Inhibitory Effects of Erythrosine/Curcumin Derivatives/Nano-Titanium Dioxide-Mediated Photodynamic Therapy on Candida albicans
Abstract
:1. Introduction
2. Results
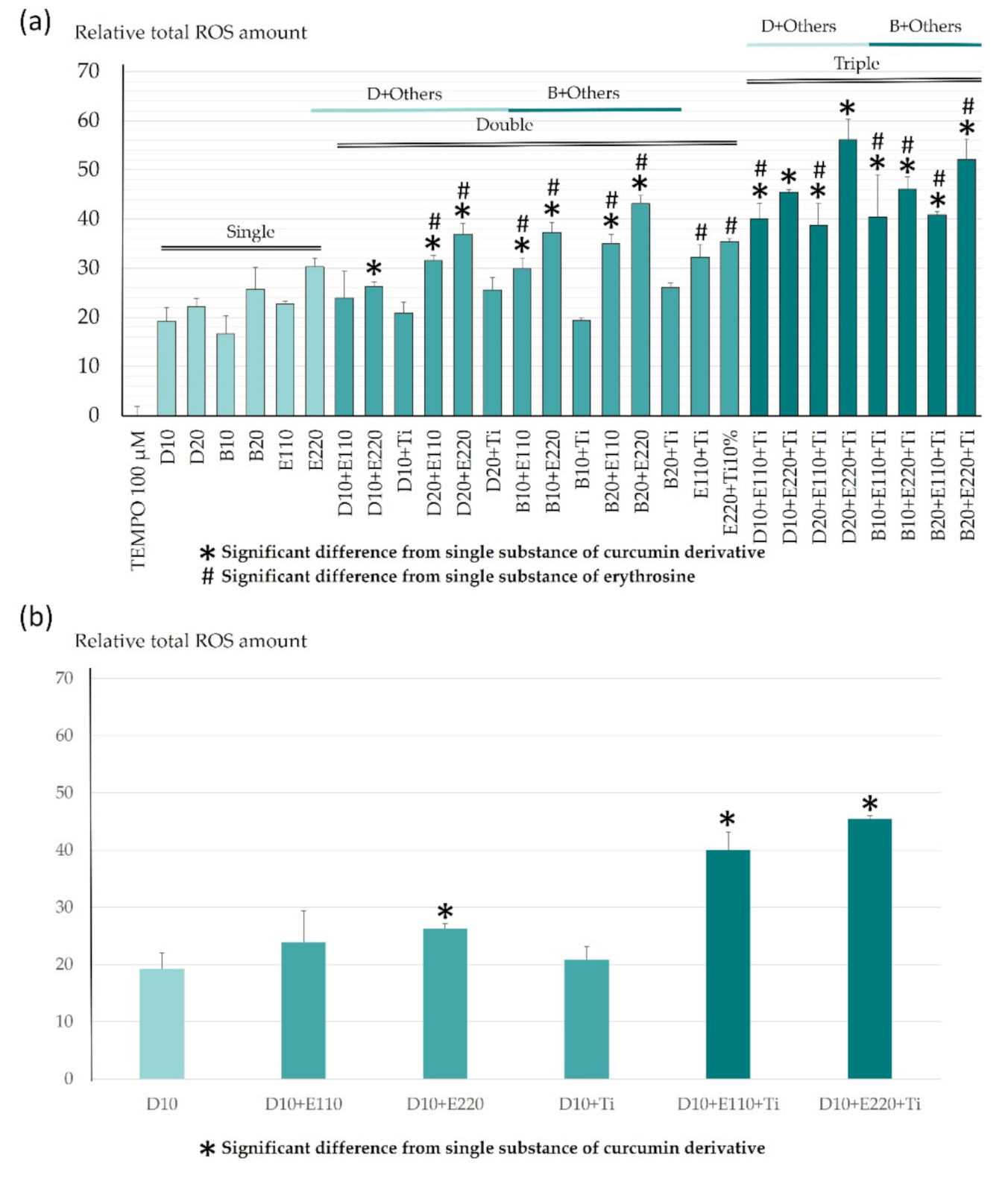
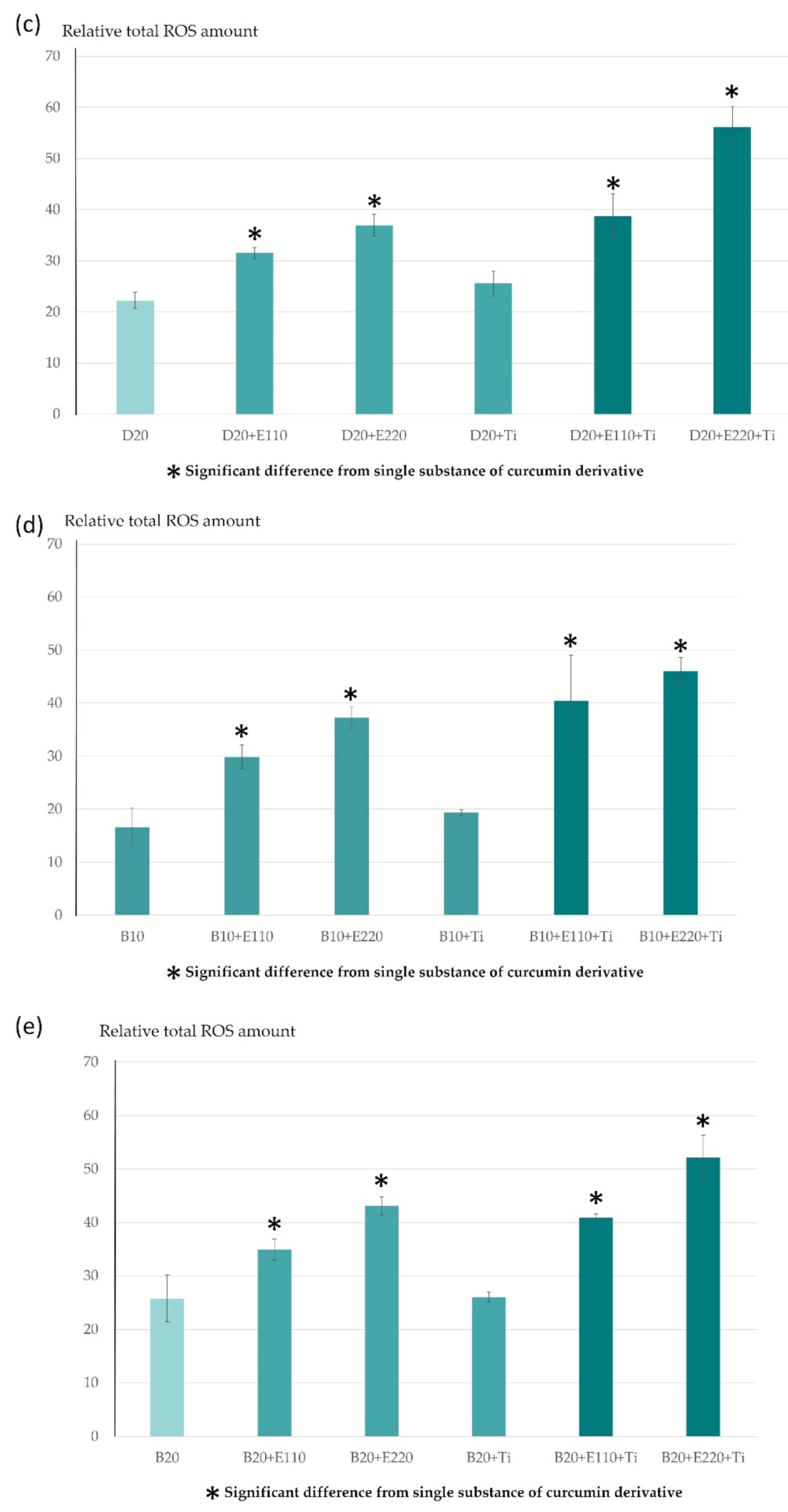
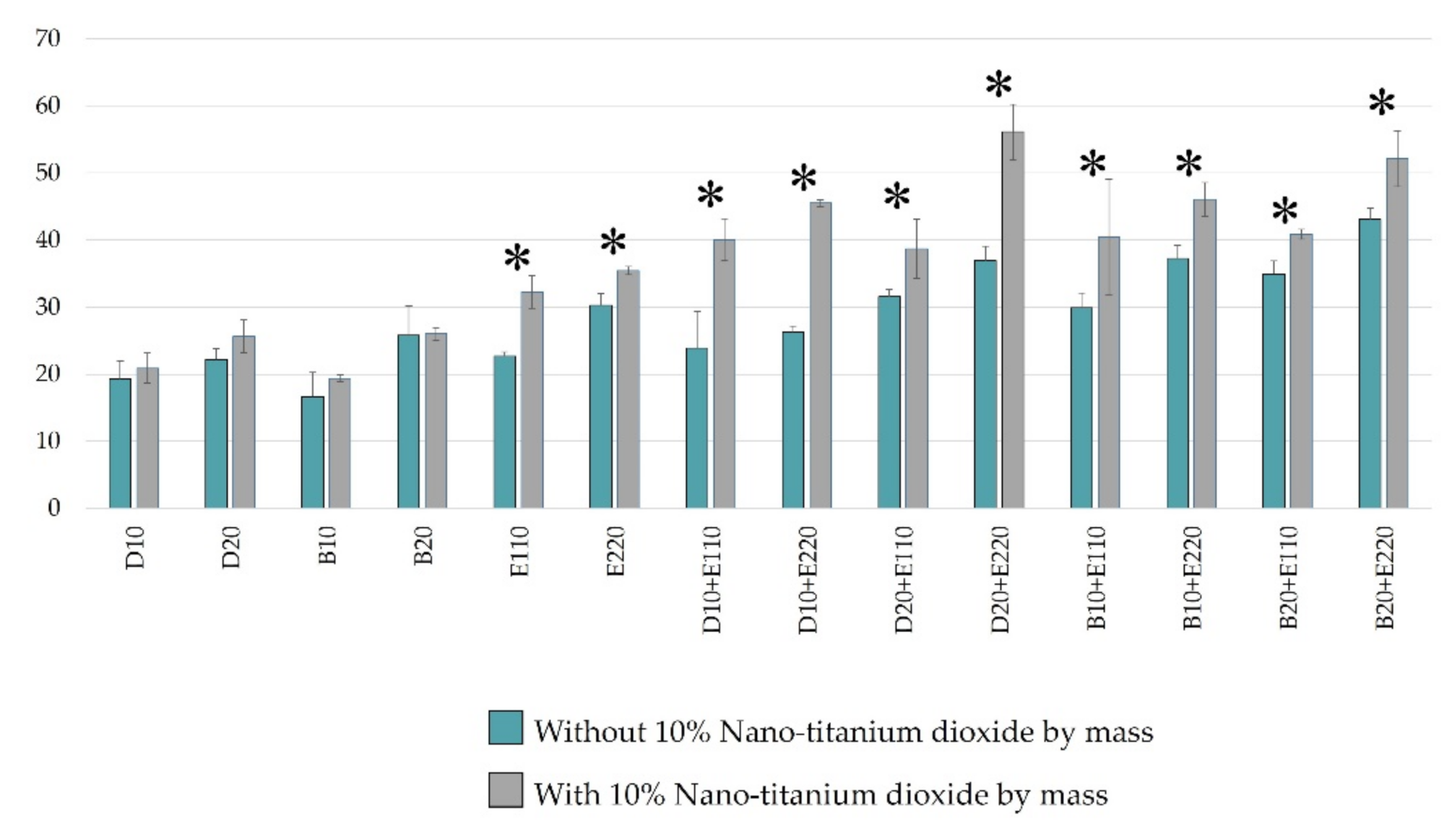
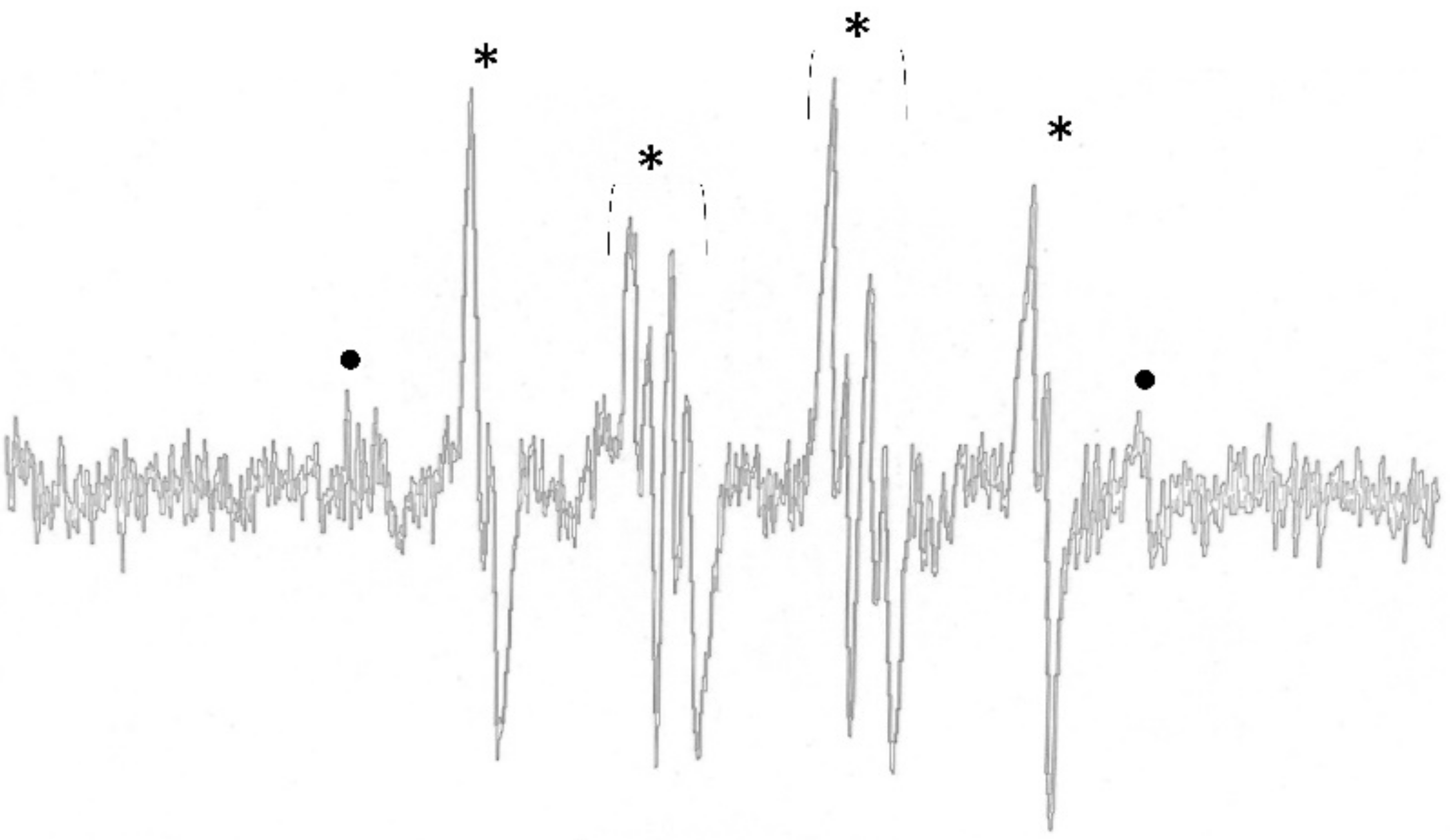
2.1. ROS Determination Using Electron Paramagnetic Resonance Spectrometer
2.2. Viability of Gingival Fibroblast Cells
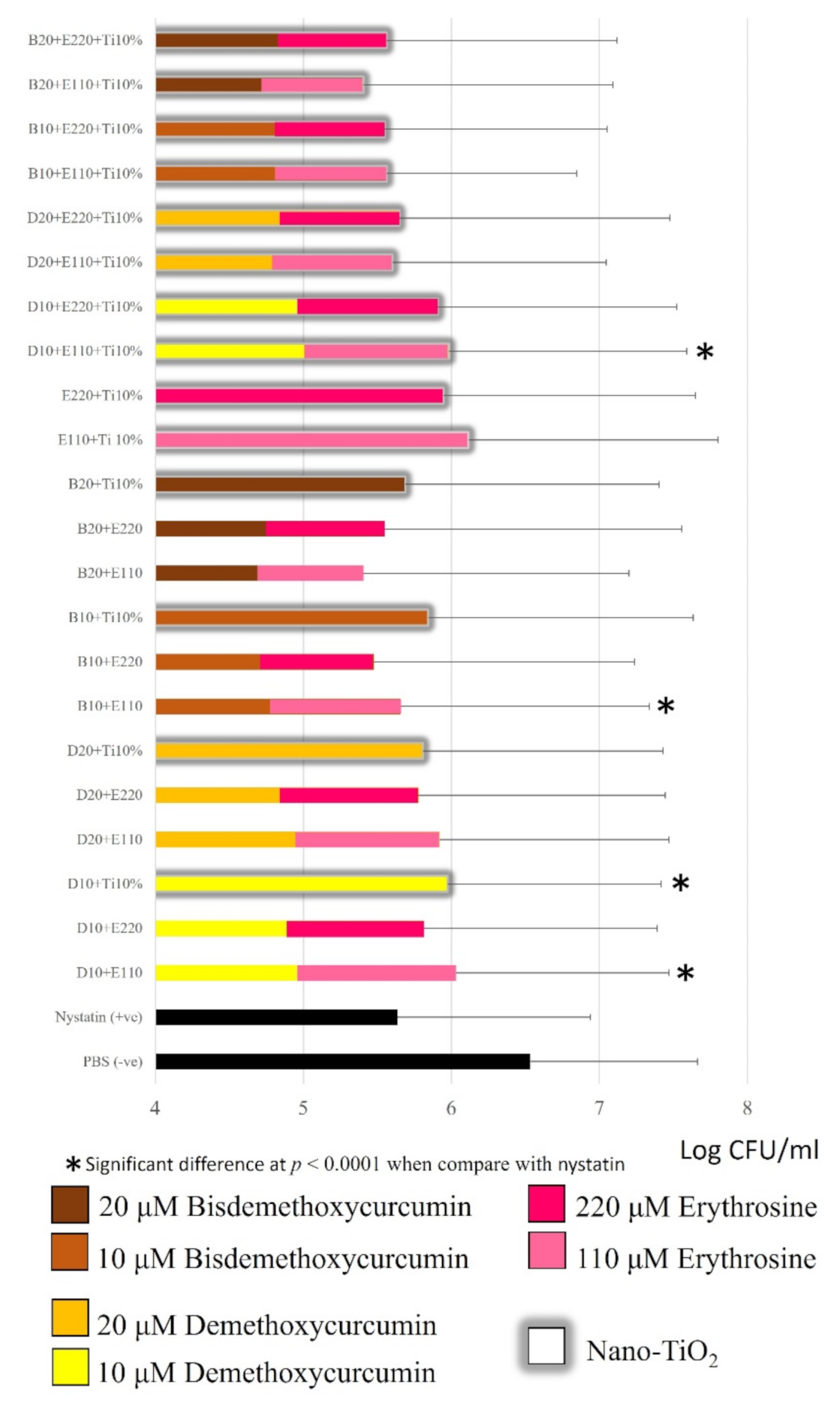
2.3. Candida Albicans Inhibition
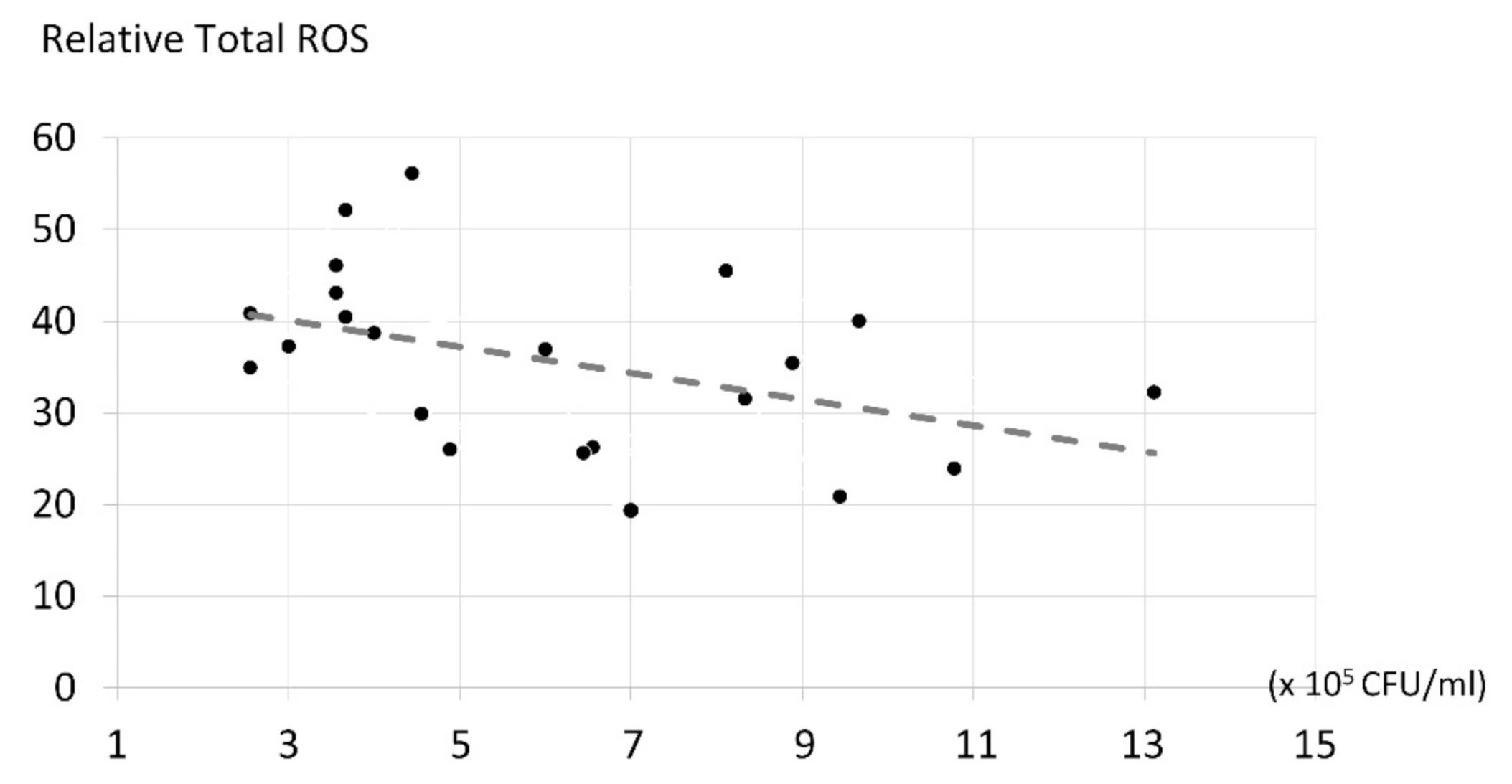
2.4. The Correlation of ROS Production and Candida albicans Inhibition
3. Discussion
3.1. Candida albicans Inhibition
3.2. ROS in Photodynamic Treatment
3.3. The Effect of the Methoxy Group in Curcuminoids’ Structure on ROS Production and Antifungal Activity
3.4. Influence of Nano-Titanium Dioxide on ROS Production and Antifungal Activity
3.5. Viability of Gingival Fibroblast Cell.
3.6. The Correlation of ROS Production and Candida albicans Inhibition
3.7. Limitation of the Study
4. Materials and Methods
4.1. Photosensitizers Preparation
- (1)
- Single 10 or 20 µM demethoxycurcumin and 110 or 220 µM erythrosine;
- (2)
- 10 or 20 µM demethoxycurcumin with/without 110 or 220 µM erythrosine with 10% nano-titanium dioxide;
- (3)
- 10 or 20 µM demethoxycurcumin with/without 110 or 220 µM erythrosine without nano-titanium dioxide;
- (4)
- 10 or 20 µM bismethoxycurcumin with/without 110 or 220 µM erythrosine with 10% nano-titanium dioxide;
- (5)
- 10 or 20 µM bismethoxycurcumin with/without 110 or 220 µM erythrosine without nano-titanium dioxide.
4.2. Dental light Optic Preparation
4.3. Determination of ROS Production Using the Electron Paramagnetic Resonance (EPR) Technique
4.3.1. Total ROS Production
4.3.2. ROS Measurement of Type I Photodynamic Reaction
4.4. Toxicity Testing of A Test Photosensitizer Containing Curcumin Derivatives, Erythrosine, and Nano-Titanium Dioxide to Gingival Fibroblast Cell by PrestoBlue®
4.4.1. Test Photosensitizers Used in the Experimental Group
4.4.2. Gingival Fibroblast Cell Culture Conditions
4.4.3. Study Process
4.5. Candida albicans Inhibition Assay
4.5.1. Test Photosensitizers Used in the Experimental Group
4.5.2. Candida albicans Biofilm Culture
4.5.3. Study Process
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yang, Y.L. Virulence factors of Candida species. J. Microbiol. Immunol. Infect. 2003, 36, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.P. Nosocomial candidemia: Risk factors and attributable mortality. Clin. Infect. Dis. 1995, 20, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Urzua, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Coccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med. Mycol. 2008, 46, 783–793. [Google Scholar] [CrossRef]
- Jarvensivu, A.; Hietanen, J.; Rautemaa, R.; Sorsa, T.; Richardson, M. Candida yeasts in chronic periodontitis tissues and subgingival microbial biofilms in vivo. Oral Dis. 2004, 10, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Rams, T.E.; Listgarten, M.A. Yeasts, enteric rods and pseudomonads in the subgingival flora of severe adult periodontitis. Oral. Microbiol. Immunol. 1988, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidova, T.N.; Hamblin, M.R. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 2004, 17, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef]
- Babilas, P.; Karrer, S.; Sidoroff, A.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology—An update. Photodermatol. Photoimmunol. Photomed. 2005, 21, 142–149. [Google Scholar] [CrossRef]
- Costa, A.C.; de Campos Rasteiro, V.M.; Pereira, C.A.; da Silva Hashimoto, E.S.; Beltrame, M., Jr.; Junqueira, J.C.; Jorge, A.O. Susceptibility of Candida albicans and Candida dubliniensis to erythrosine- and LED-mediated photodynamic therapy. Arch. Oral Biol. 2011, 56, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.C.; Rasteiro, V.M.; Pereira, C.A.; Rossoni, R.D.; Junqueira, J.C.; Jorge, A.O. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses 2012, 55, 56–63. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.; Brunetti, I.L.; Costa, C.A.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Arrieta, A.F.; Lerner, D. Studies on curcumin and curcuminoids. Part XXIV: Characterization of the spectroscopic properties of the naturally occurring curcuminoids and selected derivatives. Pharmazie 1995, 50, 689–693. [Google Scholar]
- Walker, J. Carbohydrates. Available online: http://thebiologyprimer.com/carbohydrates/ (accessed on 15 June 2018).
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food. Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Leite, D.P.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: A randomized controlled trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Mu, T.; Sun, H.; Zhang, M.; Wang, C. Sweet Potato Processing Technology, 1st ed.; Academic Press: Beijing, China, 2017; Volume 1, pp. 49–119. [Google Scholar]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [Green Version]
- Gázquez, M.J.; Bolívar, J.P.; García-Tenorio, R.; Galan, F.V. A review of the production cycle of titanium dioxide pigment. Mater. Sci. Appl. 2014, 5, 441–458. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, N.; Pax, R.A.; Gray, E.M. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Teerakapong, A.; Damrongrungruang, T.; Sattayut, S.; Morales, N.P.; Sangpanya, A.; Tanapoomchai, M. Fungicidal effect of combined nano TiO2 with erythrosine for mediated photodynamic therapy on Candida albicans: An in vitro study. Lasers Dent. Sci. 2017, 1, 101–106. [Google Scholar] [CrossRef]
- Bugelski, P.J.; Atif, U.; Molton, S.; Toeg, I.; Lord, P.G.; Morgan, D.G. A strategy for primary high throughput cytotoxicity screening in pharmaceutical toxicology. Pharm. Res. 2000, 17, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Umebayashi, Y.; Nishisaka, T. Comparison of phototoxicity mechanism between pulsed and continuous wave irradiation in photodynamic therapy. J. Photochem. Photobiol. B 1999, 53, 53–59. [Google Scholar] [CrossRef]
- Klimenko, V.V.; Knyazev, N.A.; Moiseenko, F.V.; Rusanov, A.A.; Bogdanov, A.A.; Dubina, M.V. Pulse mode of laser photodynamic treatment induced cell apoptosis. Photodiagnosis Photodyn. Ther. 2016, 13, 101–107. [Google Scholar] [CrossRef]
- Ding, P.; Di, J.; Chen, X.; Zhao, J.; Gu, K.; Zhang, Y.; Yin, S.; Liu, G.; Xia, J.; Li, H. Partially etched Bi2O2CO3 by metal chloride for enhanced reactive oxygen species generation: A tale of two strategies. Appl. Catal. B Environ. 2019, 245, 325–333. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [Green Version]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin derivatives as photosensitizers in photodynamic therapy: Photophysical properties and in vitro studies with prostate cancer cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-light active titanium dioxide nanomaterials with bactericidal properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Ordoudi, S.A.; Tsimidou, M.Z. Consideration of fluorescence properties for the direct determination of erythrosine in saffron in the presence of other synthetic dyes. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk. Assess. 2011, 28, 417–422. [Google Scholar] [CrossRef]
- Werts, M.H.; Raimbault, V.; Texier-Picard, R.; Poizat, R.; Français, O.; Griscom, L.; Navarro, J.R.G. Quantitative full-colour transmitted light microscopy and dyes for concentration mapping and measurement of diffusion coefficients in microfluidic architectures. Lab Chip 2012, 12, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Péret-Almeida, L.; Cherubino, A.P.F.; Alves, R.J.; Dufossé, L.; Glória, M.B.A. Separation and determination of the physico-chemical characteristics of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Res. Int. 2005, 38, 1039–1044. [Google Scholar] [CrossRef]
- Kloesch, B.; Gober, L.; Loebsch, S.; Vcelar, B.; Helson, L.; Steiner, G. In vitro study of a liposomal curcumin formulation (Lipocurc™): Toxicity and biological activity in synovial fibroblasts and macrophages. In Vivo 2016, 30, 413–419. [Google Scholar]
- Mpountoukas, P.; Pantazaki, A.; Kostareli, E.; Christodoulou, P.; Kareli, D.; Poliliou, S.; Mourelatos, C.; Lambropoulou, V.; Lialiaris, T. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem. Toxicol. 2010, 48, 2934–2944. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Kim, C. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Nardi, G.; Manet, I.; Monti, S.; Miranda, M.A.; Lhiaubet-Vallet, V. Scope and limitations of the TEMPO/EPR method for singlet oxygen detection: The misleading role of electron transfer. Free. Radic. Biol. Med. 2014, 77, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yip, H.K.; Samaranayake, Y.H.; Yau, J.Y.; Samaranayake, L.P. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 2003, 41, 2961–2967. [Google Scholar] [CrossRef] [Green Version]
- Thein, Z.M.; Samaranayake, Y.H.; Samaranayake, L.P. In vitro biofilm formation of Candida albicans and non-albicans Candida species under dynamic and anaerobic conditions. Arch. Oral Biol. 2007, 52, 761–767. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanpittaya, K.; Teerakapong, A.; Morales, N.P.; Hormdee, D.; Priprem, A.; Weera-archakul, W.; Damrongrungruang, T. Inhibitory Effects of Erythrosine/Curcumin Derivatives/Nano-Titanium Dioxide-Mediated Photodynamic Therapy on Candida albicans. Molecules 2021, 26, 2405. https://doi.org/10.3390/molecules26092405
Kanpittaya K, Teerakapong A, Morales NP, Hormdee D, Priprem A, Weera-archakul W, Damrongrungruang T. Inhibitory Effects of Erythrosine/Curcumin Derivatives/Nano-Titanium Dioxide-Mediated Photodynamic Therapy on Candida albicans. Molecules. 2021; 26(9):2405. https://doi.org/10.3390/molecules26092405
Chicago/Turabian StyleKanpittaya, Kasama, Aroon Teerakapong, Noppawan Phumala Morales, Doosadee Hormdee, Aroonsri Priprem, Wilawan Weera-archakul, and Teerasak Damrongrungruang. 2021. "Inhibitory Effects of Erythrosine/Curcumin Derivatives/Nano-Titanium Dioxide-Mediated Photodynamic Therapy on Candida albicans" Molecules 26, no. 9: 2405. https://doi.org/10.3390/molecules26092405