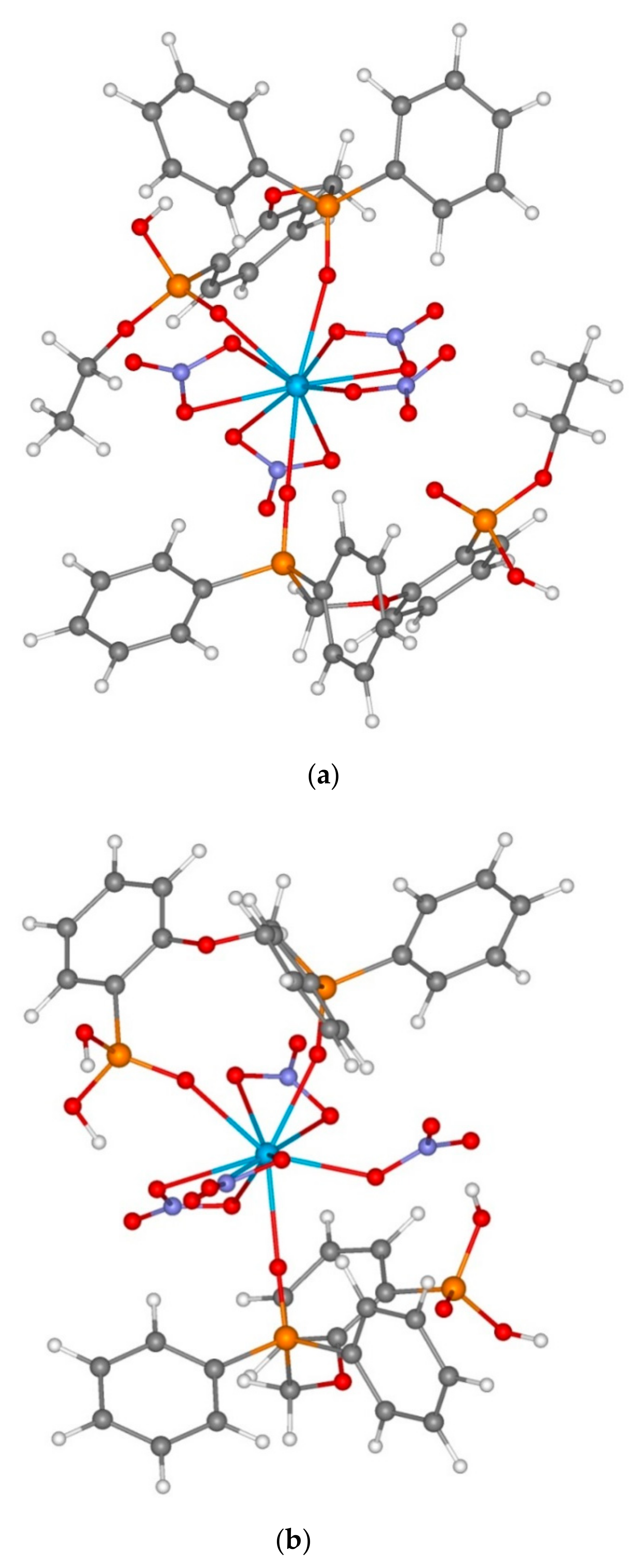
3.1. X-ray Crystallography of Compounds 6 and 7
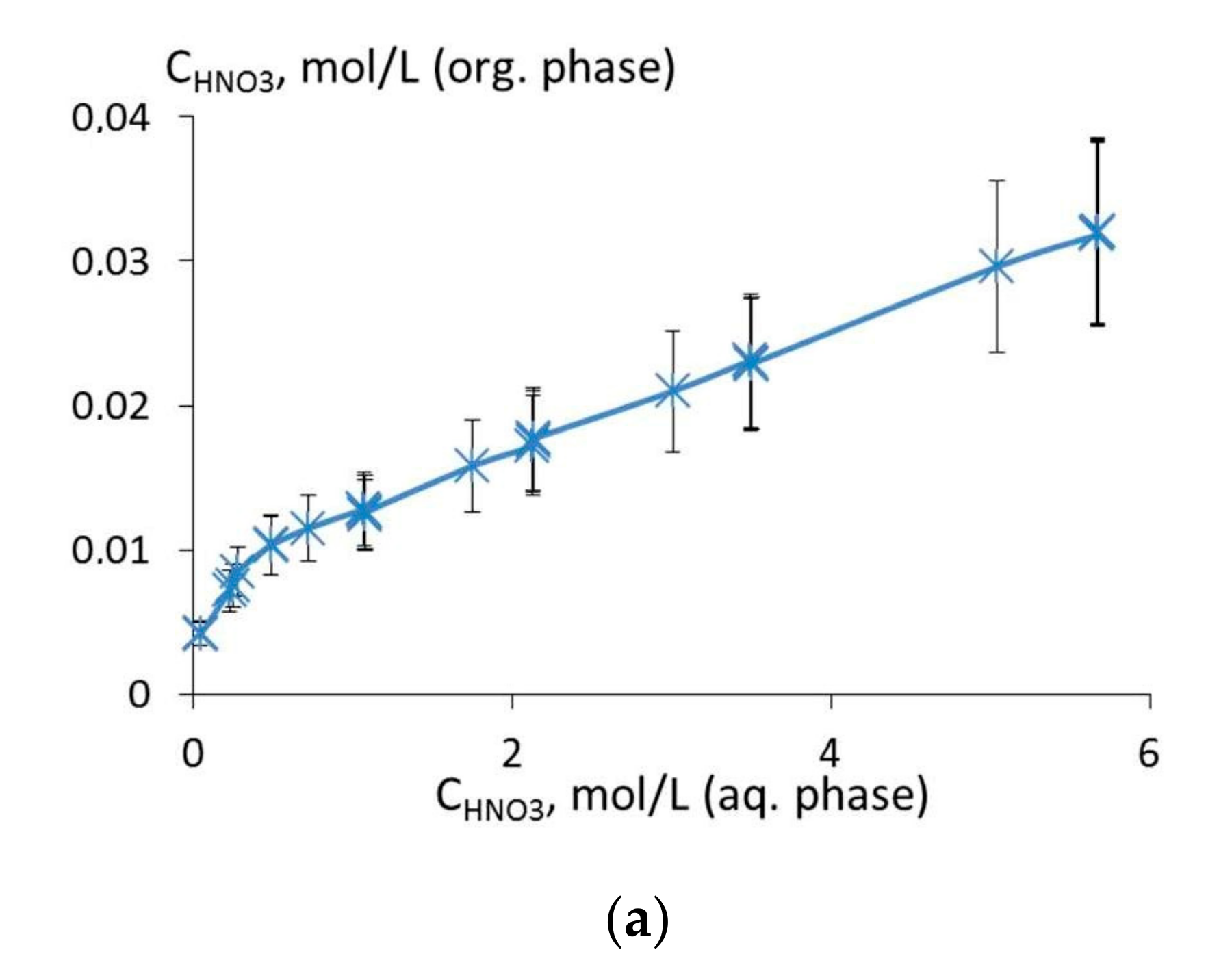
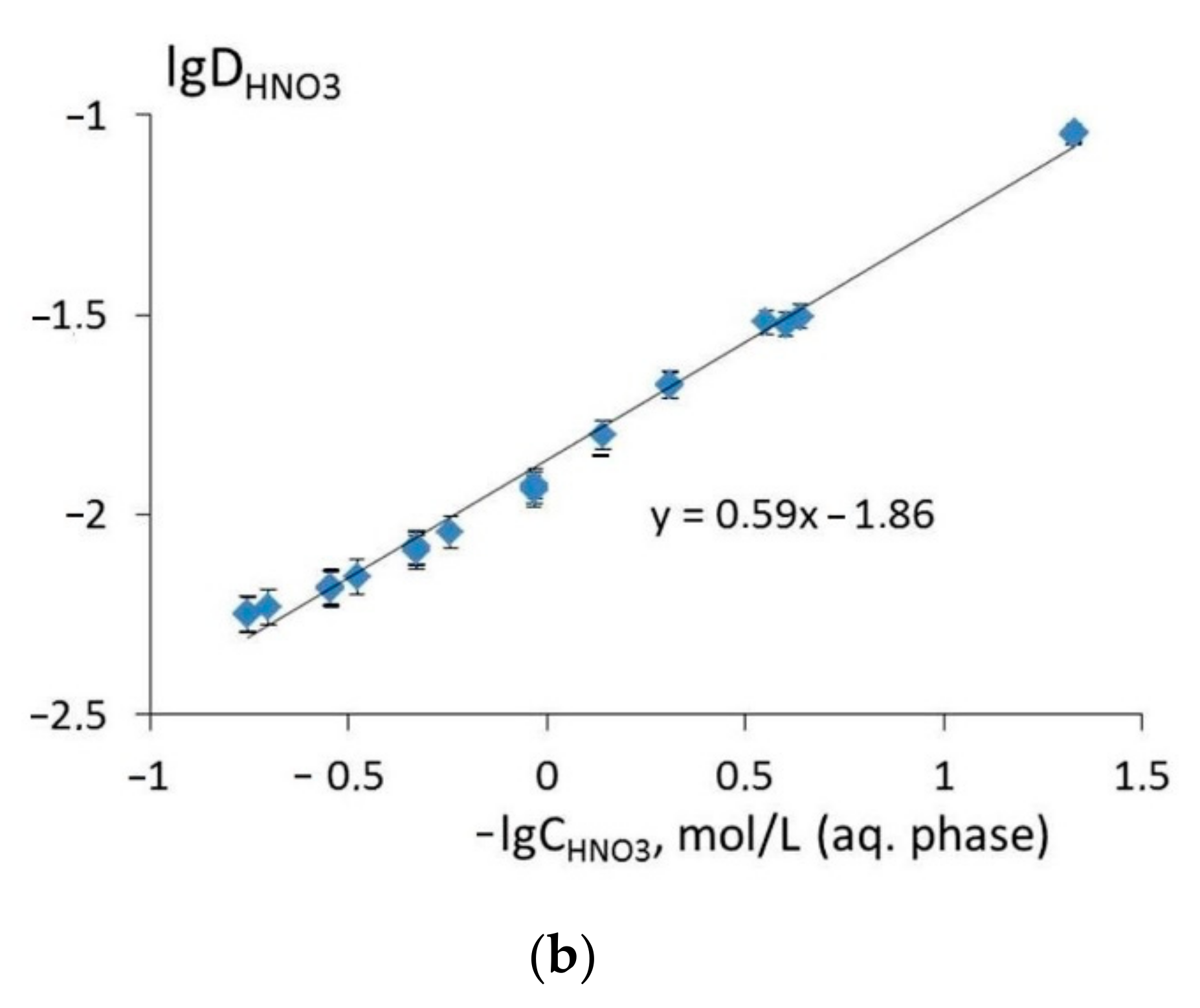
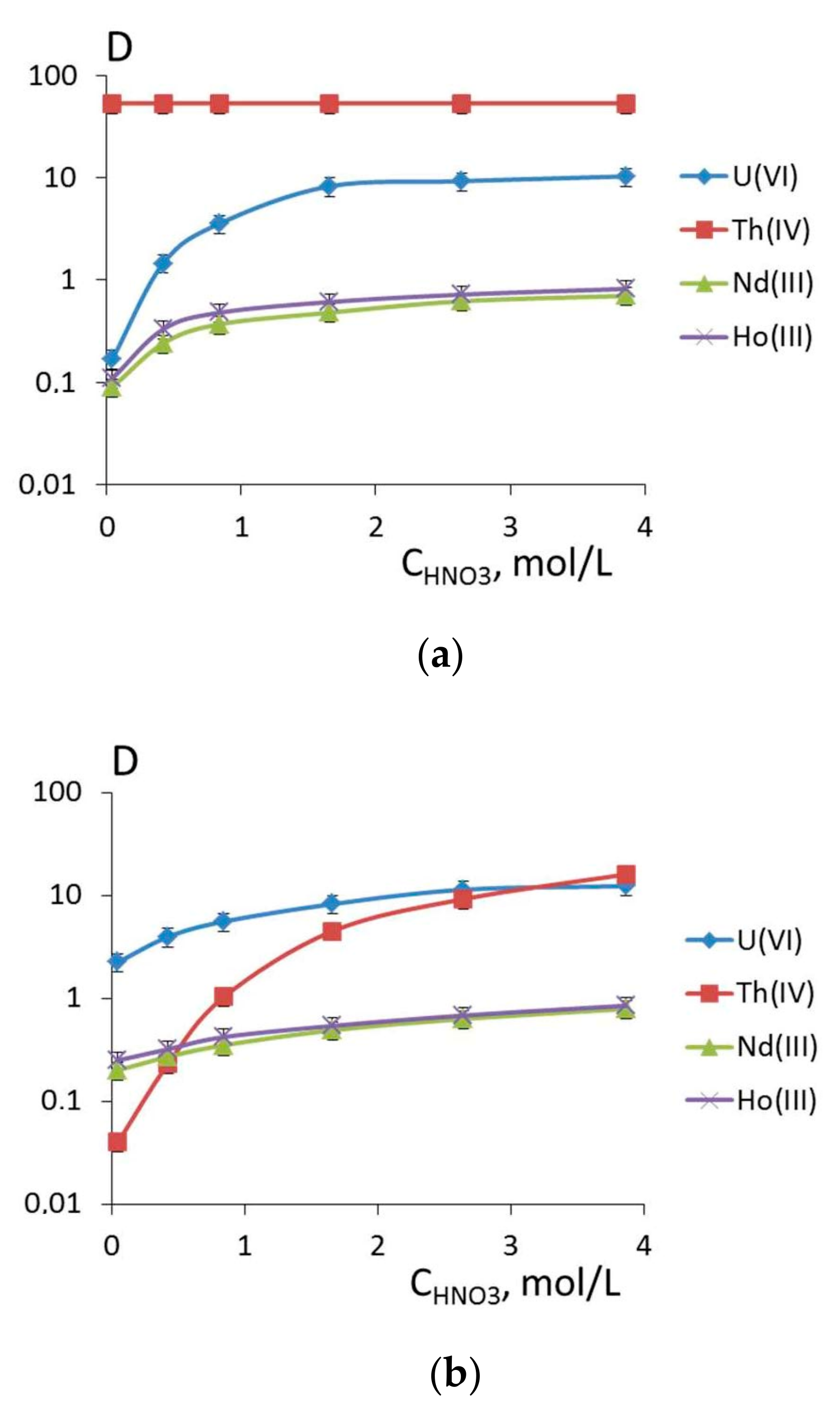
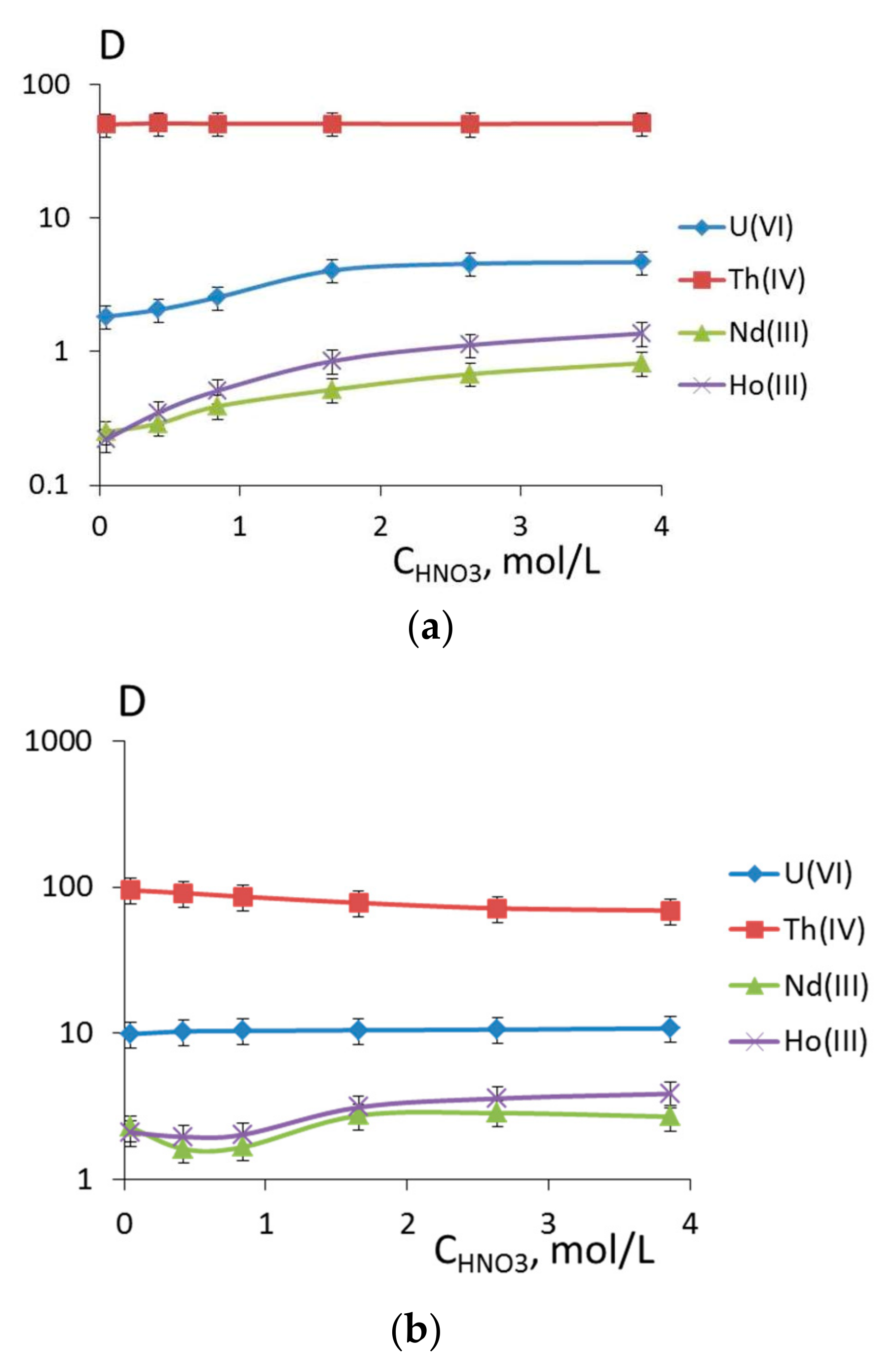
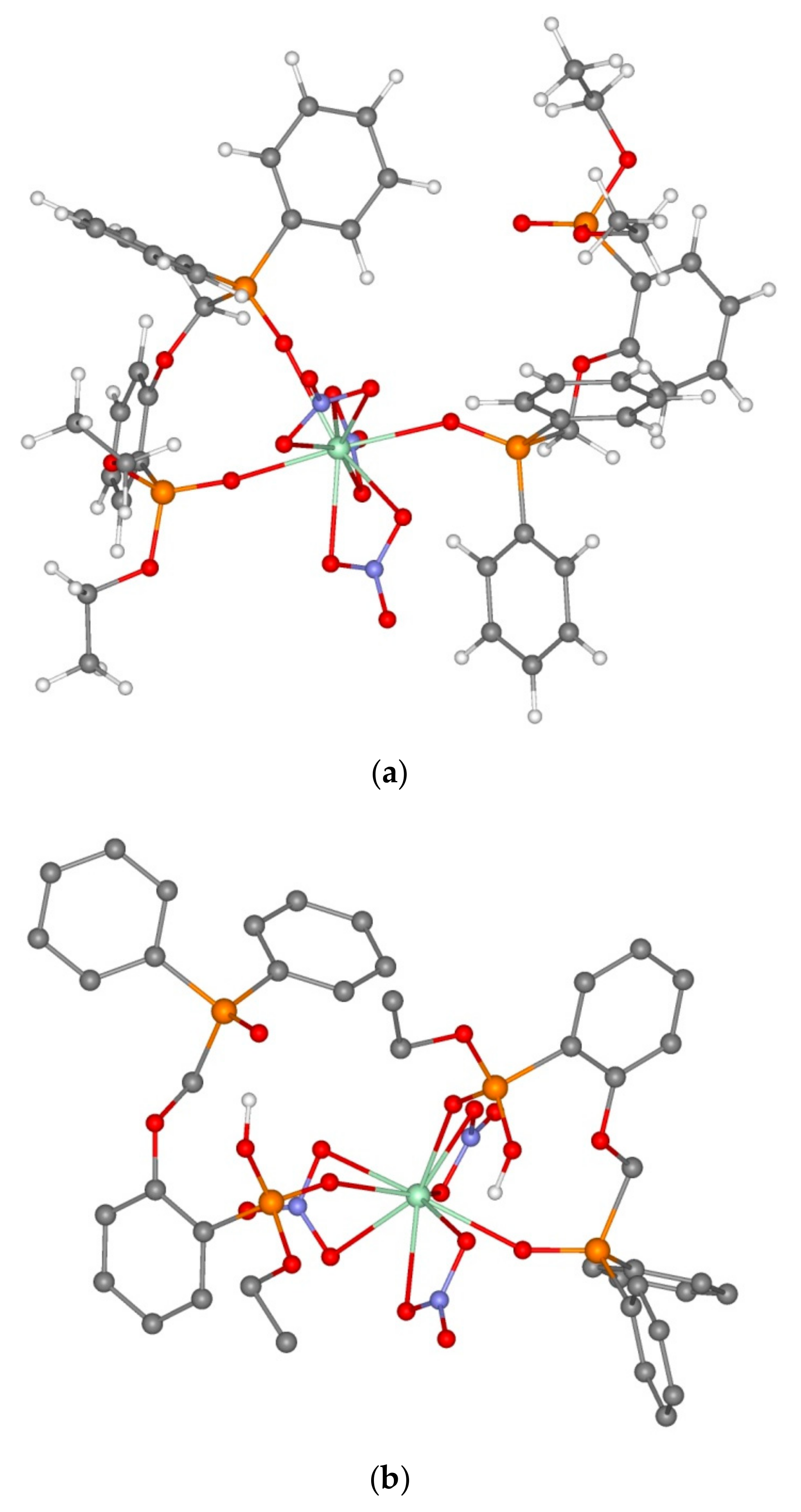
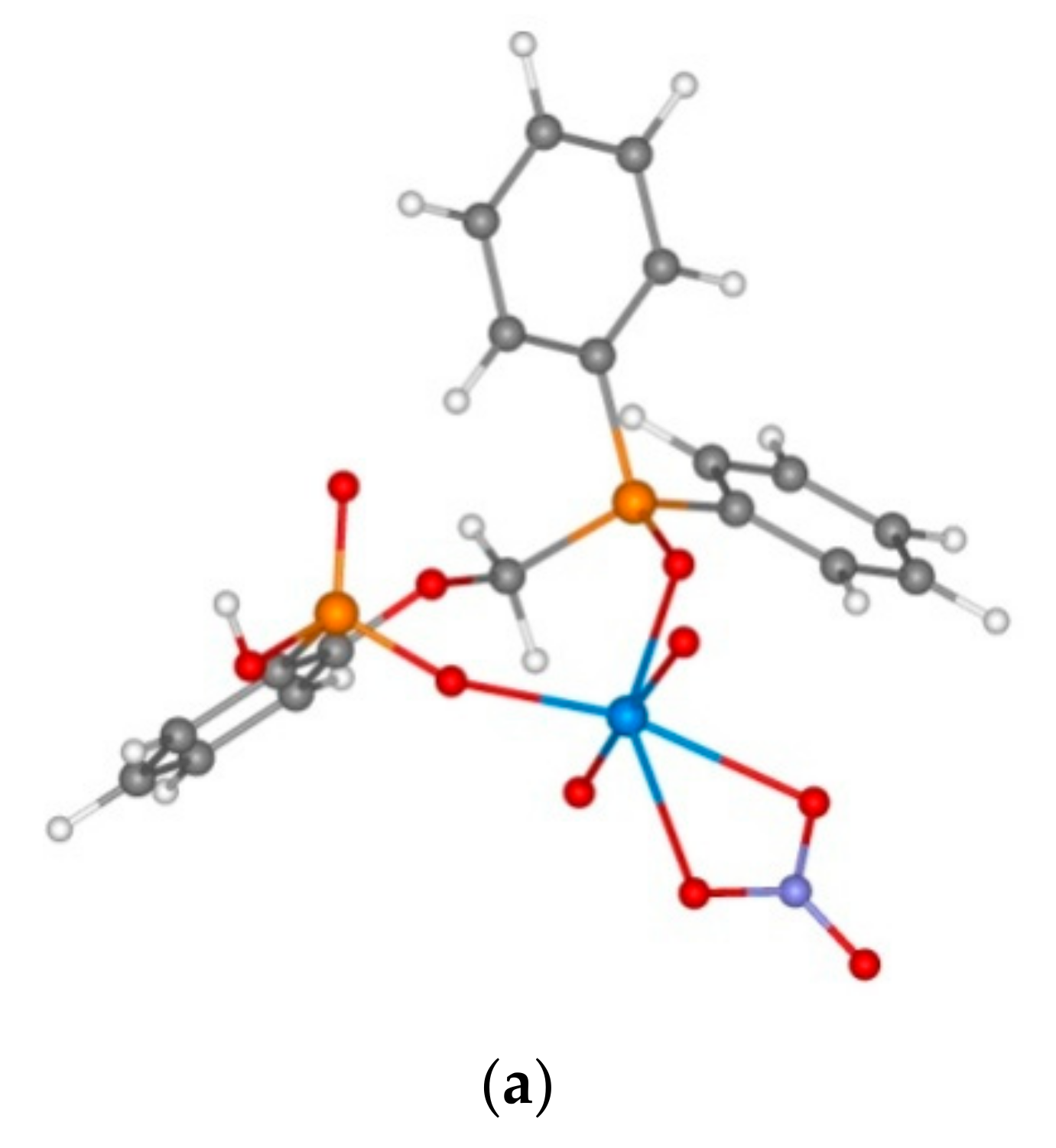
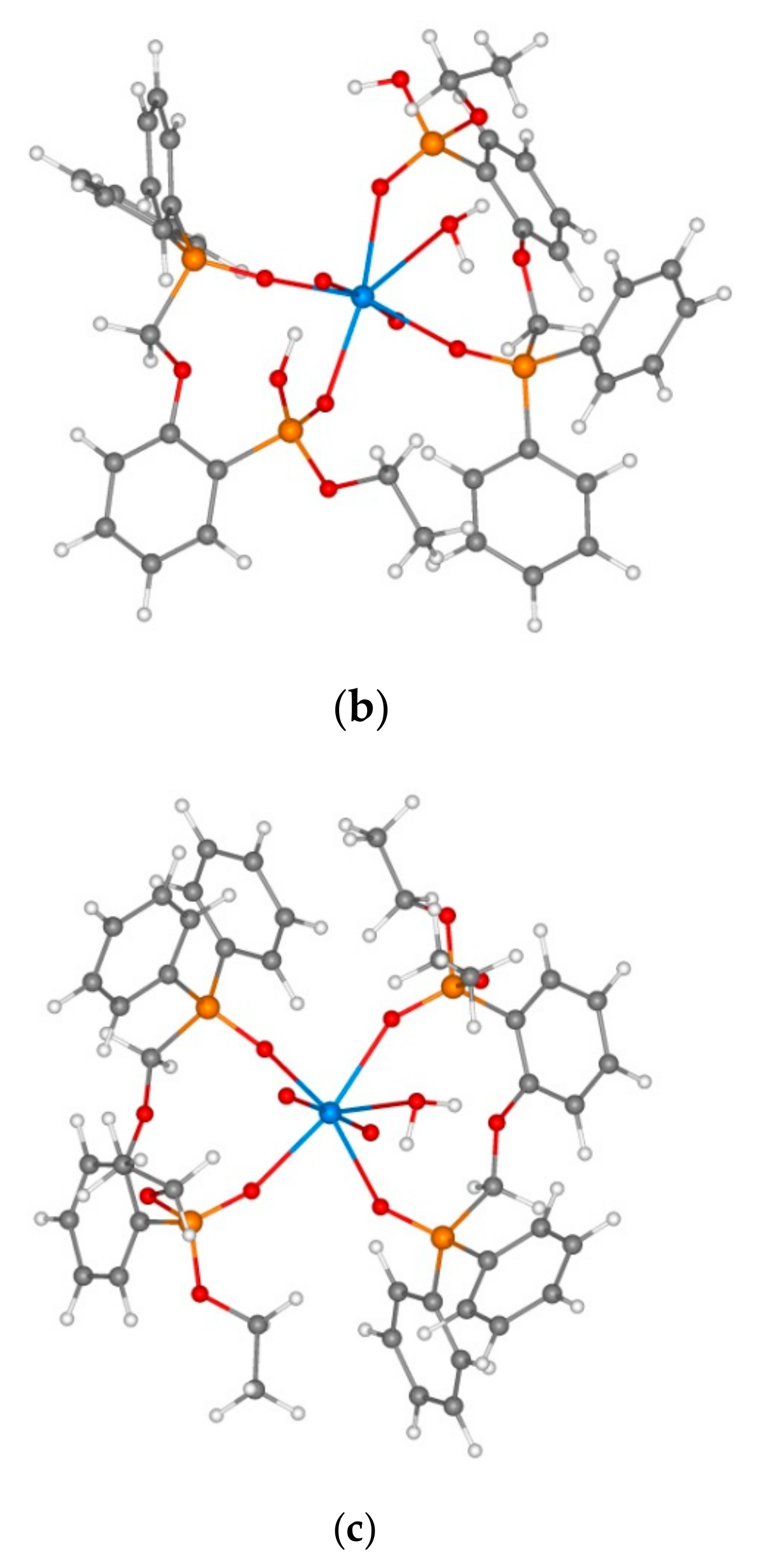
3.2. Extraction of Th(IV), Nd(III), U(VI), and Ho(III) and Quantum Chemical Modelling for the Structure of Extracted Complexes
3.3. The Comparison of Extraction Ability of Compounds 1 and 5–7 with Commercially Available Extractants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Paulick, H.; Machacek, E. The global rare earth element exploration boom: An analysis of resources outside of China and discussion of development perspectives. Resour. Policy 2017, 52, 134–153. [Google Scholar] [CrossRef]
- Hill, C. Overview of Recent Advances in An(III)/Ln(III) Separations. In Ion Exchange and Solvent Extraction: A Series of Advances; Moyer, B.A., Ed.; CRC Press: Boca Raton, FL, USA, 2010; Volume 19, pp. 119–194. [Google Scholar]
- Tachimori, S.; Morita, Y. Overview of solvent extraction chemistry for reprocessing. Ion Exch. Solvent Extr. 2010, 19, 15–17. [Google Scholar]
- Leoncini, A.; Huskens, J.; Verboom, W. Ligands for f-element extraction used in the nuclear fuel cycle. Chem. Soc. Rev. 2017, 46, 7229–7273. [Google Scholar] [CrossRef] [PubMed]
- Safiulina, A.M.; Anan’ev, A.V.; Lizunov, A.V.; Tuiza, M.; Logunov, M.V.; Dvoeglazov, K.N. Experimental Modeling of Technetium(VII) Recoveryfrom Raffinates after Extractive Reprocessing of Spent Nuclear Fuel. Russ. J. Inorg. Chem. 2020, 1928–1934. [Google Scholar] [CrossRef]
- Batchu, N.K.; Li, Z.; Verbelen, B.; Binnemans, K. Structural effects of neutral organophosphorus extractants on solvent extraction of rare-earth elements from aqueous and non-aqueous nitrate solutions. Sep. Purif. Technol. 2021, 255, 117711. [Google Scholar] [CrossRef]
- Flett, D.S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. 2005, 2426–2438. [Google Scholar] [CrossRef]
- Rozen, A.M.; Krupnov, B.V. Dependence of the extraction ability of organic compounds on their structure. Russ. Chem. Rev. 1996, 65, 973–1000. [Google Scholar] [CrossRef]
- Sharova, E.V.; Artyushin, O.I.; Odinets, I.L. Synthetic routes to carbamoylmethylphosphoryl compounds—Extractants for the processing of spent nuclear fuels. Russ. Chem. Rev. 2014, 83, 95–119. [Google Scholar] [CrossRef]
- Matveev, P.; Mohapatra, P.K.; Kalmykov, S.N.; Petrov, V. Solvent extraction systems for mutual separation of Am(III) and Cm(III) from nitric acid solutions. A review of recent state-of-the-art. Solvent Extr. Ion Exch. 2020. [Google Scholar] [CrossRef]
- Jensen, M.; Chiarizia, R.; Ulicki, J.S.; Spindler, B.D.; Murphy, D.; Hossain, M.M.; Roca-Sabio, A.; Blas, A.; Rodríguez-Blas, T. Solvent Extraction Separation of Trivalent Americium from Curium and the Lanthanides. Solvent Extr. Ion Exch. 2015, 33, 329–345. [Google Scholar] [CrossRef]
- Mahanty, B.; Mohapatra, P.K.; Leoncini, A.; Huskens, J.; Verboom, W. Evaluation of three novel benzene-centered tripodal diglycolamide ligands for the pertraction of americium(III) through flat sheet membranes for nuclear waste remediation applications. Sep. Purif. Technol. 2019, 229, 115846. [Google Scholar] [CrossRef]
- Sasaki, Y.; Umetani, S. Comparison of Four Bidentate Phosphoric and Diamide Compounds for the Extractability of Actinides. J. Nucl. Sci. Technol. 2006, 43, 794–797. [Google Scholar] [CrossRef]
- Dam, H.H.; Reinhoudt, D.N.; Verboom, W. Multicoordinate ligands for actinide/lanthanide separations. Chem. Soc. Rev. 2007, 36, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Tatarinov, D.A.; Mironov, V.F.; Kostin, A.A.; Nemtarev, A.V.; Baronova, T.A.; Buzykin, B.I.; Elistratova, Y.G. A New Versatile Synthesis of Functionalized Phosphine Oxides—Efficient Ligands for Rare-Earth Metals Extraction. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 694–697. [Google Scholar] [CrossRef]
- Swinburne, A.N.; Steed, J.W. Podands. In Supramolecular Chemistry; Gale, P.A., Steed, J.W., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2012; p. smc058. ISBN 9780470746400. [Google Scholar]
- Werner, E.J.; Biros, S.M. Supramolecular ligands for the extraction of lanthanide and actinide ions. Org. Chem. Front. 2019, 6, 2067–2094. [Google Scholar] [CrossRef]
- Borisova, N.E.; Safiullina, A.M.; Lizunov, A.V.; Semenov, A.A.; Grigor’ev, M.S.; Reshetova, M.D.; Litvinov, I.A.; Tatarinov, D.A.; Mironov, V.F. Metal-Promoted Extraction Deprotonation of Bidentate Organophosphoric Reagents: Recovery of Uranium, Thorium, and Lanthanides. Russ. J. Inorg. Chem. 2019, 64, 414–422. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Kandwal, P.; Iqbal, M.; Huskens, J.; Murali, M.S.; Verboom, W. A novel CMPO-functionalized task specific ionic liquid: Synthesis, extraction and spectroscopic investigations of actinide and lanthanide complexes. Dalton Trans. 2013, 42, 4343–4347. [Google Scholar] [CrossRef]
- Charpentier, C.; Salaam, J.; Lecointre, A.; Jeannin, O.; Nonat, A.; Charbonnière, L.J. Phosphonated Podand Type Ligand for the Complexation of Lanthanide CationsPhosphonated Podand Type Ligand for the Complexation of Lanthanide Cations: Phosphonated Podand Type Ligand for the Complexation of Lanthanide Cations. Eur. J. Inorg. Chem. 2019, 2019, 2168–2174. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Baulin, V.E. Extraction of Metal Chloride Complexes by Phosphoryl-Containing Podands. Solvent Extr. Ion Exch. 1996, 14, 227–245. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Baulin, V.E. Extraction of Metal Chloride Complexes by Phosphoryl-Containing Azapodands. Solvent Extr. Ion Exch. 1996, 14, 689–704. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Baulin, V.E. Extraction of Rare-Earth Elements from Nitric Solutions by Phosphoryl-Containing Podands. Solvent Extr. Ion Exch. 1999, 17, 1423–1444. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Fedoseev, A.M.; Rodygina, N.I.; Baulin, V.E. Effect of the Structure of o-Methyleneoxyphenyldiphosphine Dioxides on Their Extractive Power and Selectivity in Nitric Acid Solutions. Radiochemistry 2005, 47, 177–180. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Yarkevich, A.N.; Baulin, V.E. Extraction of U(VI) and Th(IV) from nitric acid solutions with tetraaryl-substituted (o-phenyleneoxymethylene)diphosphine dioxides. Radiochemistry 2009, 51, 359–364. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Baulin, V.E.; Yarkevich, A.N.; Safronova, Z.V. Extraction of Lanthanides(III) from Aqueous Nitrate Media with Tetra-(p-tolyl)[(o-Phenylene)Oxymethylene] Diphosphine Dioxide. Solvent Extr. Ion Exch. 2009, 27, 551–578. [Google Scholar] [CrossRef]
- Demin, S.V.; Zhilov, V.I.; Nefedov, S.E.; Baulin, V.E.; Tsivadze, A.Y. Extraction of rare earth elements with 1-(diphenylphosphorylmethoxy)-2-diphenylphosphoryl-4-ethylbenzene with the use of 1,1,7-trihydrododecafluoroheptanol as a solvent. Russ. J. Inorg. Chem. 2012, 57, 897–902. [Google Scholar] [CrossRef]
- Polyakova, I.N.; Baulin, V.E.; Ivanova, I.S.; Pyatova, E.N.; Sergienko, V.S.; Tsivadze, A.Y. Crystal and molecular structure of the coordination compounds of Er3+ with 1-(methoxydiphenylphosphoryl)-2-diphenylphosphorylbenzene [ErL21(NO3)2]2[Er(NO3)2(H2O)5]0.333(NO3)2.333 2.833H2O and its ethyl substituted derivative [ErL22(NO3)2][Er(NO3)5]0.5 · 0.5H2O. Crystallogr. Rep. 2015, 60, 57–62. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Baulin, V.E.; Kalashnikova, I.P.; Kirillov, E.V.; Kirillov, S.V.; Rychkov, V.N.; Tsivadze, A.Y. Extraction of rare earths and scandium by 2-phosphorylphenoxyacetic acid amides in the presence of ionic liquids. Russ. J. Inorg. Chem. 2016, 61, 377–383. [Google Scholar] [CrossRef]
- Safiulina, A.M.; Matveeva, A.G.; Ivanets, D.V.; Kudryavtsev, E.M.; Grigor’ev, M.S.; Baulin, V.E.; Tsivadze, A.Y. Phosphoryl-containing acidic podands as extractants for recovery of f-elements: Synthesis and comparison of podands different in polyether chain length and structure. Russ. Chem. Bull. 2015, 64, 161–168. [Google Scholar] [CrossRef]
- Safiulina, A.M.; Matveeva, A.G.; Ivanets, D.V.; Kudryavtsev, E.M.; Baulin, V.E.; Tsivadze, A.Y. Phosphoryl-containing acidic podands as extractants for recovery of f-elements: Synthesis and comparison of podands different in terminal group structure. Russ. Chem. Bull. 2015, 64, 169–175. [Google Scholar] [CrossRef]
- Timofeeva, G.I.; Matveeva, A.G.; Safiulina, A.M.; Ivanets, D.V.; Kudryavtsev, E.M.; Baulin, V.E.; Tsivadze, A.Y. Phosphoryl-containing acidic podands as extractants for recovery of f-elements: Dependence of the degree of association of podands on the nature of substituent and concentration in water–methanol solutions. Russ. Chem. Bull. 2015, 64, 224–227. [Google Scholar] [CrossRef]
- Laikov, D.N. A new class of atomic basis functions for accurate electronic structure calculations of molecules. Chem. Phys. Lett. 2005, 416, 116–120. [Google Scholar] [CrossRef]
- Laikov, D.N. Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets. Chem. Phys. Lett. 1997, 281, 151–156. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Bruker. APEX2; Bruker: Madison, WI, USA, 2006. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Savvin, S.B. Arsenazo III; Atomizdat: Moscow, Russia, 1966; p. 256. [Google Scholar]
- Hamed, M.M.; Aglan, R.F. Removal of Arsenazo-III from liquid radioactive waste by cloud point extraction. J. Radioanal. Nucl. Chem. 2019, 321, 917–926. [Google Scholar] [CrossRef]


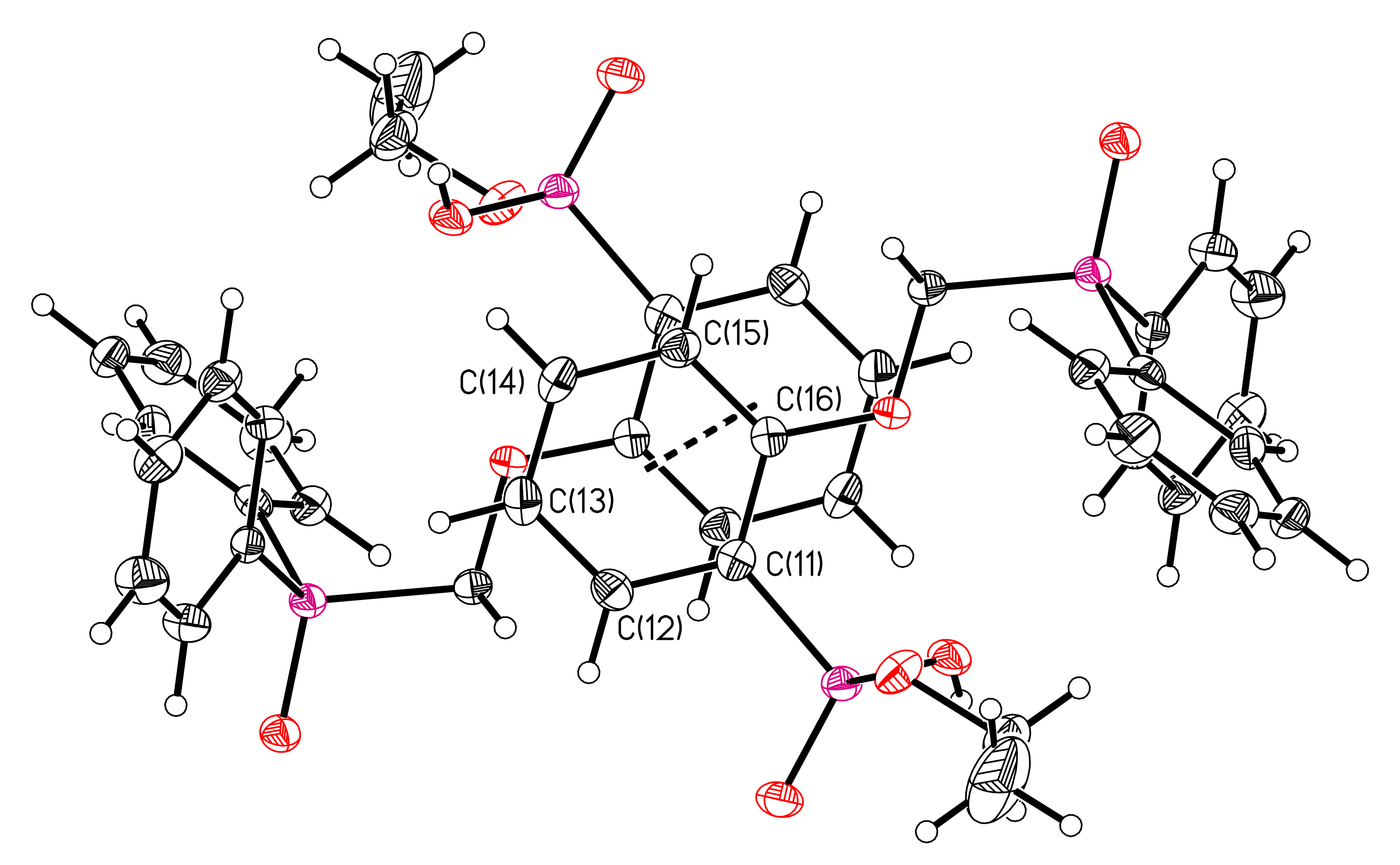




| Parameter | 6 | 7 |
|---|---|---|
| Molecular formula | C21H22O5P2 | C19H18O5P2 |
| M | 416.32 | 388.27 |
| Syngony, space group, Z | Monoclinic, P21/n, 4 | Monoclinic, P21/c, 4 |
| a, Å | 8.0671(13) | 13.0212 (3) |
| b, Å | 18.393(4) | 11.2464 (3) |
| c, Å | 14.410(3) | 12.5224 (3) |
| β, degrees | 92.309(8) | 105.213 (1) |
| V, Å3 | 2136.5(7) | 1769.54 (8) |
| Dx, g/cm3 | 1.294 | 1.457 |
| Radiation, λ, Å | MoKα, 0.71073 | MoKα, 0.71073 |
| T, K | 100(2) | 100 (2) |
| Size of crystal, mm | 0.40 × 0.10 × 0.10 | 0.26 × 0.20 × 0.18 |
| θmax, degrees | 27.5 | 30 |
| h, k, l range | −10 ≤ h ≤ 6, −23 ≤ k ≤ 20, −17 ≤ l ≤ 18 | −18 ≤ h ≤ 18, −15 ≤ k ≤ 15, −17 ≤ l ≤ 17 |
| Number of reflections: Measured/independent, Rint | 14159/4729, 0.0759 | 46482/5118, 0.0325 |
| Number of refined parameters | 257 | 243 |
| R(F), wR(F2) [I > 2σ(I)] | 0.0499, 0.0978 | 0.0312, 0.0794 |
| R(F), wR(F2) [whole array] | 0.0960, 0.1153 | 0.0377, 0.0833 |
| S | 0.988 | 1.052 |
| Δρmax, Δρmin, e/Å3 | 0.358, −0.418 | 0.472, −0.268 |
| D–H…A | D–H, Å | H…A, Å | D…A, Å | D–H…A, Degrees | Symmetry Operation for A |
|---|---|---|---|---|---|
| 6 | |||||
| O(2)-H(2)...O(5) | 0.78(3) | 1.78(3) | 2.562(2) | 177(4) | 1 + x, y, z |
| C(36)-H(36A)...O(2) | 0.95 | 2.34 | 3.196(3) | 149 | |
| 7 | |||||
| O(2)-H(2)...O(1) | 0.80(3) | 1.77(3) | 2.5725(13) | 176(3) | 2-x, 1-y, 1-z |
| O(3)-H(3)...O(5) | 0.79(3) | 1.74(3) | 2.5190(12) | 169(3) | |
| 1 | 5 | 6 | 7 | |
|---|---|---|---|---|
| q O=PAr3 | −0.4801 | −0.4735 | −0.5100 | −0.5182 |
| q O=P(R2)CH2 | −0.4701 | −0.4698 | −0.4901 | −0.4816 |
| H…O=P | - | - | 1.647 (164.167) | 1.636 (169.931) |
| No. | Complexes | ΔE, kcal/mol (Equation (1) or (2)) | Th-O=PCH2, Å | Th-O=P(OEt) | Th-O=PPh2 | Th…O |
|---|---|---|---|---|---|---|
| 1 | 1Th(NO3)3+ | −126.63 | 2.338 | 2.307 | 4.236 | |
| 2 | (7H)Th(NO3)3 | −211.43 | 2.368 | 2.216 | 4.309 | |
| 3 | 6Th(NO3)3 | −211.18 | 2.369 | 2.204 | 4.298 | |
| 4 | 5Th(NO3)3+ | −119.77 | 2.330 | 2.354 | 4.291 | |
| 5 | 12Th(NO3)3+ | −180.12 | 2.417 | 2.436 | 4.388/4.385 | |
| 6 | 12Th(NO3)4 | −60.40 | 2.458/2.500 | 2.675/2.652 | 4.582/4.787 | |
| 7 | (7H2)2Th(NO3)4 | −54.92 | 2.439/2.398 | 2.465/4.987 | 4.656/5.419 | |
| 8 | (6H)2Th(NO3)4 | −42.43 | 2.535/2.429 | 2.519/3.796 | 4.669/5.073 | |
| 9 | 52Th(NO3)4 | −56.12 | 2.524/2.445 | 2.519/3.811 | 4.613/5.116 |
| No. | Complexes | ΔE, kcal/mol (Equation (3)) | Nd-O=PCH2, Å | Nd-O=P(OEt), Å | Nd-O=PPh2, Å | Nd…O, Å |
|---|---|---|---|---|---|---|
| 1 | 12Nd(NO3)3 | −72.48 | 2.528/2.467 | 2.408/5.938 | 4.296/5.645 | |
| 2 | (7H)2Nd(NO3)3 | −69.29 | 2.446/3.913 | 2.384/2.642 | 4.715/5.787 | |
| 3 | (6H)2Nd(NO3)3 | −69.05 | 2.457/5.294 | 2.490/2.445 | 4.013/5.689 | |
| 4 | 52Nd(NO3)3 | −63.04 | 2.504/2.486 | 2.453/6.794 | 4.474/5.782 |
| No. | Complexes | ΔE, kcal/mol (Equations (4)–(6)) | U-O=PCH2, Å | U-O=P(OEt), Å | U-O=PPh2, Å | U…O, Å |
|---|---|---|---|---|---|---|
| 1 | 1UO2(NO3)+ | −134.91 | 2.325 | 2.285 | 3.852 | |
| 2 | 7UO2(NO3) | −218.23 | 2.354 | 2.207 | 3.563 | |
| 3 | 6UO2(NO3) | −216.38 | 2.368 | 2.215 | 3.383 | |
| 4 | 5UO2(NO3)+ | −128.83 | 2.322 | 2.303 | 3.534 | |
| 5 | 12UO22+ | −393.01 | 2.337/2.337 | 2.302/2.302 | 4.188/4.187 | |
| 6 | (7H)2UO2 | −586.22 | 2.399/2.356 | 2.222/2.233 | 4.057/3.800 | |
| 7 | 6UO2 | −579.00 | 2.415/2.366 | 2.244/2.244 | 4.197/3.754 | |
| 8 | 52UO22+ | −377.95 | 2.319/2.321 | 2.325/2.327 | 4.127/4.111 | |
| 9 | (7H2)2UO22+ | −357.87 | 2.320/2.340 | 2.290/2.297 | 3.986/3.830 | |
| 10 | (6H)2UO22+ | −359.77 | 2.314/2.319 | 2.323/2.336 | 4.120/3.722 | |
| 11 | 12UO2(H2O)2+ | −395.09 | 2.350/2.445 | 2.325/2.346 | 4.321/3.625 | |
| 12 | (7H2)2UO2(H2O)2+ | −376.28 | 2.311/2.316 | 2.294/2.293 | 4.006/4.878 | |
| 13 | (6H)2UO2(H2O)2+ | −591.61 | 2.350/2.485 | 2.418/2.484 | 4.118/3.644 | |
| 14 | 52UO2(H2O)2+ | −596.81 | 2.348/2.476 | 2.454/2.448 | 4.341/3.642 |
| 1 | 5 | 6 | 7 | |
|---|---|---|---|---|
| U-OH2, Å | 2.768 | 2.855 | 2.840 | 4.878 |
| HO-H…O, Å | - | 1.968/2.103 | 1.936 | 1.519 |
| HO-H…O, deg. | - | 150.5/121.2 | 131.59 | 176.38 |
| Compound | U(VI) | Th(IV) | Nd(III) | Ho(III) | ||||
| CHNO3, mol/L | ||||||||
| 0.045 | 3.86 | 0.045 | 3.86 | 0.045 | 3.86 | 0.045 | 3.86 | |
| Distribution Ratio (D) | ||||||||
| 1 | 0.17 | 10.3 | 53 | 53 | 0.09 | 0.7 | 0.11 | 0.82 |
| 5 | 2.24 | 12.3 | 0.040 | 16 | 0.20 | 0.79 | 0.25 | 0.85 |
| 11 | 0.019 | 0.38 | 0 | 0.19 | 0 | 0.35 | 0.04 | 0.42 |
| 12 | 0.05 | 0.60 | 0.030 | 0.20 | 0 | 0.33 | 0 | 0.38 |
| Compound | U(VI) | Th(IV) | Nd(III) | Ho(III) | ||||
| CHNO3, mol/L | ||||||||
| 0.045 | 3.86 | 0.045 | 3.86 | 0.045 | 3.86 | 0.045 | 3.86 | |
| Distribution Ratio (D) | ||||||||
| 6 | 1.83 | 4.68 | 50 | 51 | 0.25 | 0.82 | 0.22 | 1.37 |
| 7 | 9.88 | 10.8 | 95 | 70 | 2.28 | 2.69 | 2.1 | 3.86 |
| 8 | 10 | 0.62 | 74 | 0.056 | 0.11 | 1.08 | 0.24 | 1.68 |
| 9 | 1.08 | 0.49 | 52 | 0.05 | 0.24 | 0.71 | 0.06 | 0.73 |
| 10 | 0.023 | 0.47 | 53 | 0 | 0.13 | 0.49 | 0.07 | 1.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safiulina, A.; Borisova, N.; Grigoriev, M.; Baulin, D.; Baulin, V.; Tsivadze, A. Design of Extractants for F-Block Elements in a Series of (2-(Diphenylphosphoryl)methoxyphenyl)diphenylphosphine Oxide Derivatives: Synthesis, Quantum-Chemical, and Extraction Studies. Molecules 2021, 26, 2217. https://doi.org/10.3390/molecules26082217
Safiulina A, Borisova N, Grigoriev M, Baulin D, Baulin V, Tsivadze A. Design of Extractants for F-Block Elements in a Series of (2-(Diphenylphosphoryl)methoxyphenyl)diphenylphosphine Oxide Derivatives: Synthesis, Quantum-Chemical, and Extraction Studies. Molecules. 2021; 26(8):2217. https://doi.org/10.3390/molecules26082217
Chicago/Turabian StyleSafiulina, Alfiya, Nataliya Borisova, Mikhail Grigoriev, Dmitriy Baulin, Vladimir Baulin, and Aslan Tsivadze. 2021. "Design of Extractants for F-Block Elements in a Series of (2-(Diphenylphosphoryl)methoxyphenyl)diphenylphosphine Oxide Derivatives: Synthesis, Quantum-Chemical, and Extraction Studies" Molecules 26, no. 8: 2217. https://doi.org/10.3390/molecules26082217
APA StyleSafiulina, A., Borisova, N., Grigoriev, M., Baulin, D., Baulin, V., & Tsivadze, A. (2021). Design of Extractants for F-Block Elements in a Series of (2-(Diphenylphosphoryl)methoxyphenyl)diphenylphosphine Oxide Derivatives: Synthesis, Quantum-Chemical, and Extraction Studies. Molecules, 26(8), 2217. https://doi.org/10.3390/molecules26082217






