Structural Insights into the Host–Guest Complexation between β-Cyclodextrin and Bio-Conjugatable Adamantane Derivatives
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Preparation of β-CD and Adamantane Inclusion Complexes
2.3. 1H-NMR and FT-IR Characterization Data for the Adamantane Inclusion Complexes
2.4. X-ray Crystal Structure Determinations
2.5. Isothermal Titration Calorimetry (ITC) Measurements
3. Results and Discussion
3.1. Synthesis and Spectroscopic Characterization
3.2. Isothermal Titration Calorimetry (ITC) Analysis of 4 and 5
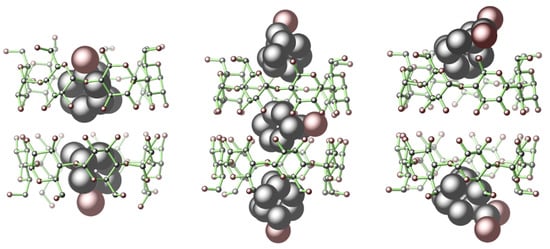
3.3. Single-Crystal Structure Analysis of 1–6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Saenger, W.; Jacob, J.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the common cyclodextrins and their larger analoguesbeyond the doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef]
- Eftink, M.R.; Andy, M.L.; Bystrom, K.; Perlmutter, H.D.; Kristol, D.S. Cyclodextrin inclusion complexes: Studies of the variation in the size of alicyclic guests. J. Am. Chem. Soc. 1989, 111, 6765–6772. [Google Scholar] [CrossRef]
- Cromwell, W.C.; Bystrom, K.; Eftink, M.R. Cyclodextrin-adamantanecarboxylate inclusion complexes: Studies of the variation in cavity size. J. Phys. Chem. 1985, 89, 326–332. [Google Scholar] [CrossRef]
- Krois, D.; Brinker, U.H. Induced Circular Dichroism and UV−Vis Absorption Spectroscopy of Cyclodextrin Inclusion Complexes: Structural Elucidation of Supramolecular Azi-adamantane (Spiro[adamantane-2,3‘-diazirine]). J. Am. Chem. Soc. 1998, 120, 11627–11632. [Google Scholar] [CrossRef]
- Gelb, R.I.; Schwartz, L.M. Complexation of adamantane-ammonium substrates by beta-cyclodextrin and itsO-methylated derivatives. J. Incl. Phenom. Macrocycl. Chem. 1989, 7, 537–543. [Google Scholar] [CrossRef]
- Dasgupta, S.; Wu, J. Formation of [2]rotaxanes by encircling [20], [21] and [22]crown ethers onto the dibenzylammonium dumbbell. Chem. Sci. 2011, 3, 425–432. [Google Scholar] [CrossRef]
- Park, H.J.; Suh, M.P. Enhanced isosteric heat of H2 adsorption by inclusion of crown ethers in a porous metal–organic framework. Chem. Commun. 2012, 48, 3400–3402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liang, Z.; Zhang, B.; Wang, Q.; Lee, J.; Li, F.; Wang, Q.; Ma, D.; Ling, D. Supramolecular Container-Mediated Surface Engineering Approach for Regulating the Biological Targeting Effect of Nanoparticles. Nano Lett. 2020, 20, 7941–7947. [Google Scholar] [CrossRef]
- Frank, N.; Dallmann, A.; Braun-Cula, B.; Herwig, C.; Limberg, C. Mercaptothiacalixarenes steer 24 copper(I) centers to form a hollow-sphere structure featuring Cu2S2 motifs with exceptionally short Cu⋅⋅⋅Cu distances. Angew. Chem. Int. Ed. 2020, 59, 6735–6739. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, M.H.; Mutihac, L.; Vicens, J.; Kim, J.S. Host–guest sensing by calixarenes on the surfaces. Chem. Soc. Rev. 2012, 41, 1173–1190. [Google Scholar] [CrossRef]
- Dai, F.-R.; Wang, Z. Modular Assembly of Metal–Organic Supercontainers Incorporating Sulfonylcalixarenes. J. Am. Chem. Soc. 2012, 134, 8002–8005. [Google Scholar] [CrossRef]
- Xian, S.; Webber, M.J. Temperature-responsive supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Feng, H.; Zhao, W.; Huang, C.; Redshaw, C.; Tao, Z.; Xiao, X. Amino acid recognition by a fluorescent chemosensor based on cucurbit[8]uril and acridine hydrochloride. Anal. Chim. Acta 2020, 1135, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Q.; Gao, Z.; Wang, H.; Shi, B.; Wu, Y.; Shangguan, L.; Hong, X.; Wang, F.; Huang, F. Pillararene Host–Guest Complexation Induced Chirality Amplification: A New Way to Detect Cryptochiral Compounds. Angew. Chem. Int. Ed. 2020, 59, 10868–10872. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Naugolny, A.; Feldman, M.; Herzog, I.M.; Fridman, M.; Cohen, Y. Cationic Pillararenes Potently Inhibit Biofilm Formation without Affecting Bacterial Growth and Viability. J. Am. Chem. Soc. 2016, 138, 754–757. [Google Scholar] [CrossRef]
- Zhou, Y.; Jie, K.; Zhao, R.; Li, E.; Huang, F. Highly Selective Removal of Trace Isomers by Nonporous Adaptive Pillararene Crystals for Chlorobutane Purification. J. Am. Chem. Soc. 2020, 142, 6957–6961. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2020, 32, e1806158. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, D.; Tao, H.; Li, G.; Liu, R.; Dou, Y.; Jin, T.; Li, L.; Huang, J.; Hu, H.; et al. Cyclodextrin-Derived Intrinsically Bioactive Nanoparticles for Treatment of Acute and Chronic Inflammatory Diseases. Adv. Mater. 2019, 31, e1904607. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, I.M.; Yannakopoulou, K. Porphyrinoid–Cyclodextrin Assemblies in Biomedical Research: An Update. J. Med. Chem. 2019, 63, 3391–3424. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Ariza, R.; Graña-Suárez, L.; Verboom, W.; Huskens, J. Cyclodextrin-based supramolecular nanoparticles for biomedical applications. J. Mater. Chem. B 2017, 5, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef] [PubMed]
- Numanoğlu, U.; Şen, T.; Tarimci, N.; Kartal, M.; Koo, O.M.Y.; Önyüksel, H. Use of cyclodextrins as a cosmetic delivery system for fragrance materials: Linalool and benzyl acetate. AAPS PharmSciTech 2007, 8, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikder, T.; Rahman, M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nat. Cell Biol. 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ling, Y.; Alsbaiee, A.; Li, C.; Helbling, D.E.; Dichtel, W.R. β-Cyclodextrin Polymer Network Sequesters Perfluorooctanoic Acid at Environmentally Relevant Concentrations. J. Am. Chem. Soc. 2017, 139, 7689–7692. [Google Scholar] [CrossRef] [Green Version]
- Santana, I.; Wu, H.; Hu, P.; Giraldo, J.P. Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.; Zheng, Z.; Wang, J.; Wang, Q.; Hu, X.; Tang, G.; Bai, H. Enhanced antitumour effect for hepatocellular carcinoma in the advanced stage using a cyclodextrin-sorafenib-chaperoned inclusion complex. Biomater. Sci. 2019, 7, 4758–4768. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Zhang, N.-Y.; Xiao, K.; Yu, Q.; Liu, Y. Photo-controlled reversible microtubule assembly mediated by paclitax-el-modified cyclodextrin. Angew. Chem. Int. Ed. 2018, 57, 8649–8653. [Google Scholar] [CrossRef]
- Stricker, L.; Fritz, E.-C.; Peterlechner, M.; Doltsinis, N.L.; Ravoo, B.J. Arylazopyrazoles as Light-Responsive Molecular Switches in Cyclodextrin-Based Supramolecular Systems. J. Am. Chem. Soc. 2016, 138, 4547–4554. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-D.; Tang, G.-P.; Chu, P.K. Cyclodextrin-Based Host–Guest Supramolecular Nanoparticles for Delivery: From Design to Applications. Accounts Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, X.; Dou, Y.; He, B.; Liu, L.; Wei, Z.; Li, J.; Wang, C.; Mao, C.; Zhang, J.; et al. A pH-responsive cyclodextrin-based hybrid nanosystem as a nonviral vector for gene delivery. Biomater. 2013, 34, 4159–4172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, R.; Lv, P.; Wang, Q.; Zheng, J.; Feng, B.; Yang, B. Cyclodextrin-based biological stimuli-responsive carriers for smart and precision medicine. Biomater. Sci. 2017, 5, 1736–1745. [Google Scholar] [CrossRef]
- Vícha, R.; Rouchal, M.; Kozubková, Z.; Kuřitka, I.; Marek, R.; Branná, P.; Čmelík, R. Novel adamantane-bearing anilines and properties of their supramolecular complexes with β-cyclodextrin. Supramol. Chem. 2011, 23, 663–677. [Google Scholar] [CrossRef]
- Enright, G.D.; Udachin, K.A.; Ripmeester, J.A. Complex packing motifs templated by a pseudo-spherical guest: The structure of β-cyclodextrin–adamantaneinclusion compounds. CrystEngComm 2010, 12, 1450–1453. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Sun, M.; Li, Y.; Tang, G. Evaluation of molecular chaperone drug function: Regorafenib and β-cyclodextrins. Colloids Surf. B 2017, 153, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, K.; Sun, X.; Li, Y.; Fu, Q.; Liang, T.; Tang, G. A redox-sensitive, oligopeptide-guided, self-assembling, and effi-ciency-enhanced (ROSE) system for functional delivery of microRNA therapeutics for treatment of hepatocellular carcinoma. Biomaterials 2016, 104, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-D.; Fan, H.; Ping, Y.; Liang, W.-Q.; Tang, G.-P.; Li, J. Cationic supramolecular nanoparticles for co-delivery of gene and anticancer drug. Chem. Commun. 2011, 47, 5572–5574. [Google Scholar] [CrossRef]
- Wen, Y.; Bai, H.; Zhu, J.; Song, X.; Tang, G.; Li, J. A supramolecular platform for controlling and optimizing molecular architectures of siRNA targeted delivery vehicles. Sci. Adv. 2020, 6, eabc2148. [Google Scholar] [CrossRef]
- Harata, K. Crystallographic analysis of the thermal motion of the inclusion complex of cyclomaltoheptaose (β-cyclodextrin) with hexamethylenetetramine. Carbohydr. Res. 2003, 338, 353–359. [Google Scholar] [CrossRef]
- Rüdiger, V.; Eliseev, A.; Simova, S.; Schneider, H.-J.; Blandamer, M.J.; Cullis, P.M.; Meyer, A.J. Conformational, calorimetric and NMR spectroscopic studies on inclusion complexes of cyclodextrins with substituted phenyl and adamantane derivatives. J. Chem. Soc. Perkin Trans. 2 1996, 2119–2123. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of Cyclodextrins and Cyclodextrin Complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef] [PubMed]
- Ejchart, A.; Koźmiński, W. NMR of Cyclodextrins and Their Complexes. In Cyclodextrins and Their Complexes; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2006; pp. 231–254. [Google Scholar]
- Higashi, T. ABSCOR. Rigaku Corporation, Tokyo, Japan. ABSCOR. Rigaku Corporation, Tokyo, Japan 1995
- Bruker. APEX2, SAINT and SADABS. Bruker ACS Inc., Madison, Wisconsin, USA. 2014
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGXandORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the programPLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-M.; Xu, X.; Yu, Q.; Liu, Y.-H.; Zhang, Y.-H.; Chen, L.-X.; Liu, Y. Reversing the Cytotoxicity of Bile Acids by Supramolecular Encapsulation. J. Med. Chem. 2017, 60, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K.; Mazzaferro, S. How to conduct and interpret ITC experiments accurately for cyclodextrin–guest interactions. Drug Discov. Today 2012, 17, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Furlanetto, S.; Pinzauti, S. Differential scanning calorimetry as an analytical tool in the study of drug-cyclodextrin interactions. J. Therm. Anal. Calorim. 2003, 73, 635–646. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Harries, D.; Rau, A.D.C.; Parsegian, V.A. Solutes Probe Hydration in Specific Association of Cyclodextrin and Adamantane. J. Am. Chem. Soc. 2005, 127, 2184–2190. [Google Scholar] [CrossRef]
- Brett, T.J.; Alexander, J.M.; Stezowski, J.J. Chemical insight from crystallographic disorder-structural studies of supramolecular photochemical systems. Part 2. The β-cyclodextrin–4,7-dimethylcoumarin inclusion complex: A new β-cyclodextrin dimer packing type, unanticipated photoproduct formation, and an examination of guest influence on β-CD dimer packing. J. Chem. Soc. Perkin Trans. 2 2000, 2, 1095–1103. [Google Scholar] [CrossRef]
- Harata, K. Crystallographic study of cyclodextrins and their inclusion complexes. In Cyclodextrins and their complexes; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2006; pp. 147–198. [Google Scholar]
- Caira, M.R. Structural Aspects of Crystalline Derivatized Cyclodextrins and Their Inclusion Complexes. Curr. Org. Chem. 2011, 15, 815–830. [Google Scholar] [CrossRef]
- Caira, M.R. Polymorphism and isostructurality of cyclodextrins and their inclusion complexes. Acta Crystallogr. A 2006, 62, s76. [Google Scholar] [CrossRef] [Green Version]
- Mentzafos, D.; Mavridis, I.M.; Le Bas, G.; Tsoucaris, G. Structure of the 4-tert-butylbenzyl alcohol–β-cyclodextrin complex. Common features in the geometry of β-cyclodextrin dimeric complexes. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1991, 47, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Lindner, K.; Saenger, W. β-Cyclodextrin dodecahydrate: Crowding of water molecules within a hydrophobic cavity. Angew. Chem. Int. Ed. 1978, 17, 694–695. [Google Scholar] [CrossRef]
- Ohira, A.; Sakata, M.; Taniguchi, I.; Hirayama, C.; Kunitake, M. Comparison of nanotube structures constructed from α-, β-, and γ-cyclodextrins by potential-controlled adsorption. J. Am. Chem. Soc. 2003, 125, 5057–5065. [Google Scholar] [CrossRef]
- Yee, E.M.; Hook, J.M.; Bhadbhade, M.M.; Vittorio, O.; Kuchel, R.P.; Brandl, M.B.; Tilley, R.D.; Black, D.S.; Kumar, N.; Eugene, Y.M. Preparation, characterization and in vitro biological evaluation of (1:2) phenoxodiol-β-cyclodextrin complex. Carbohydr. Polym. 2017, 165, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; McGown, L.B. Molecular nanotube aggregates of β- and γ-cyclodextrins linked by diphenylhexatrienes. Science 1994, 264, 249–251. [Google Scholar] [CrossRef]
- Aree, T. Inclusion complex of β-cyclodextrin with coffee chlorogenic acid: New insights from a combined crystallographic and theoretical study. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chao, M.-Y.; Liu, Y.; Xu, B.-W.; Zhang, W.-H.; Young, D.J. An N,N′-diethylformamide solvent-induced conversion cascade within a metal–organic framework single crystal. Chem. Commun. 2020, 56, 5877–5880. [Google Scholar] [CrossRef]
- Chao, M.-Y.; Chen, J.; Hao, Z.-M.; Tang, X.-Y.; Ding, L.; Zhang, W.-H.; Young, D.J.; Lang, J.-P. A Single-Crystal to Single-Crystal Conversion Scheme for a Two-Dimensional Metal–Organic Framework Bearing Linear Cd3 Secondary Building Units. Cryst. Growth Des. 2019, 19, 724–729. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Ding, N.-N.; Zhang, W.-H.; Chen, J.-X.; Young, D.J.; Hor, T.S.A. Stitching 2D Polymeric Layers into Flexible Interpenetrated Metal-Organic Frameworks within Single Crystals. Angew. Chem. Int. Ed. 2014, 53, 4628–4632. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.; Steinrauf, L.K.; Van Etten, R.L. Interrelated space groups observed for complexes of cycloheptaamylose with small organic molecules. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1968, 24, 1560–1562. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Sabesan, M.N. Structure of a complex of cycloheptaamylose with 1-adamantanecarboxylic acid. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 3063–3069. [Google Scholar] [CrossRef]





| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Formula Weight | 2574.41 | 2726.69 | 2723.73 | 2630.47 | 2494.20 | 2522.28 |
| Empirical formula | 2C42H70O35∙ 2C10H16O | 2C42H70O35∙ 3C10H16O | 2C42H70O35∙3C10 H17N | 2C42H70O35∙ 2C11H16O2 | 2C42H70O35∙ C12H16O4 | 2C42H70O35∙ C14H20O4 |
| Crystal system | triclinic | orthorhombic | orthorhombic | orthorhombic | triclinic | triclinic |
| Space group | P1 | C2221 | C2221 | C2221 | P1 | P1 |
| a (Å) | 15.3986(9) | 23.8095(5) | 19.2469(5) | 19.1225(4) | 15.2993(8) | 15.2868(3) |
| b (Å) | 15.4175(8) | 19.2806(4) | 23.9691(5) | 24.2639(6) | 15.4429(7) | 15.4435(3) |
| c (Å) | 17.9599(10) | 32.4542(9) | 32.5978(9) | 32.6433(8) | 18.0837(10) | 18.0918(4) |
| α (°) | 113.169(2) | 90 | 90 | 90.00 | 99.751(4) | 100.0500(10) |
| β (°) | 99.213(2) | 90 | 90 | 90.00 | 113.551(5) | 113.0330(10) |
| γ (°) | 103.219(2) | 90 | 90 | 90.00 | 102.813(4) | 102.4900(10) |
| V (Å3) | 3664.1(4) | 14,898.5(6) | 15,038.4(7) | 15,146.0(6) | 3656.6(4) | 3674.56(13) |
| Z | 1 | 1 | 4 | 4 | 1 | 1 |
| ρcalc (g cm–3) | 1.167 | 1.097 | 1.031 | 1.075 | 1.133 | 1.026 |
| F(000) | 1372 | 5235 | 4754 | 5212 | 1324 | 1204 |
| µ (cm–1) | 0.545 | 0.520 | 0.802 | 0.093 | 0.099 | 0.504 |
| Total reflections | 50,836 | 96,200 | 72,586 | 55,633 | 27,091 | 46,007 |
| Unique reflections | 26,218 | 14,176 | 13,298 | 13,052 | 21,115 | 27,008 |
| Observed reflections | 19,199 | 11,540 | 12,601 | 9831 | 16,593 | 25,912 |
| Rint | 0.0715 | 0.0782 | 0.0408 | 0.0361 | 0.0387 | 0.0400 |
| Variables | 1568 | 774 | 773 | 739 | 1676 | 1387 |
| R1 a | 0.1024 | 0.082400853 | 0.0801 | 0.0883 | 0.0758 | 0.0648 |
| wR2b | 0.2643 | 0.2371 | 0.2303 | 0.2630 | 0.2080 | 0.1891 |
| GOF c | 1.027 | 1.071 | 1.051 | 1.153 | 1.027 | 1.028 |
| Guest | Guest:β-CD | Space Group | Packing Mode | |
|---|---|---|---|---|
| 1 | adm-1-OH | 2:2 | P1 | CH |
| 2 | adm-2-OH | 3:2 | C2221 | CB |
| 3 | adm-1-NH2 | 3:2 | C2221 | CB |
| 4 | adm-1-COOH | 2:2 | C2221 | CB |
| 5 | adm-1,3-diCOOH | 1:2 | P1 | CH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-W.; Yu, K.-X.; Ji, X.-Y.; Bai, H.; Zhang, W.-H.; Hu, X.; Tang, G. Structural Insights into the Host–Guest Complexation between β-Cyclodextrin and Bio-Conjugatable Adamantane Derivatives. Molecules 2021, 26, 2412. https://doi.org/10.3390/molecules26092412
Wang J-W, Yu K-X, Ji X-Y, Bai H, Zhang W-H, Hu X, Tang G. Structural Insights into the Host–Guest Complexation between β-Cyclodextrin and Bio-Conjugatable Adamantane Derivatives. Molecules. 2021; 26(9):2412. https://doi.org/10.3390/molecules26092412
Chicago/Turabian StyleWang, Jian-Wei, Ka-Xi Yu, Xin-Yuan Ji, Hongzhen Bai, Wen-Hua Zhang, Xiurong Hu, and Guping Tang. 2021. "Structural Insights into the Host–Guest Complexation between β-Cyclodextrin and Bio-Conjugatable Adamantane Derivatives" Molecules 26, no. 9: 2412. https://doi.org/10.3390/molecules26092412
APA StyleWang, J.-W., Yu, K.-X., Ji, X.-Y., Bai, H., Zhang, W.-H., Hu, X., & Tang, G. (2021). Structural Insights into the Host–Guest Complexation between β-Cyclodextrin and Bio-Conjugatable Adamantane Derivatives. Molecules, 26(9), 2412. https://doi.org/10.3390/molecules26092412