1. Introduction
Applications of rare-earth elements (REEs) in various high technology industries are conditioned by their unique physical properties. Satisfying the needs of various industries, such as metallurgy, nuclear energy, manufacture of optical, magnetic, luminescent, and laser materials, and petrochemicals most often requires high purity individual REEs. Among the rare earth elements neodymium is important for doping alloys [
1] and manufacturing permanent magnets [
2], laser production [
3], and preparing catalysts [
4,
5]. Due to their similar physical and chemical properties the recovery of individual rare earth metals is a complex and yet partially unresolved problem [
6]. The most important REEs minerals processed in industrial scale are monazite, bastnesite, and xenotime [
7]. However, due to the growing global demand for REEs their recovery from alternative secondary resources such as phosphate ores [
8], coal combustion products [
9], permanent magnets [
10], mine tailings [
11], etc. has become more relevant. At the same time, the need to extract REE from secondary resources gives rise to new scientific challenges for the development of new effective approaches to the extraction of REE due to the complex multicomponent composition of these resources and the extremely low content of REE in them [
12].
Currently, extraction chromatography methods based on the use of highly selective solvent-impregnated resins (SIRs) are becoming increasingly important for solving problems of concentration, recovery, and separation of elements with similar properties [
13]. Extraction chromatography combines the efficiency of liquid-liquid extraction with the simplicity and convenience of performing adsorption methods [
14]. However, for the successful implementation of the extraction chromatography process, it is necessary to develop new extraction chromatographic materials.
Synthetically available SIRs in which a selective organic ligand (extractant) is non-covalently fixed on the surface of an inert support are most widely used in extraction chromatography [
15]. An important aspect of the development of effective SIRs is the optimization of their stationary phase composition, which includes both the choice of a selective organic extractant and a suitable diluent. The latter can affect both the efficiency of metal recovery (values of distribution coefficients and resin capacity), and the selectivity of metal separation (values of metal separation factors) [
16,
17]. The choice of organic extractants and diluents for preparation of SIRs is commonly performed by using the results of preliminary experiments of liquid–liquid extraction.
For REEs liquid-liquid extraction and separation alkyl-substituted phosphorus acids are used such as di-(2-ethylhexyl)phosphoric acid (DEHPA) [
18], 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester (PC88A) [
19], bis-(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272) [
20], as well as neutral organophosphorus extractants, for example, tributylphosphate (TBP) [
21], trialkylphosphine oxides (Cyanex 923) [
22], dicarboxylic acid amides such as N,N-dioctyldiglycolamic acid (DODGAA) [
23], and N,N,N′,N′-tetraoctyldiglycolamide (TODGA) [
8],. However, these extractants do not always allow solving the problem of selective separation of individual REEs.
The development of organic synthesis has led to the creation of new synthetically available polydentate extractants such as phosphorylpodands of neutral [
24,
25,
26,
27], and acidic type [
28,
29,
30]. They possess high extraction ability and selectivity in relation to a wide range of chemical elements. Varying of substituents at the phosphoryl group and the design of polyether chain are effective approaches toward obtaining of phosphorylpodands with suitable extraction properties in relation to various metals, REEs inclusively. In a review [
31] the applications of series of phosphorylpodands as extractants in impregnated resins for processing radioactive waste are reported.
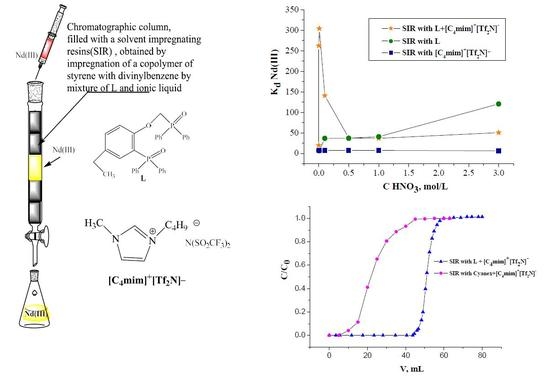
Short-chain phosphorylpodand (2-diphenylphosphoryl-4-ethylphenoxy)-methyl)diphenylphosphine oxide (L) (
Figure 1) is a promissing extractant for REEs recovery from nitric acid solution.
In our previous report, we studied the effect of substituents at phosphoryl groups of compound L on the efficiency of liquid-liquid extraction of REEs [
32]. Additionally, extraction of REEs, U(VI), and Th(IV) from perchlorate solutions into dichloroethane was investigated. The stoichiometry of the extractable complexes was determined, and the influence of the aqueous phase composition on the efficiency and selectivity of the extraction of U(VI), Th(IV), and REEs into the organic phase was explored [
33]. The solution of L in 1,1,7-trihydrododecafluoroheptanol have also been investigated for rare earth element extraction from nitric acid media. It was shown that the values of distribution coefficients of REEs are negligible at nitric acid concentration lower 1 mol/L. Distribution coefficient sharply increases with nitric acid concentration from 1 mol/L and reaches 5.5 for the yttrium subgroup elements at HNO
3 concentration of 6 mol/L. The rare earth elements of the yttrium subgroup were found to be extracted much better than the rare earth elements of the cerium one under the same conditions. Additionally, the values of distribution coefficients in both subgroups smoothly rise with atomic number of element. It was established using the method of extraction equilibrium shift that the metal: L ratio in extracted complexes is 1:2 irrespective of the nature of the rare earth element. The structure of the complex of Yb with L was determined by an X-ray diffraction study [
34]. The complex of L with neodymium was studied by X-ray structural analysis and IR spectroscopy [
35].
Recently increasing attention is focused on using of ionic liquids as promising dilutents both in liquid–liquid extraction [
36,
37,
38], and as components of SIRs [
39,
40,
41,
42]. In both cases, the using of ionic liquids leads to a significant improvement of REEs recovery efficiency. Unlike traditional organic solvents, ionic liquids are not flammable or toxic, and have low pressure of vapor and high electric conductivity [
43]. In the composition of SIRs ionic liquids may be used both independently [
42,
44] and as mixture with different extractants [
41,
45,
46,
47]. In the present report we are interested in the second case, that’s why first we consider extraction of REEs by such systems.
Numerous reports on the liquid–liquid extraction of REEs with different neutral extractants in the presence of variously structured ionic liquids have shown that the higher the hydrophilicity of the cation and the hydrophobicity of the anion in ionic liquids, the greater is the extraction of REEs [
38,
48,
49,
50]. Varying cations and anions of various structures yields ionic liquids with suitable properties necessary for certain application [
43,
51,
52]. An important feature of ionic liquids is that their components can serve as hydrophobic counterions in the extracted metal complexes during their recovery from aqueous phase by various extractants -[
36]. The most pronounced improvement in the efficiency of REE recovery in liquid-liquid extraction was achieved in the presence of ionic liquids of bis [(trifluoromethyl) sulfonyl] imide-1-alkyl-3-methylimidazolium derivatives ([C
nmim]
+[Tf
2N]
− (where
n = 4, 6, 8))].
Recently, we have found that liquid-liquid extraction of REEs with solutions of some neutral phosphorylpodands in dichloroethane from nitric acid media significantly increases in the presence of [C
4mim]
+[Tf
2N]
− [
53,
54,
55].
In this report, novel SIRs containing (2-diphenylphosphoryl)-4-ethylphenoxy)methyl) diphenylphosphine oxide and ionic liquid [C4mim]+[Tf2N]− were obtained and the features of the chromatographic extraction of Nd (III) from nitric acid solutions were studied as well. The selectivity of Nd(III) recovery at the presence of La(III), Dy(III), and Tm(III) was examined. The comparison of recovery efficiency of Nd(III) by novel SIR and resin based on the mixture of Cyanex 923 (a mixture of monodentate trialkylphosphine oxides) and [C4mim]+[Tf2N]− was performed.
3. Experimental Section
3.1. Synthesis
The structure and the purity degree of synthesized compound L was ascertained with NMR spectroscopy and elemental analysis data. The content of C, H was established with standard methods using a Carlo Erba CHN analyzer (Erba Group, Brno, Czech Republic). NMR spectra were registered with a CXP-200 or Bruker-DXP-200 (200 MHz) instrument (Bruker, MA, USA) with tetramethylsilane as the internal standard, while for 31P NMR 85% H3PO4 was used as reference. The control of composition of reaction mixture was conducted by the method of thin-layer chromatography on Silufol plates (Merck, NJ, USA). The mixture chloroform:isopropanol = 10:1 was used as eluent. The development of chromatograms was performed by fuming iodine.
((2-Diphenylphosphoryl)-4-ethylphenoxy)methyl)diphenylphosphine oxide (L). A mixture of 5.0 g (15.5 mmoL) (5-ethyl-2-hydroxyphenyl)diphenylphosphine oxide, 6.00 g (15.5 mmoL) diphenylphosphoryl)methylbebzenesulfonate and 5.1 g (15.5 mmoL) anhydrous cesium carbonate in 45 mL dioxane was heated and stirred at 100 °C for 10 h. The reaction mixture was diluted by 50 mL of water, acidified by adding concentrated HCl to pH 1, and extracted by CHCl3 (3 × 25 mL). The organic layer was separated, washed with water, and evaporated under reduced pressure to give 7.25 g (86%) of crude product. After recrystallization from a benzene-hexane mixture (1:1), 6.6 g (79%) of compound L was obtained, mp = 166–168 °C (The melting temperature is established with a short Anschutz thermometer). It was found, %: C 73.63, 73.50; H 5.35, 5.60; P 11.49, 11.39. For C33H30O3P2. It was calculated, %: C 73.87; H 5.64; P 11.55. 1H-NMR δ, ppm (CDCl3): 1.12 t (6H, 3JH_H = 7.02 Hz CH3CH2_Ar), 2.55 q (2H, 3JH-H = 7.58 Hz CH3CH2Ar), 4.49 d (2H, 2JH-P = 5.18 Hz OCH2P(O)Ph2), 7.07 m (1H, Ar-H), 7.30–7.65 m (22H, Ar–H). 31P-NMR, δ, ppm (CDCl3): 28.13, 29.92.
Bis[(trifluoromethyl)sulfonyl]imide-1-buthyl-3-methylimidazolium was provided by Sorbent-Technologies, Ltd. (Moscow, Russia). This compound was “purim” grade and assay an ≥99% (GC). The purity of synthesized compounds was ≥99.0% (according to the NMR data and elemental analysis results).
3.2. Preparation of Solutions and Analysis
Nitric acid solutions of Nd(III) were prepared by dissolving precisely weighed portions of Nd
2O
3 (purity > 99.9%, Aldrich, Germany)in nitric acid solutions of the corresponding concentrations. Nitric acid solutions were prepared by diluting concentrated HNO
3. The concentrations of the obtained diluted solutions of HNO
3 were determined by titration with the standard solution of NaOH in the presence of bromothymol blue. Solutions of Arsenazo M (ACS Reagent grade, Acros Organics, Belgium were prepared by dissolving precisely weighed portions of the reagent in distilled water. Solutions of EDTA (“purum” grade, Acros Organics) were prepared similarly. The concentrations of Nd(III) in the eluates were evaluated spectrophotometrically using Arsenazo M] [
70]. All reagents used were analytical grade. The measurements of optical density of Nd(III) solutions flowing from the column were performed automatically with a spectrophotometric detector and software. With concentration of HNO
3 in eluates exceeding 0.5 mol/L, the concentration of Nd(III) in such solutions was detected spectrophotometrically using specific absorption spectrum of Nd(III) (
Figure 10), with the wavelength of 576 nm] [
71].
3.3. Preparation of SIRs
The studied SIRs (
Table 5) were prepared with method described earlier in [
38]. Weighed portions of L and ionic liquid [C
4mim]
+[Tf
2N]
− (
Table 6) were dissolved in 30 mL CHCl
3 and mixed with the suspension of copolymer of styrene with divinylbenzene LPS-500 (specific surface area is 570 m
2/g, diameter of pores is 3–50 μm, size of particles is 40–70 μm) (provided by RossPolimer, Moscow, Russia) in approx. 20 mL CHCl
3. The resulting mixture was stirred in the rotating flask of the rotary evaporator, and then CHCl
3 was removed with vacuum at 50 °C. Having collected all the condensate and not seeing bubbles in the suspension, the SIR was stirred in complete vacuum at 40–50 °C for 30 min for full removal of CHCl
3.
3.4. Equipment
The recovery of Nd(III) was studied in dynamic mode on an automatic chromatographic device manufactured by Knauer (Germany), consisting of three high pressure pumps, dosing valve, chromatography column, and a spectrophotometric detector. The recovery of Nd(III) was carried out using a plastic column with the length of 100 mm and the internal diameter equaling 4 mm, respectively. The column was packed with resins by the “dry method”, loading dry resin inside the column in small portions and compacting it by a glass rod. The physical constants of the prepared columns are presented in
Table 7.
3.5. Batch Uptake of Nd(III)
The chromatographic column stuffed with SIRs (
Table 1) was rinsed using a peristaltic pump by HNO
3 solution of a chosen concentration with a flow rate of 1 mL/min for 1 h. Then Nd(III) solution of a certain concentration, which was previously determined by titration with standard solution of EDTA] [
72], in HNO
3 of the same concentration as on the rinsing step was constantly passed through the column until the complete SIR saturation with a flow rate of 0.5 mL/min. The Nd(III) concentrations in eluates which left the column were automatically determined by the spectrophotometric method. The obtained frontal loading curves (
Figure 11) were used to calculate the values of distribution coefficients and the capacity of the SIRs.
The dynamic distribution coefficients (K
d, mL/g) were calculated per Equation (3)] [
60]:
where V
0.5 is the volume of solution until half breakthrough of metal, mL; m
e is the mass of extractant in the resin, g.
The values of separation factors (β) were calculated per Equation (4):
where K
d1 and K
d2 are distribution coefficients of separating metals
The values of the synergy effect (SE) were calculated per Equation (4):
where K
d(L+IL), K
d L and K
d IL (mg/L) are values of the distribution coefficient obtained for SIRs impregnated by mixture of phosphorylpodand L with ionic liquid, phosphorylpodand L alone and ionic liquid alone, respectively.