Prebiotic Route to Thymine from Formamide—A Combined Experimental–Theoretical Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Detection of Uracil and Thymine in Heat-Treated Formamide Samples
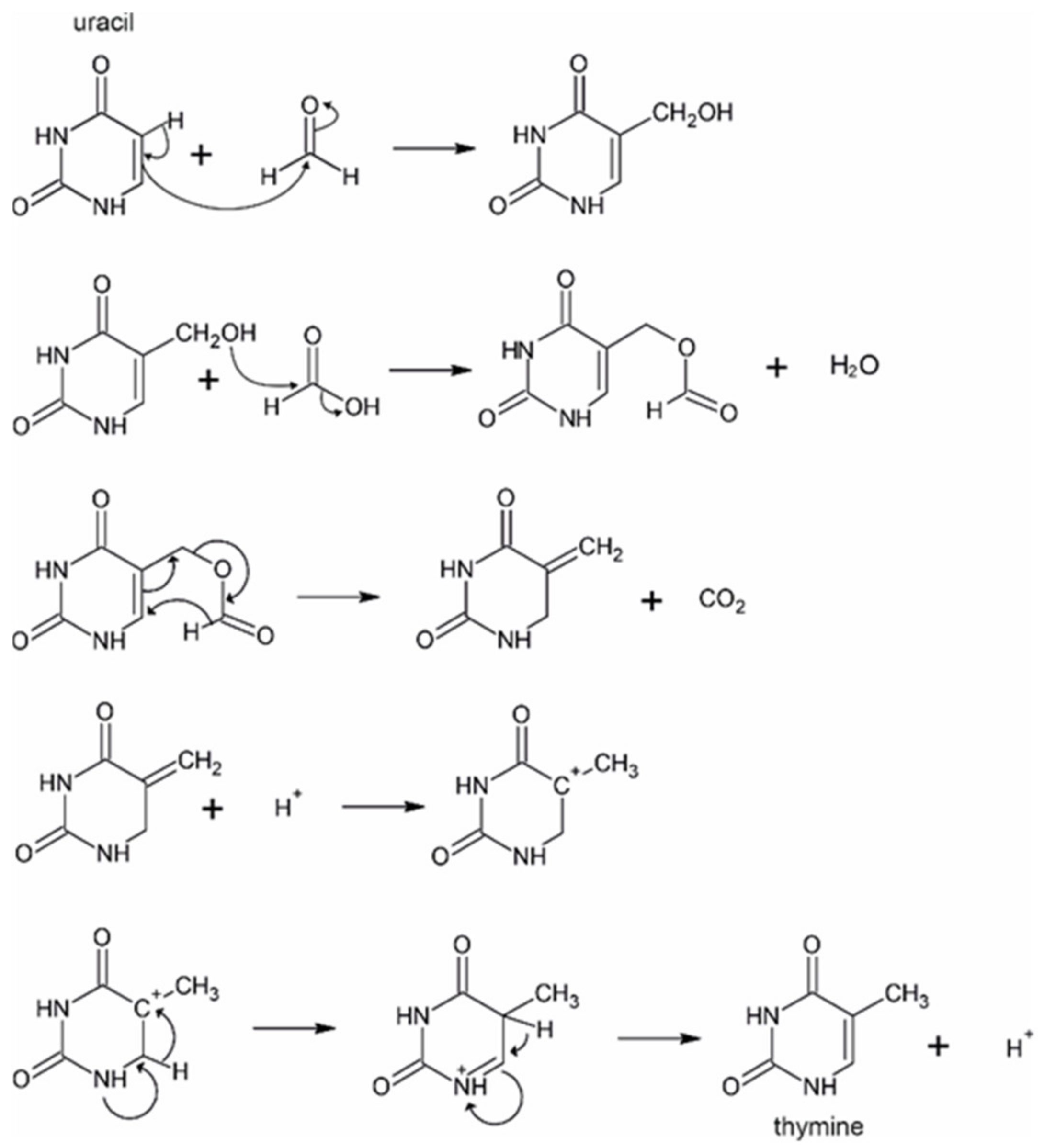
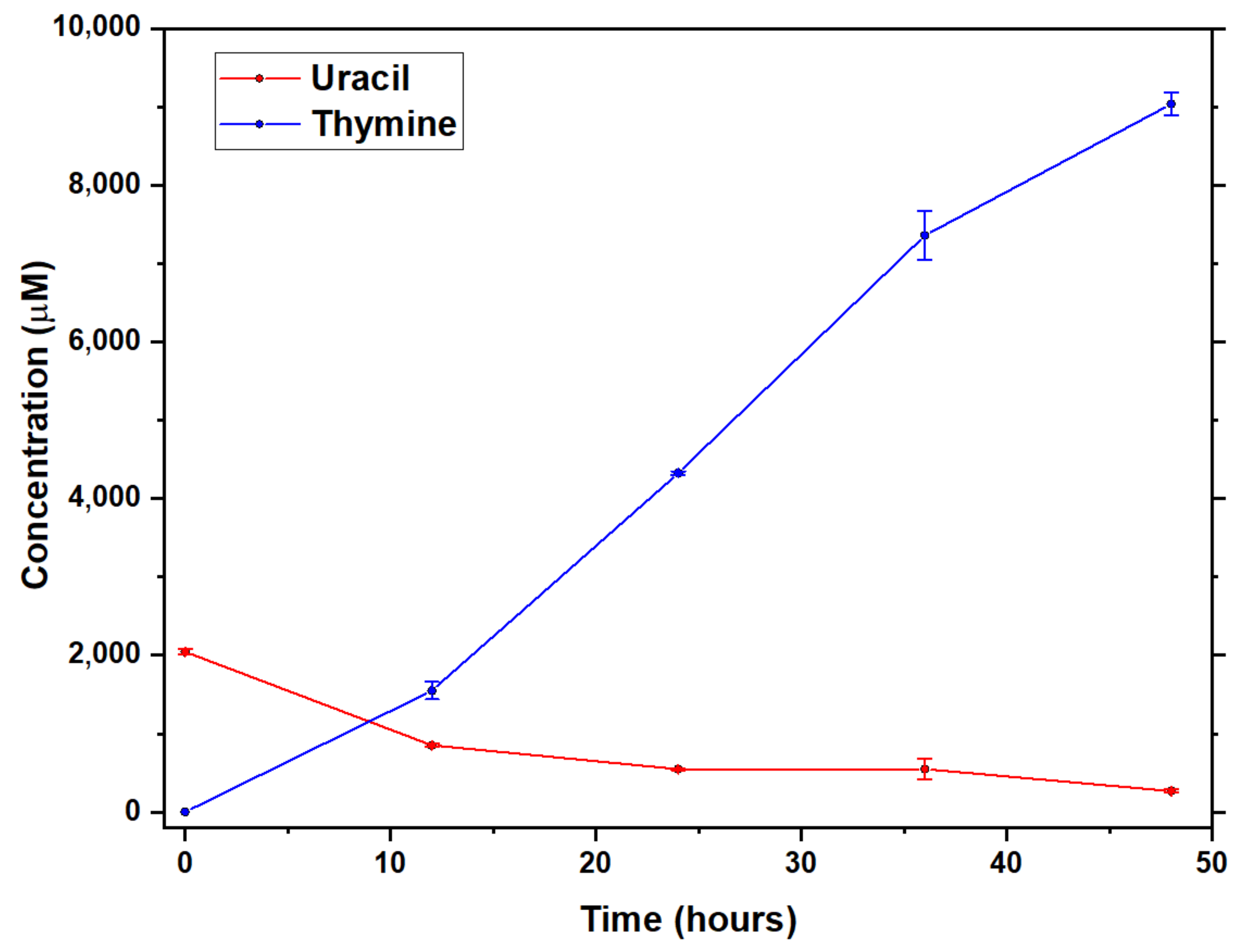
2.2. Conversion of Uracil to Thymine in the Presence of Added Formic Acid and Formaldehyde in Formamide Solution
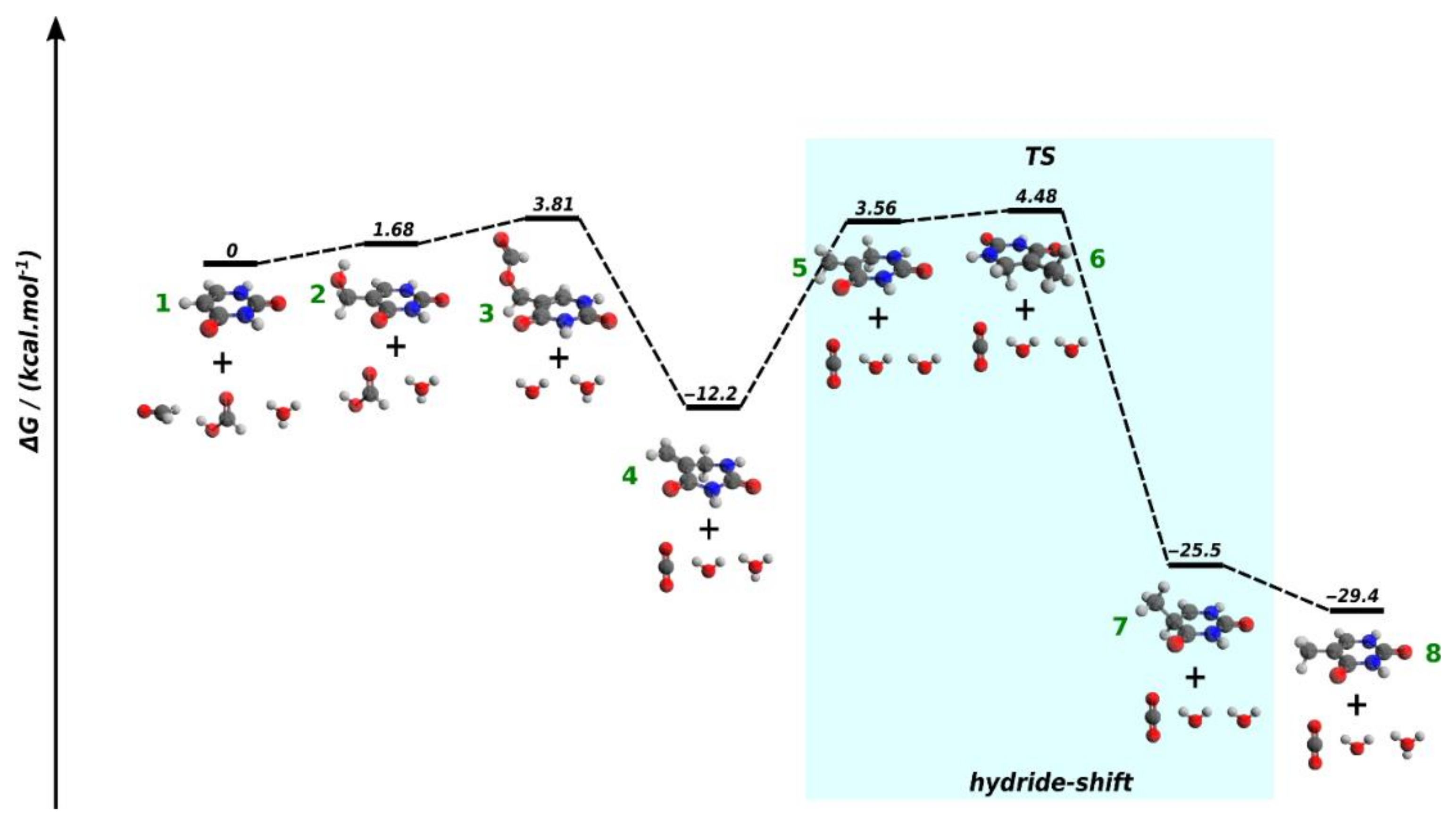
2.3. DFT Modeling of the Conversion of Uracil to Thymine
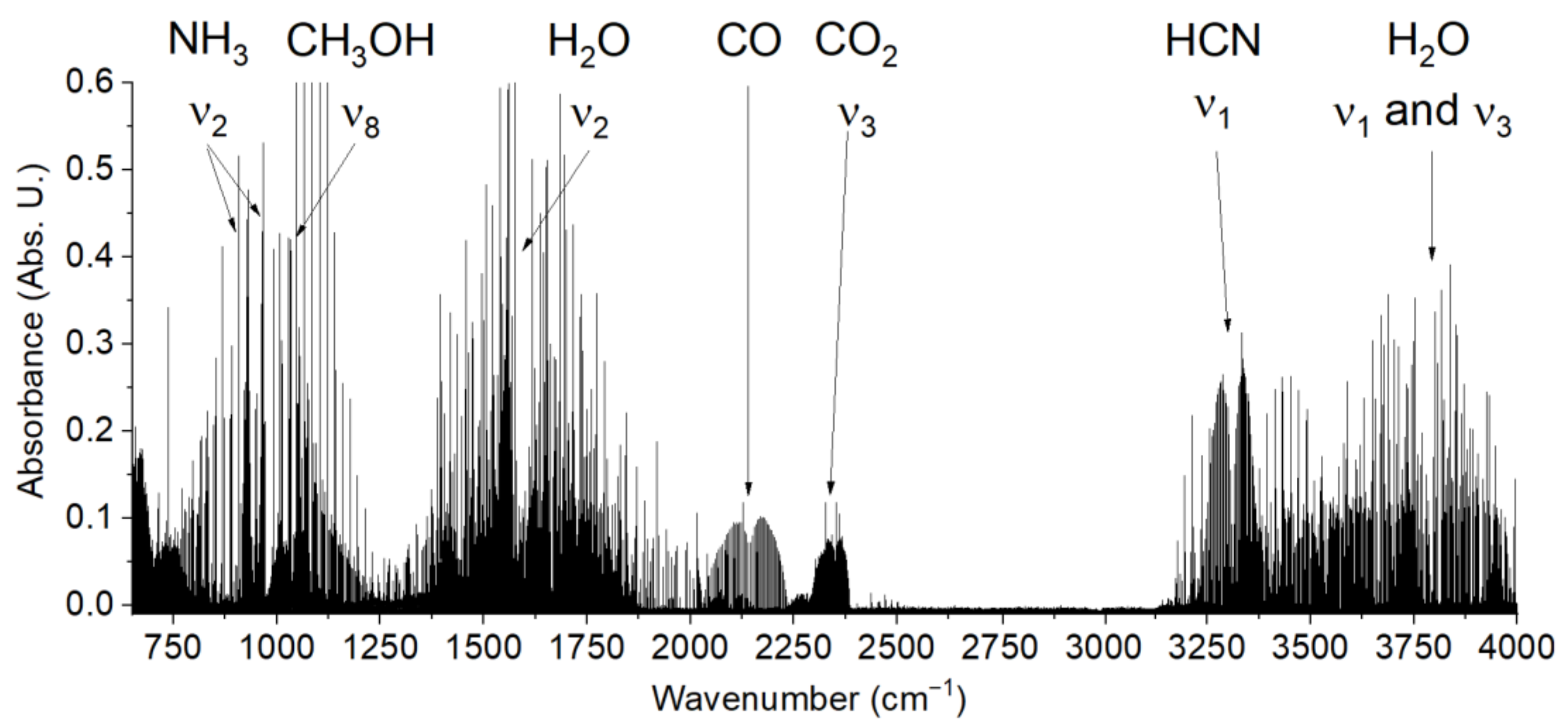
2.4. Formic Acid and Formaldehyde from Formamide without Added Catalysts
3. Materials and Methods
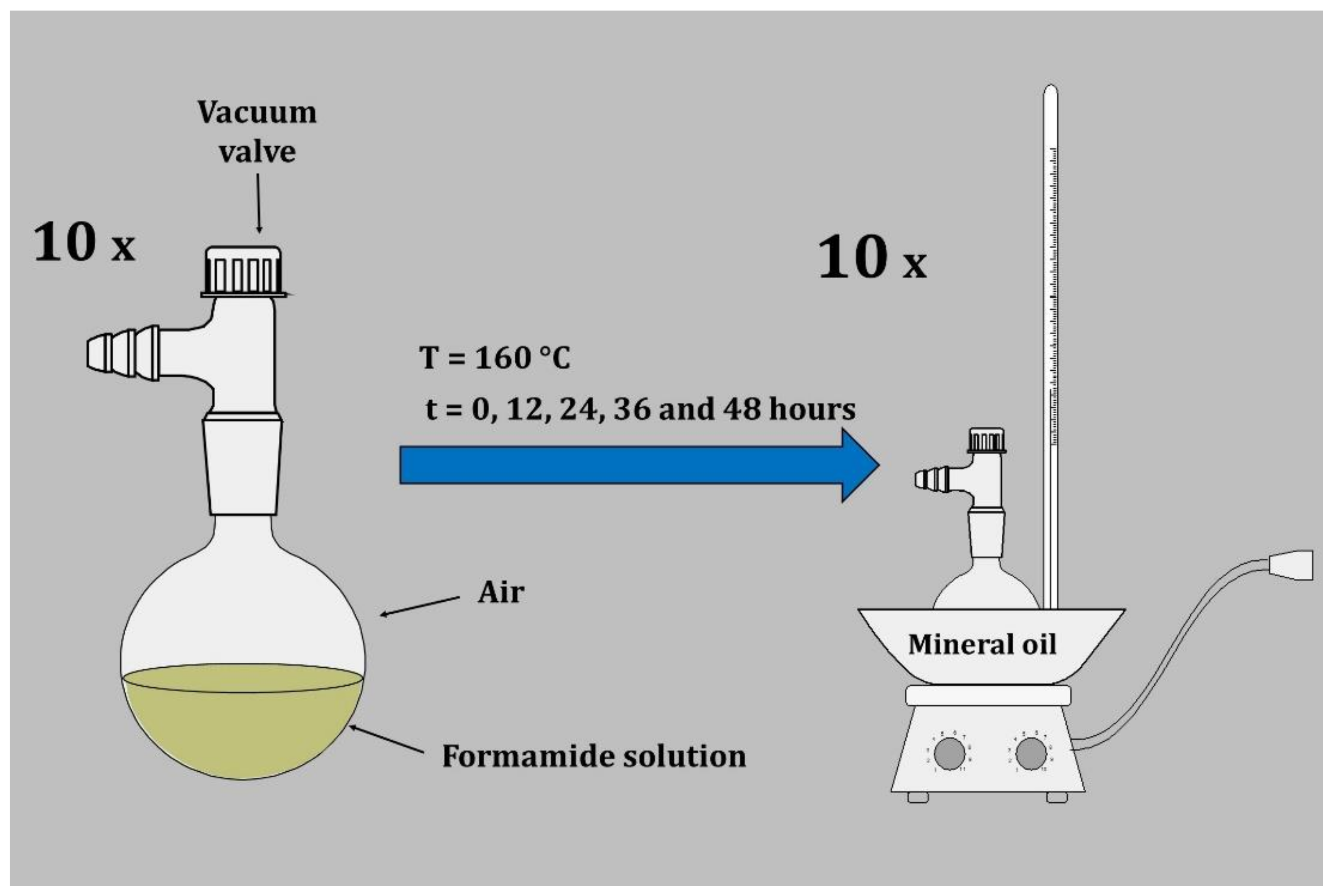
3.1. Sample Preparation
3.1.1. Formamide Thermolysis
3.1.2. Reaction of Uracil with Formaldehyde and Formic Acid in Formamide Solution
3.2. Micellar Electrokinetic Capillary Chromatography (MEKC)
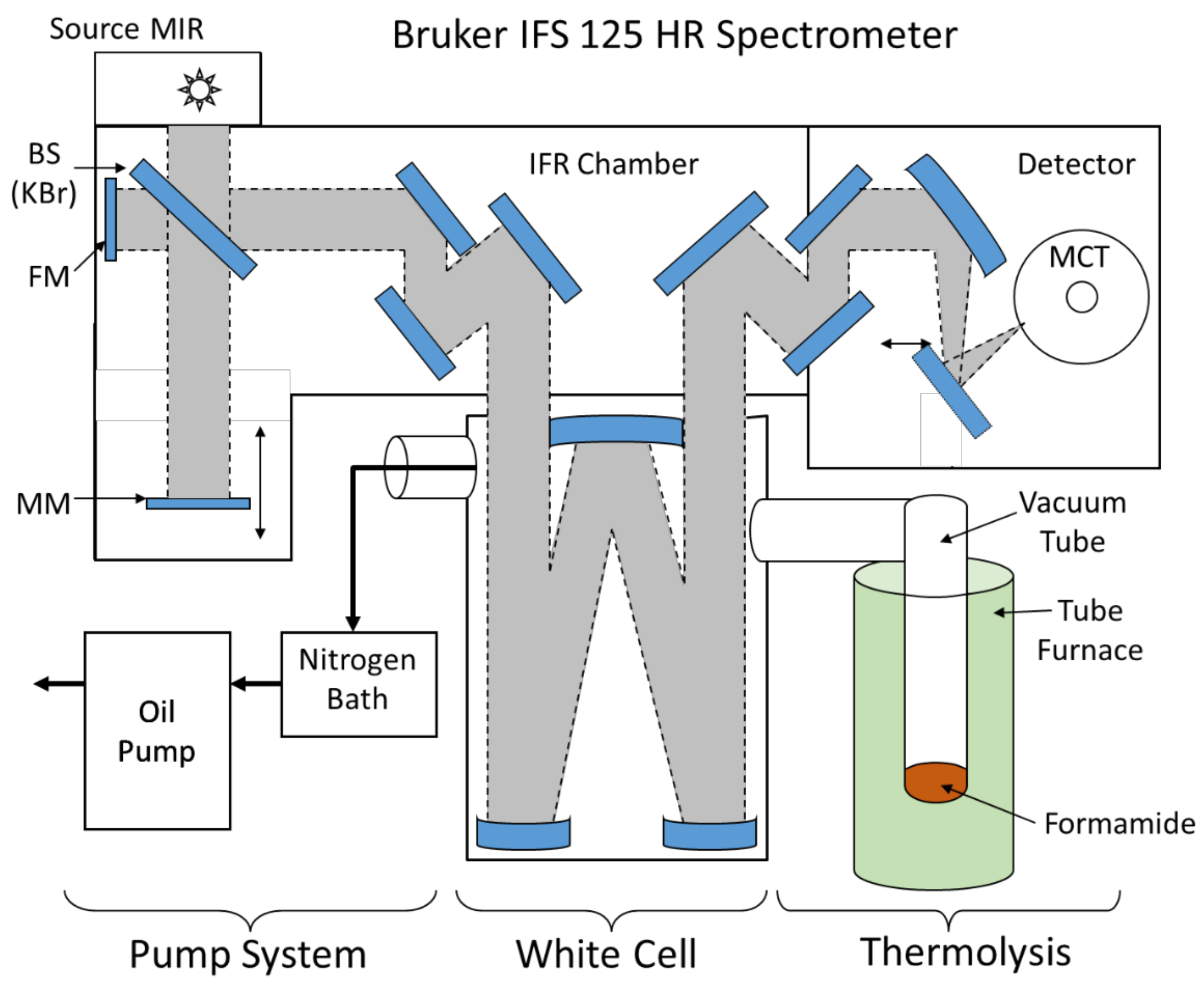
3.3. Fourier-Transform Infrared (FTIR) Spectroscopy
3.4. Quantum Chemical (QM) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbert, W. Origin of life: The RNA world. Nat. Cell Biol. 1986, 319, 618. [Google Scholar] [CrossRef]
- Rich, A. On the problems of evolution and biochemical information transfer. In Horizons in Biochemistry; Kasha, M., Pullman, B., Eds.; Academic Press: New York, NY, USA, 1962; pp. 103–126. [Google Scholar]
- Kruse, F.M.; Teichert, J.S.; Trapp, O. Prebiotic nucleoside synthesis: The selectivity of simplicity. Chem. Eur. J. 2020, 26, 14776–14790. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chmela, V.; Green, N.J.; Russell, D.A.; Janicki, M.J.; Góra, R.W.; Szabla, R.; Bond, A.D.; Sutherland, J.D. Selective prebiotic formation of RNA pyrimidine and DNA purine nucleosides. Nat. Cell Biol. 2020, 582, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Green, N.J.; Gibard, C.; Krishnamurthy, R.; Sutherland, J.D. Prebiotic phosphorylation of 2-thiouridine provides either nucleotides or DNA building blocks via photoreduction. Nat. Chem. 2019, 11, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Teichert, J.S.; Kruse, F.M.; Trapp, O. Direct Prebiotic Pathway to DNA Nucleosides. Angew. Chem. Int. Ed. 2019, 58, 9944–9947. [Google Scholar] [CrossRef] [PubMed]
- Inostroza-Rivera, R.; Herrera, B.; Toro-Labbé, A. Using the reaction force and the reaction electronic flux on the proton transfer of formamide derived systems. Phys. Chem. Chem. Phys. 2014, 16, 14489–14495. [Google Scholar] [CrossRef]
- Yamada, H.; Hirobe, M.; Higashiyama, K.; Takahashi, H.; Suzuki, K.T. Detection of carbon-13-nitrogen-15 coupled units in adenine derived from doubly labeled hydrogen cyanide or formamide. J. Am. Chem. Soc. 1978, 100, 4617–4618. [Google Scholar] [CrossRef]
- Yamada, H.; Hirobe, M.; Okamoto, T. Formamide Reaction. III. Studies on the Reaction Mechanism of Purine Ring Formation and the Reaction of Formamide with Hydrogen Cyanide. Yakugaku Zasshi 1980, 100, 489–492. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Costanzo, G.; Negri, R.; Mauro, E. A possible prebiotic synthesis of purine, adenine, cytosine, and 4(3H)-pyrimidone from formamide: Implications for the origin of life. Bioorg. Med. Chem. 2001, 9, 1249–1253. [Google Scholar] [CrossRef]
- Saladino, R.; Ciambecchini, U.; Crestini, C.; Costanzo, G.; Negri, R.; Di Mauro, E. One-Pot TiO2-Catalyzed Synthesis of Nucleic Bases and Acyclonucleosides from Formamide: Implications for the Origin of Life. ChemBioChem 2003, 4, 514–521. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Costanzo, G.; Di Mauro, E. Formamide Chemistry and the Origin of Informational Polymers. Chem. Biodivers. 2007, 4, 694–720. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, G.; Pino, S.; Costanzo, G.; Di Mauro, E. Genetics first or metabolism first? The formamide clue. Chem. Soc. Rev. 2012, 41, 5526–5565. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Nesvorný, D.; Šponer, J.; Kubelík, P.; Michalčíková, R.; Shestivská, V.; Šponer, J.E.; Civiš, S. High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc. Natl. Acad. Sci. USA 2015, 112, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Carota, E.; Botta, G.; Kapralov, M.; Timoshenko, G.N.; Rozanov, A.Y.; Krasavin, E.; Di Mauro, E. Meteorite-catalyzed syntheses of nucleosides and of other prebiotic compounds from formamide under proton irradiation. Proc. Natl. Acad. Sci. USA 2015, 112, E2746–E2755. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, A.; Hrnčířová, J.; Jankovič, L.; Nejdl, L.; Civiš, S.; Ivanek, O.; Shestivska, V.; Knížek, A.; Kubelík, P.; Šponer, J.; et al. Prebiotic synthesis at impact craters: The role of Fe-clays and iron meteorites. Chem. Comm. 2019, 55, 10563–10566. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Delfino, M.; Di Mauro, E. Meteorites as Catalysts for Prebiotic Chemistry. Chem. A Eur. J. 2013, 19, 16916–16922. [Google Scholar] [CrossRef] [PubMed]
- Signorile, M.; Pantaleone, S.; Balucani, N.; Bonino, F.; Martra, G.; Ugliengo, P. Monitoring the Reactivity of Formamide on Amorphous SiO2 by In-Situ UV-Raman Spectroscopy and DFT Modeling. Molecules 2020, 25, 2274. [Google Scholar] [CrossRef]
- Ferus, M.; Michalčíková, R.; Shestivská, V.; Šponer, J.; Šponer, J.E.; Civiš, S. High-Energy Chemistry of Formamide: A Simpler Way for Nucleobase Formation. J. Phys. Chem. A 2014, 118, 719–736. [Google Scholar] [CrossRef]
- Robertson, M.; Miller, S. Prebiotic synthesis of 5-substituted uracils: A bridge between the RNA world and the DNA-protein world. Science 1995, 268, 702–705. [Google Scholar] [CrossRef]
- Enchev, V.; Angelov, I.; Dincheva, I.; Stoyanova, N.; Slavova, S.; Rangelov, M.; Markova, N. Chemical evolution: From formamide to nucleobases and amino acids without the presence of catalyst. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef]
- Šebesta, F.; Brela, M.Z.; Diaz, S.; Miranda, S.; Murray, J.S.; Gutiérrez-Oliva, S.; Toro-Labbé, A.; Michalak, A.; Burda, J.V. The influence of the metal cations and microhydration on the reaction trajectory of the N3 ↔ O2 thymine proton transfer: Quantum mechanical study. J. Comput. Chem. 2017, 38, 2680–2692. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nat. Cell Biol. 2009, 459, 239–242. [Google Scholar] [CrossRef]
- Sponer, J.E.; Sponer, J.; Fuentes-Cabrera, M. Prebiotic routes to nucleosides: A quantum chemical insight into the energetics of the multistep reaction pathways. Chem. Eur. J. 2011, 17, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; Patanè, G.; Compagnini, G. Synthesis of HCN Polymer from Thermal Decomposition of Formamide. J. Macromol. Sci. Part. A 2009, 46, 1039–1048. [Google Scholar] [CrossRef]
- Gibson, H.W. Chemistry of formic acid and its simple derivatives. Chem. Rev. 1969, 69, 673–692. [Google Scholar] [CrossRef]
- Czaun, M.; Goeppert, A.; Kothandaraman, J.; May, R.B.; Haiges, R.; Prakash, G.K.S.; Olah, G.A. Formic Acid As a Hydrogen Storage Medium: Ruthenium-Catalyzed Generation of Hydrogen from Formic Acid in Emulsions. ACS Catal. 2013, 4, 311–320. [Google Scholar] [CrossRef]
- Chauvier, C.; Imberdis, A.; Thuéry, P.; Cantat, T. Catalytic disproportionation of formic acid to methanol by using recyclable silylformates. Angew. Chem. Int. Edit. 2020, 59, 14019–14023. [Google Scholar] [CrossRef] [PubMed]
- Zemankova, K.; Nejdl, L.; Bezdekova, J.; Vodova, M.; Petera, L.; Pastorek, A.; Civis, S.; Kubelik, P.; Ferus, M.; Adam, V.; et al. Micellar electrokinetic chromatography as a powerful analytical tool for research on prebiotic chemistry. Microchem. J. 2021, 106022. [Google Scholar] [CrossRef]
- Jmol: An Open-Source Java Viewer for Chemical Structures in 3D. Available online: http://www.jmol.org/ (accessed on 8 April 2021).
- Frisch, M.J.T.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density—Functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799–805. [Google Scholar] [CrossRef]
| Bases | Regression Equation | R | Detection Limit (µM) | Migration Time (min) |
|---|---|---|---|---|
| Thymine | y = 163.51x − 0.1331 | 0.999 | 0.6 | 11.5 |
| Uracil | y = 216.05x − 0.1199 | 0.999 | 0.4 | 14.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petera, L.; Mrazikova, K.; Nejdl, L.; Zemankova, K.; Vaculovicova, M.; Pastorek, A.; Civis, S.; Kubelik, P.; Heays, A.; Cassone, G.; et al. Prebiotic Route to Thymine from Formamide—A Combined Experimental–Theoretical Study. Molecules 2021, 26, 2248. https://doi.org/10.3390/molecules26082248
Petera L, Mrazikova K, Nejdl L, Zemankova K, Vaculovicova M, Pastorek A, Civis S, Kubelik P, Heays A, Cassone G, et al. Prebiotic Route to Thymine from Formamide—A Combined Experimental–Theoretical Study. Molecules. 2021; 26(8):2248. https://doi.org/10.3390/molecules26082248
Chicago/Turabian StylePetera, Lukáš, Klaudia Mrazikova, Lukas Nejdl, Kristyna Zemankova, Marketa Vaculovicova, Adam Pastorek, Svatopluk Civis, Petr Kubelik, Alan Heays, Giuseppe Cassone, and et al. 2021. "Prebiotic Route to Thymine from Formamide—A Combined Experimental–Theoretical Study" Molecules 26, no. 8: 2248. https://doi.org/10.3390/molecules26082248
APA StylePetera, L., Mrazikova, K., Nejdl, L., Zemankova, K., Vaculovicova, M., Pastorek, A., Civis, S., Kubelik, P., Heays, A., Cassone, G., Sponer, J., Ferus, M., & Sponer, J. (2021). Prebiotic Route to Thymine from Formamide—A Combined Experimental–Theoretical Study. Molecules, 26(8), 2248. https://doi.org/10.3390/molecules26082248








