Group Contribution Estimation of Ionic Liquid Melting Points: Critical Evaluation and Refinement of Existing Models
Abstract
:1. Introduction
2. Results and Discussion
2.1. Database Development and Refinement
2.2. Model Evaluation and Comparison
2.3. Refinement and Reparametrization of GCM1
3. Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| AARD | Average Absolute Relative Deviation |
| BETI | Bis((perfluoroethyl)sulfonyl)imide |
| CAMD | Computer Aided Molecular Design |
| DSC | Differential Scanning Calorimetry |
| GCM | Group Contribution Method |
| IL | Ionic Liquid |
| MAE | Mean Absolute Error |
| NTf2 | Bis((trifluoromethyl)sulfonyl)imide |
| OTf | Trifluoromethanesulfonate |
| QSPR | Quantitative Structure Property Relationship |
| RNN | Recurrent Neural Network |
| SGD | Stochastic Gradient Descent |
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krossing, I.; Slattery, J.M.; Daguenet, C.; Dyson, P.J.; Oleinikova, A.; Weingärtner, H. Why Are Ionic Liquids Liquid? A Simple Explanation Based on Lattice and Solvation Energies. J. Am. Chem. Soc. 2006, 128, 13427–13434. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Ozokwelu, D.; Zhang, S.; Okafor, O.C.; Cheng, W.; Litombe, N. Chapter 5—Separation Science and Technology. In Novel Catalytic and Separation Processes Based on Ionic Liquids; Ozokwelu, D., Zhang, S., Okafor, O.C., Cheng, W., Litombe, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 193–202. ISBN 978-0-12-802027-2. [Google Scholar]
- Chen, H.; He, Y.; Zhu, J.; Alias, H.; Ding, Y.; Nancarrow, P.; Hardacre, C.; Rooney, D.; Tan, C. Rheological and heat transfer behaviour of the ionic liquid, [C4mim][NTf2]. Int. J. Heat Fluid Flow 2008, 29, 149–155. [Google Scholar] [CrossRef]
- Bendová, M.; Čanji, M.; Wagner, Z.; Bogdanov, M.G. Ionic Liquids as Thermal Energy Storage Materials: On the Importance of Reliable Data Analysis in Assessing Thermodynamic Data. J. Solut. Chem. 2019, 48, 949–961. [Google Scholar] [CrossRef]
- Nancarrow, P.; Al-Othman, A.; Mital, D.K.; Döpking, S. Comprehensive analysis and correlation of ionic liquid conductivity data for energy applications. Energy 2021, 220, 119761. [Google Scholar] [CrossRef]
- Mohammed, H.; Al-Othman, A.; Nancarrow, P.; Elsayed, Y.; Tawalbeh, M. Enhanced proton conduction in zirconium phosphate/ionic liquids materials for high-temperature fuel cells. Int. J. Hydrogen Energy 2021, 46, 4857–4869. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. Acs Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef]
- Nancarrow, P.; Mohammed, H. Ionic Liquids in Space Technology—Current and Future Trends. ChemBioEng Rev. 2017, 4, 106–119. [Google Scholar] [CrossRef]
- Schubert, T.J.S. Commercial Production of Ionic Liquids. In Commercial Applications of Ionic Liquids; Shiflett, M.B., Ed.; Green Chemistry and Sustainable Technology; Springer International Publishing: Cham, Switherlands, 2020; pp. 191–208. ISBN 978-3-030-35245-5. [Google Scholar]
- Ionic Liquids. Available online: https://chemicals.basf.com/global/en/Intermediates/Product_groups/Ionic_liquids.html (accessed on 14 March 2021).
- Zhu, J.; Bai, L.; Chen, B.; Fei, W. Thermodynamical properties of phase change materials based on ionic liquids. Chem. Eng. J. 2009, 147, 58–62. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. Ionic liquids. Clean Prod. Process. 1999, 1, 223–236. [Google Scholar] [CrossRef]
- Niedermeyer, H.; Hallett, J.P.; Villar-Garcia, I.J.; Hunt, P.A.; Welton, T. Mixtures of ionic liquids. Chem. Soc. Rev. 2012, 41, 7780–7802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gani, R. Group contribution-based property estimation methods: Advances and perspectives. Curr. Opin. Chem. Eng. 2019, 23, 184–196. [Google Scholar] [CrossRef]
- Karelson, M.; Lobanov, V.S.; Katritzky, A.R. Quantum-Chemical Descriptors in QSAR/QSPR Studies. Chem. Rev. 1996, 96, 1027–1044. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Lomaka, A.; Petrukhin, R.; Jain, R.; Karelson, M.; Visser, A.E.; Rogers, R.D. QSPR Correlation of the Melting Point for Pyridinium Bromides, Potential Ionic Liquids. J. Chem. Inf. Comput. Sci. 2002, 42, 71–74. [Google Scholar] [CrossRef]
- Lazzús, J.A. A group contribution method to predict the melting point of ionic liquids. Fluid Phase Equilibria 2012, 313, 1–6. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Ilani-Kashkouli, P.; Mohammadi, A.H. Computation of normal melting temperature of ionic liquids using a group contribution method. Fluid Phase Equilibria 2012, 329, 1–7. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, J.A.P. Extension of the Ye and Shreeve group contribution method for density estimation of ionic liquids in a wide range of temperatures and pressures. Fluid Phase Equilibria 2008, 263, 26–32. [Google Scholar] [CrossRef]
- Jacquemin, J.; Ge, R.; Nancarrow, P.; Rooney, D.W.; Costa Gomes, M.F.; Pádua, A.A.H.; Hardacre, C. Prediction of Ionic Liquid Properties. I. Volumetric Properties as a Function of Temperature at 0.1 MPa. J. Chem. Eng. Data 2008, 53, 716–726. [Google Scholar] [CrossRef]
- Jacquemin, J.; Nancarrow, P.; Rooney, D.W.; Costa Gomes, M.F.; Husson, P.; Majer, V.; Pádua, A.A.H.; Hardacre, C. Prediction of Ionic Liquid Properties. II. Volumetric Properties as a Function of Temperature and Pressure. J. Chem. Eng. Data 2008, 53, 2133–2143. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. A New Group Contribution Method For Prediction of Density of Pure Ionic Liquids over a Wide Range of Temperature and Pressure. Ind. Eng. Chem. Res. 2012, 51, 591–604. [Google Scholar] [CrossRef]
- Evangelista, N.S.; do Carmo, F.R.; de Santiago-Aguiar, R.S.; de Sant’Ana, H.B. Development of a new group contribution method based on GCVOL Model for the estimation of pure ionic liquid density over a wide range of temperature and pressure. Ind. Eng. Chem. Res. 2014, 53, 9506–9512. [Google Scholar] [CrossRef]
- Součková, M.; Klomfar, J.; Pátek, J. Group contribution and parachor analysis of experimental data on density and surface tension for members of the homologous series of 1-C -3-methylimidazolium chlorides. Fluid Phase Equilibria 2017, 454, 43–56. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, J.A.P. A group contribution method for viscosity estimation of ionic liquids. Fluid Phase Equilibria 2008, 266, 195–201. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Ilani-Kashkouli, P.; Mohammadi, A.H.; Ramjugernath, D.; Richon, D. Development of a group contribution method for determination of viscosity of ionic liquids at atmospheric pressure. Chem. Eng. Sci. 2012, 80, 326–333. [Google Scholar] [CrossRef]
- Fatehi, M.-R.; Raeissi, S.; Mowla, D. Estimation of viscosity of binary mixtures of ionic liquids and solvents using an artificial neural network based on the structure groups of the ionic liquid. Fluid Phase Equilibria 2014, 364, 88–94. [Google Scholar] [CrossRef]
- Lazzús, J.A.; Pulgar-Villarroel, G. A group contribution method to estimate the viscosity of ionic liquids at different temperatures. J. Mol. Liq. 2015, 209, 161–168. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, J.A.P. A group contribution method for heat capacity estimation of ionic liquids. Ind. Eng. Chem. Res. 2008, 47, 5751–5757. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Toro, A.; Rojas, R.E. Prediction of the heat capacity of ionic liquids using the mass connectivity index and a group contribution method. J. Chem. Thermodyn. 2011, 43, 1068–1073. [Google Scholar] [CrossRef]
- Sattari, M.; Gharagheizi, F.; Ilani-Kashkouli, P.; Mohammadi, A.H.; Ramjugernath, D. Development of a group contribution method for the estimation of heat capacities of ionic liquids. J. Therm. Anal. Calorim 2014, 115, 1863–1882. [Google Scholar] [CrossRef]
- Nancarrow, P.; Lewis, M.; AbouChacra, L. Group Contribution Methods for Estimation of Ionic Liquid Heat Capacities: Critical Evaluation and Extension. Chem. Eng. Technol. 2015, 38, 632–644. [Google Scholar] [CrossRef]
- Ge, R.; Hardacre, C.; Jacquemin, J.; Nancarrow, P.; Rooney, D.W. Heat Capacities of Ionic Liquids as a Function of Temperature at 0.1 MPa. Measurement and Prediction. J. Chem. Eng. Data 2008, 53, 2148–2153. [Google Scholar] [CrossRef]
- Lazzús, J.A. A group contribution method to predict the thermal conductivity λ(T,P) of ionic liquids. Fluid Phase Equilibria 2015, 405, 141–149. [Google Scholar] [CrossRef]
- Atashrouz, S.; Mozaffarian, M.; Pazuki, G. Modeling the Thermal Conductivity of Ionic Liquids and Ionanofluids Based on a Group Method of Data Handling and Modified Maxwell Model. Ind. Eng. Chem. Res. 2015, 54, 8600–8610. [Google Scholar] [CrossRef]
- Wu, K.-J.; Zhao, C.-X.; He, C.-H. Development of a group contribution method for determination of thermal conductivity of ionic liquids. Fluid Phase Equilibria 2013, 339, 10–14. [Google Scholar] [CrossRef]
- Joback, K.G.; Reid, R.C. Estimation of Pure-Component Properties from Group-Contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Lydersen, A.L.; Engineering Experiment Station. Estimation of Critical Properties of Organic Compounds by the Method of Group Contibutions; University of Wisconsin: Madison, WI, USA, 1955. [Google Scholar]
- Huo, Y.; Xia, S.; Zhang, Y.; Ma, P. Group Contribution Method for Predicting Melting Points of Imidazolium and Benzimidazolium Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 2212–2217. [Google Scholar] [CrossRef]
- Aguirre, C.L.; Cisternas, L.A.; Valderrama, J.O. Melting-Point Estimation of Ionic Liquids by a Group Contribution Method. Int. J. 2012, 33, 34–46. [Google Scholar] [CrossRef]
- Chen, Y.; Kontogeorgis, G.M.; Woodley, J.M. Group Contribution Based Estimation Method for Properties of Ionic Liquids. Ind. Eng. Chem. Res. 2019, 58, 4277–4292. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Jain, R.; Lomaka, A.; Petrukhin, R.; Karelson, M.; Visser, A.E.; Rogers, R.D. Correlation of the melting points of potential ionic liquids (imidazolium bromides and benzimidazolium bromides) using the CODESSA program. J. Chem. Inf. Comput. Sci. 2002, 42, 225–231. [Google Scholar] [CrossRef]
- Trohalaki, S.; Pachter, R. Prediction of Melting Points for Ionic Liquids. QSAR Comb. Sci. 2005, 24, 485–490. [Google Scholar] [CrossRef]
- Farahani, N.; Gharagheizi, F.; Mirkhani, S.A.; Tumba, K. Ionic liquids: Prediction of melting point by molecular-based model. Thermochim. Acta 2012, 549, 17–34. [Google Scholar] [CrossRef]
- Bini, R.; Chiappe, C.; Duce, C.; Micheli, A.; Solaro, R.; Starita, A.; Tiné, M.R. Ionic liquids: Prediction of their melting points by a recursive neural network model. Green Chem. 2008, 10, 306–309. [Google Scholar] [CrossRef]
- Carrera, G.; Aires-de-Sousa, J. Estimation of melting points of pyridinium bromide ionic liquids with decision trees and neural networks. Green Chem. 2005, 7, 20–27. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Eike, D.M.; Brennecke, J.F.; Maginn, E.J. Predicting melting points of quaternary ammonium ionic liquids. Green Chem. 2003, 5, 323–328. [Google Scholar] [CrossRef]
- Varnek, A.; Kireeva, N.; Tetko, I.V.; Baskin, I.I.; Solov’ev, V.P. Exhaustive QSPR Studies of a Large Diverse Set of Ionic Liquids: How Accurately Can We Predict Melting Points? J. Chem. Inf. Model. 2007, 47, 1111–1122. [Google Scholar] [CrossRef]
- López-Martin, I.; Burello, E.; Davey, P.N.; Seddon, K.R.; Rothenberg, G. Anion and cation effects on imidazolium salt melting points: A descriptor modelling study. Chemphyschem 2007, 8, 690–695. [Google Scholar] [CrossRef]
- Yan, C.; Han, M.; Wan, H.; Guan, G. QSAR correlation of the melting points for imidazolium bromides and imidazolium chlorides ionic liquids. Fluid Phase Equilibria 2010, 292, 104–109. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Campusano, R.A. Melting properties of molten salts and ionic liquids. Chemical homology, correlation, and prediction. Comptes Rendus Chim. 2016, 19, 654–664. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol Tech. 2010, 21, 167–193. [Google Scholar] [PubMed]
- Kočí, V.; Fořt, J.; Maděra, J.; Scheinherrová, L.; Trník, A.; Černý, R. Correction of errors in DSC measurements using detailed modeling of thermal phenomena in calorimeter-sample system. IEEE Trans. Instrum. Meas. 2020, 69, 8178–8186. [Google Scholar] [CrossRef]
- Preiss, U.P.; Beichel, W.; Erle, A.M.T.; Paulechka, Y.U.; Krossing, I. Is universal, simple melting point prediction possible? ChemPhysChem 2011, 12, 2959–2972. [Google Scholar] [CrossRef]
- Yamamoto, H. Structure Properties Relationship of Ionic Liquid. J. Comput. Aided Chem. 2006, 7, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Maginn, E.J. Molecular dynamics study of the effect of alkyl chain length on melting points of [CnMIM][PF6] ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 13489–13499. [Google Scholar] [CrossRef]
- NIST Ionic Liquids Database–ILThermo (v.2.0). 2020. Available online: https://ilthermo.boulder.nist.gov/ (accessed on 10 October 2020).
- Zhang, S.; Zhou, Q.; Lu, X.; Song, Y.; Wang, X. (Eds.) Introduction to properties of ionic liquid mixtures. In Physicochemical Properties of Ionic Liquid Mixtures; Springer: Dordrecht, The Netherlands, 2016; pp. 1–53. ISBN 978-94-017-7573-1. [Google Scholar]
- Krolikowska, M. (Solid + liquid) and (liquid + liquid) phase equilibria of (IL + water) binary systems. The influence of the ionic liquid structure on mutual solubility. Fluid Phase Equilibria 2014, 361, 273–281. [Google Scholar] [CrossRef]
- Yoshida, Y.; Muroi, K.; Otsuka, A.; Saito, G.; Takahashi, M.; Yoko, T. 1-Ethyl-3-methylimidazolium based ionic liquids containing cyano groups: Synthesis, characterization, and crystal structure. Inorg. Chem. 2004, 43, 1458–1462. [Google Scholar] [CrossRef]
- Wasserscheid, P.; van Hal, R.; Bösmann, A. 1-n-Butyl-3-methylimidazolium ([bmim]) octylsulfate—an even ‘greener’ionic liquid. Green Chem. 2002, 4, 400–404. [Google Scholar] [CrossRef]
- Domańska, U.; Bogel-Łukasik, E.; Bogel-Łukasik, R. 1-Octanol/Water Partition Coefficients of 1Alkyl-3-methylimidazolium Chloride. Chem. A Eur. J. 2003, 9, 3033–3041. [Google Scholar] [CrossRef]
- Couadou, E.; Jacquemin, J.; Galiano, H.; Hardacre, C.; Anouti, M. A comparative study on the thermophysical properties for two bis [(trifluoromethyl) sulfonyl] imide-based ionic liquids containing the trimethyl-sulfonium or the trimethyl-ammonium cation in molecular solvents. J. Phys. Chem. B 2013, 117, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-B.; Matsumoto, H.; Tatsumi, K. A new class of hydrophobic ionic liquids: Trialkyl (2-methoxyethyl) ammonium perfluoroethyltrifluoroborate. Chem. Lett. 2004, 33, 886–887. [Google Scholar] [CrossRef]
- Sun, J.; Macfarlane, D.R.; Forsyth, M. A new family of ionic liquids based on the 1-alkyl-2-methyl pyrrolinium cation. Electrochim. Acta 2003, 48, 1707–1711. [Google Scholar] [CrossRef]
- Sakal, S.A.; Lu, Y.; Jiang, X.; Shen, C.; Li, C. A promising ionic liquid [BMIM][FeCl4] for the extractive separation of aromatic and aliphatic hydrocarbons. J. Chem. Eng. Data 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Wlazło, M.; Marciniak, A.; Zawadzki, M.; Dudkiewicz, B. Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 4-(3-hydroxypropyl)-4-methylmorpholinium bis (trifluoromethylsulfonyl)-amide. J. Chem. Thermodyn. 2015, 86, 154–161. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Mikkola, J.-P.; Virtanen, P.; Sjöholm, R. Aliquat 336®—a versatile and affordable cation source for an entirely new family of hydrophobic ionic liquids. Green Chem. 2006, 8, 250–255. [Google Scholar] [CrossRef]
- Cooper, E.I.; Angell, C.A. Ambient temperature plastic crystal fast ion conductors (PLICFICS). Solid State Ion. 1986, 18–19, 570–576. [Google Scholar] [CrossRef]
- Melo, C.I.; Bogel-Łukasik, R.; da Ponte, M.N.; Bogel-Łukasik, E. Ammonium ionic liquids as green solvents for drugs. Fluid Phase Equilibria 2013, 338, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Takaizumi, K.; Wakabayashi, T. Apparent molal volumes of phosphonium halides at 15, 25 and 35 °C. J. Solut. Chem. 1980, 9, 809–818. [Google Scholar] [CrossRef]
- De Roche, J.; Gordon, C.M.; Imrie, C.T.; Ingram, M.D.; Kennedy, A.R.; Lo Celso, F.; Triolo, A. Application of complementary experimental techniques to characterization of the phase behavior of [C16mim][PF6] and [C14mim][PF6]. Chem. Mater. 2003, 15, 3089–3097. [Google Scholar] [CrossRef]
- Marszalek, M.; Fei, Z.; Zhu, D.-R.; Scopelliti, R.; Dyson, P.J.; Zakeeruddin, S.M.; Grätzel, M. Application of Ionic Liquids Containing Tricyanomethanide [C(CN)3]− or Tetracyanoborate [B(CN)4]− Anions in Dye-Sensitized Solar Cells. Inorg. Chem. 2011, 50, 11561–11567. [Google Scholar] [CrossRef] [PubMed]
- Vataščin, E.; Dohnal, V. Aqueous solutions of [EMIM] 1, 1, 2, 2-tetrafluoroethanesulfonate and [EMIM] trifluoromethanesulfonate: A thermodynamic study. J. Chem. Thermodyn. 2018, 119, 114–126. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.N.; Ralys, R.V.; Schick, C.; Geppert-Rybczyńska, M.; Jayaraman, S.; Maginn, E.J. Benchmark values: Thermochemistry of the ionic liquid [C4Py][Cl]. Aust. J. Chem. 2012, 65, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Murugesan, S.; Wiencek, J.M.; Ren, R.X.; Linhardt, R.J. Benzoate-based room temperature ionic liquids—thermal properties and glycosaminoglycan dissolution. Carbohydr. Polym. 2006, 63, 268–271. [Google Scholar] [CrossRef]
- Gathergood, N.; Garcia, M.T.; Scammells, P.J. Biodegradable ionic liquids: Part I. Concept, preliminary targets and evaluation. Green Chem. 2004, 6, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Shreeve, J.M. Bridged Tetraquaternary Salts from N,N′-Polyfluoroalkyl-4,4′-bipyridine. Inorg. Chem. 2003, 42, 7416–7421. [Google Scholar] [CrossRef]
- Brünig, T.; Krekić, K.; Bruhn, C.; Pietschnig, R. Calorimetric studies and structural aspects of ionic liquids in designing sorption materials for thermal energy storage. Chemistry 2016, 22, 16200. [Google Scholar] [CrossRef] [Green Version]
- Abranches, D.O.; Silva, L.P.; Martins, M.A.R.; Fernandez, L.; Pinho, S.P.; Coutinho, J.A.P. Can cholinium chloride form eutectic solvents with organic chloride-based salts? Fluid Phase Equilibria 2019, 493, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.-J.; Kim, D.; Ra, C.-S. Carboxylation of styrene oxide catalyzed by quaternary onium salts under solvent-free conditions. Bull. Korean Chem. Soc. 2006, 27, 744–746. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Zakharov, L.S.; Kudryavtsev, I.Y. Catalytic phosphorylation of polyfluoroalkanols 13. Some ammonium and phosphonium salts as phosphorylation catalysts. Russ. Chem Bull. 1989, 38, 2553–2556. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Morita, T.; Nitta, A.; Nishikawa, K.; Westh, P.; Koga, Y. Characterization of BF4− in terms of its effect on water by the 1-propanol probing methodology. J. Mol. Liq. 2014, 198, 211–214. [Google Scholar] [CrossRef]
- Visser, A.E.; Reichert, W.M.; Swatloski, R.P.; Willauer, H.D.; Huddleston, J.G.; Rogers, R.D. Characterization of Hydrophilic and Hydrophobic Ionic Liquids: Alternatives to Volatile Organic Compounds for Liquid-Liquid Separations. In Ionic Liquids; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 818, pp. 289–308. ISBN 978-0-8412-3789-6. [Google Scholar]
- Appetecchi, G.B.; Montanino, M.; Carewska, M.; Moreno, M.; Alessandrini, F.; Passerini, S. Chemical–physical properties of bis (perfluoroalkylsulfonyl) imide-based ionic liquids. Electrochim. Acta 2011, 56, 1300–1307. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Driesen, K.; Janssen, C.R.; Van Hecke, K.; Van Meervelt, L.; Kossmann, S.; Kirchner, B.; Binnemans, K. Choline saccharinate and choline acesulfamate: Ionic liquids with low toxicities. J. Phys. Chem. B 2007, 111, 5254–5263. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Ohte, Y.; Yamamura, Y.; Tsuzuki, S.; Saito, K. Comparative study of imidazolium-and pyrrolidinium-based ionic liquids: Thermodynamic properties. J. Phys. Chem. B 2012, 116, 5406–5413. [Google Scholar] [CrossRef]
- Yasuda, T.; Kinoshita, H.; Miran, M.S.; Tsuzuki, S.; Watanabe, M. Comparative study on physicochemical properties of protic ionic liquids based on allylammonium and propylammonium cations. J. Chem. Eng. Data 2013, 58, 2724–2732. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Branco, L.C.; Crespo, J.G.; Nunes, M.C.; Raymundo, A.; Afonso, C.A. Comparison of physicochemical properties of new ionic liquids based on imidazolium, quaternary ammonium, and guanidinium cations. Chem. A Eur. J. 2007, 13, 8478–8488. [Google Scholar] [CrossRef]
- Aranowski, R.; Cichowska-Kopczyńska, I.; Dębski, B.; Jasiński, P. Conductivity and viscosity changes of imidazolium ionic liquids induced by H2O and CO2. J. Mol. Liq. 2016, 221, 541–546. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Reichert, W.M.; Nieuwenhuyzen, M.; Johnson, S.; Seddon, K.R.; Rogers, R.D. Crystal polymorphism in 1-butyl-3-methylimidazolium halides: Supporting ionic liquid formation by inhibition of crystallization. Chem. Commun. 2003, 1636–1637. [Google Scholar] [CrossRef]
- Zhou, Z.-B.; Matsumoto, H.; Tatsumi, K. Cyclic quaternary ammonium ionic liquids with perfluoroalkyltrifluoroborates: Synthesis, characterization, and properties. Chem. A Eur. J. 2006, 12, 2196–2212. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, S.; Sun, X.; Jiang, D.; Dai, S. Deep eutectic solvents formed by quaternary ammonium salts and aprotic organic compound succinonitrile. J. Mol. Liq. 2019, 274, 414–417. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Yang, M.; Yan, P.-F.; Liu, X.-M.; Tan, Z.-C.; Welz-Biermann, U. Density and surface tension of ionic liquids [C n py][NTf2] (n = 2, 4, 5). J. Chem. Eng. Data 2010, 55, 4928–4930. [Google Scholar] [CrossRef]
- Królikowska, M.; Lipiński, P.; Maik, D. Density, viscosity and phase equilibria study of {ethylsulfate-based ionic liquid+water} binary systems as a function of temperature and composition. Thermochim. Acta 2014, 582, 1–9. [Google Scholar] [CrossRef]
- Larsen, A.S.; Holbrey, J.D.; Tham, F.S.; Reed, C.A. Designing Ionic Liquids: Imidazolium Melts with Inert Carborane Anions. J. Am. Chem. Soc. 2000, 122, 7264–7272. [Google Scholar] [CrossRef] [Green Version]
- Wachter, P.; Schreiner, C.; Schweiger, H.-G.; Gores, H.J. Determination of phase transition points of ionic liquids by combination of thermal analysis and conductivity measurements at very low heating and cooling rates. J. Chem. Thermodyn. 2010, 42, 900–903. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Zhao, D.; Fei, Z.; Ohlin, C.A.; Laurenczy, G.; Dyson, P.J. Dual-functionalised ionic liquids: Synthesis and characterisation of imidazolium salts with a nitrile-functionalised anion. Chem. Commun. 2004, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Crank, J.A.; Sharma, P.S.; Wijeratne, A.B.; Adhikary, R.; Bose, S.; Armstrong, D.W.; Petrich, J.W. Dynamic solvation in phosphonium ionic liquids: Comparison of bulk and micellar systems and considerations for the construction of the solvation correlation function, C (t). J. Phys. Chem. B 2008, 112, 3390–3396. [Google Scholar] [CrossRef]
- Xue, L.; Gurung, E.; Tamas, G.; Koh, Y.P.; Shadeck, M.; Simon, S.L.; Maroncelli, M.; Quitevis, E.L. Effect of alkyl chain branching on physicochemical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2016, 61, 1078–1091. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Lu, Y.; Li, C. Effect of ionic liquid 1-methylimidazolium chloride on the vapour liquid equilibrium of water, methanol, ethanol, and {water+ ethanol} mixture. J. Chem. Thermodyn. 2011, 43, 1748–1753. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Gonfa, G.; Chong, F.K.; Ullah, Z.; Nasrullah, A.; Sarwono, A.; Ahmad, P.; Muhammad, N. Effect of structural variations on the thermophysical properties of protic ionic liquids: Insights from experimental and computational studies. J. Chem. Eng. Data 2017, 62, 2993–3003. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Veiga, H.I.; Esperança, J.M.; Rodríguez, A. Effect of temperature on the physical properties of two ionic liquids. J. Chem. Thermodyn. 2009, 41, 1419–1423. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ohte, Y.; Yamamura, Y.; Saito, K. Effects of thermal history on thermal anomaly in solid of ionic liquid compound,[C4mim][Tf2N]. Chem. Lett. 2007, 36, 1484–1485. [Google Scholar] [CrossRef]
- Wachter, P.; Schweiger, H.-G.; Wudy, F.; Gores, H.J. Efficient determination of crystallisation and melting points at low cooling and heating rates with novel computer controlled equipment. J. Chem. Thermodyn. 2008, 40, 1542–1547. [Google Scholar] [CrossRef]
- Makino, T.; Kanakubo, M.; Umecky, T.; Suzuki, A. Electrical Conductivities, Viscosities, and Densities of N-Acetoxyethyl-N, N-dimethyl-N-ethylammonium and N, N-Dimethyl-N-ethyl-N-methoxyethoxyethylammonium Bis (trifluoromethanesulfonyl) amide and Their Nonfunctionalized Analogues. J. Chem. Eng. Data 2013, 58, 370–376. [Google Scholar] [CrossRef]
- McEwen, A.B.; Ngo, H.L.; LeCompte, K.; Goldman, J.L. Electrochemical properties of imidazolium salt electrolytes for electrochemical capacitor applications. J. Electrochem. Soc. 1999, 146, 1687. [Google Scholar] [CrossRef]
- Xue, H.; Arritt, S.W.; Twamley, B.; Shreeve, J.M. Energetic salts from N-aminoazoles. Inorg. Chem. 2004, 43, 7972–7977. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Selbach, V.M.; Dullius, J.E.L.; Einloft, S.; Piatnicki, C.M.S.; Azambuja, D.S.; de Souza, R.F.; Dupont, J. Enlarged electrochemical window in dialkyl-imidazolium cation based room-temperature air and water-stable molten salts. Electrochim. Acta 1997, 42, 2533–2535. [Google Scholar] [CrossRef]
- Stolarska, O.; Rodriguez, H.; Smiglak, M. Eutectic mixtures of pyrrolidinium-based ionic liquids. Fluid Phase Equilibria 2016, 408, 1–9. [Google Scholar] [CrossRef]
- Zheng, L.; Pan, Y.; Ji, H.-X.; Ma, X.-X.; Xing, N.-N.; Guan, W. Evaluation of the Walden Product of Ionic Liquids Using Experiments and a New Theory: An Ion Exchange Transition Model. Acta Phys. Chim. Sin. 2016, 32, 2663–2670. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, J.; Liang, Y.; Zhou, H. Facile synthesis of novel ionic liquids containing dithiocarbamate. Synth. Commun. 2005, 35, 521–526. [Google Scholar] [CrossRef]
- Vieira, N.S.; Luís, A.; Reis, P.M.; Carvalho, P.J.; Lopes-Da-Silva, J.A.; Esperança, J.; Araújo, J.M.M.; Rebelo, L.P.N.; Freire, M.G.; Pereiro, A.B. Fluorination effects on the thermodynamic, thermophysical and surface properties of ionic liquids. J. Chem. Thermodyn. 2016, 97, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishi, N.; Kawakami, T.; Shigematsu, F.; Yamamoto, M.; Kakiuchi, T. Fluorine-free and hydrophobic room-temperature ionic liquids, tetraalkylammonium bis (2-ethylhexyl) sulfosuccinates, and their ionic liquid–water two-phase properties. Green Chem. 2006, 8, 349–355. [Google Scholar] [CrossRef]
- Coker, T.G.; Ambrose, J.; Janz, G.J. Fusion properties of some ionic quaternary ammonium compounds. J. Am. Chem. Soc. 1970, 92, 5293–5297. [Google Scholar] [CrossRef]
- Moura Ramos, J.J.; Afonso, C.A.M.; Branco, L.C. Glass transition relaxation and fragility in two room temperature ionic liquids. J. Therm. Anal. Calorim. 2003, 71, 659–666. [Google Scholar] [CrossRef]
- Bhatt, A.I.; May, I.; Volkovich, V.A.; Hetherington, M.E.; Lewin, B.; Thied, R.C.; Ertok, N. Group 15 quaternary alkyl bistriflimides: Ionic liquids with potential application in electropositive metal deposition and as supporting electrolytes. J. Chem. Soc., Dalton Trans. 2002, 4532–4534. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.J.; Syed, T.; Graham, B.F.; Marsh, K.N.; May, E.F. Heat capacities and low temperature thermal transitions of 1-hexyl and 1-octyl-3-methylimidazolium bis (trifluoromethylsulfonyl) amide. J. Chem. Eng. Data 2011, 56, 2153–2159. [Google Scholar] [CrossRef]
- Zimmer, M.F.; Baroody, E.E.; Carpenter, G.A.; Robb, R.A. Heat of formation of hydroxylammonium perchlorate by combustion calorimetry. J. Chem. Eng. Data 1968, 13, 212–214. [Google Scholar] [CrossRef]
- McFarlane, D.R.; Sun, J.; Golding, J.; Meakin, P.; Forsyth, M. High conductivity molten salts based on the imide ion. Electrochim. Acta 2000, 45, 1271–1278. [Google Scholar] [CrossRef]
- Anderson, J.L.; Armstrong, D.W. High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal. Chem. 2003, 75, 4851–4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, A.; Watanabe, M. Highly conductive polymer electrolytes prepared by in situ polymerization of vinyl monomers in room temperature molten salts. Electrochim. Acta 2000, 45, 1265–1270. [Google Scholar] [CrossRef]
- Matsumoto, H.; Yanagida, M.; Tanimoto, K.; Nomura, M.; Kitagawa, Y.; Miyazaki, Y. Highly conductive room temperature molten salts based on small trimethylalkylammonium cations and bis (trifluoromethylsulfonyl) imide. Chem. Lett. 2000, 29, 922–923. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, J.; Bai, L.; Wang, B.; Gao, H.; Shang, D.; Zhang, X.; Zhang, S. Highly selective capture of CO2 by ether-functionalized pyridinium ionic liquids with low viscosity. Energy Fuels 2015, 29, 6039–6048. [Google Scholar] [CrossRef]
- Tokuda, H.; Tsuzuki, S.; Susan, M.A.B.H.; Hayamizu, K.; Watanabe, M. How ionic are room-temperature ionic liquids? An indicator of the physicochemical properties. J. Phys. Chem. B 2006, 110, 19593–19600. [Google Scholar] [CrossRef] [PubMed]
- Bonhote, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Kato, H.; Miki, K.; Mukai, T.; Nishikawa, K.; Koga, Y. Hydrophobicity/hydrophilicity of 1-butyl-2, 3-dimethyl and 1-ethyl-3-methylimodazolium ions: Toward characterization of room temperature ionic liquids. J. Phys. Chem. B 2009, 113, 14754–14760. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, S.; Cheng, W.; Ren, J. Hydroxyl-functionalized ionic liquid: A novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate. Tetrahedron Lett. 2008, 49, 3588–3591. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P.; Heintz, A.; Voss, K.; Schulz, A. Imidazolium-based ionic liquids. 1-Methyl imidazolium nitrate: Thermochemical measurements and Ab initio calculations. J. Phys. Chem. B 2009, 113, 9871–9876. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Yanagida, M.; Tanimoto, K.; Kojima, T.; Tamiya, Y.; Miyazaki, Y. Improvement of Ionic Conductivity of Room Temperature Molten Salt Based on Quaternary Ammonium Cation and Imide Anion. Proc. Vol. 1999, 1999–41, 186. [Google Scholar] [CrossRef]
- Meyer, K.L.; Marasco, C.J., Jr.; Morris-Natschke, S.L.; Ishaq, K.S.; Piantadosi, C.; Kucera, L.S. In vitro evaluation of phosphocholine and quaternary ammonium containing lipids as novel anti-HIV agents. J. Med. Chem. 1991, 34, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Govinda, V.; Reddy, P.M.; Attri, P.; Venkatesu, P.; Venkateswarlu, P. Influence of anion on thermophysical properties of ionic liquids with polar solvent. J. Chem. Thermodyn. 2013, 58, 269–278. [Google Scholar] [CrossRef]
- Domańska, U.; Morawski, P. Influence of high pressure on solubility of ionic liquids: Experimental data and correlation. Green Chem. 2007, 9, 361–368. [Google Scholar] [CrossRef]
- Rauber, D.; Heib, F.; Schmitt, M.; Hempelmann, R. Influence of perfluoroalkyl-chains on the surface properties of 1-methylimidazolium bis (trifluoromethanesulfonyl) imide ionic liquids. J. Mol. Liq. 2016, 216, 246–258. [Google Scholar] [CrossRef]
- Chun, S.; Dzyuba, S.V.; Bartsch, R.A. Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether. Anal. Chem. 2001, 73, 3737–3741. [Google Scholar] [CrossRef]
- Dzyuba, S.V.; Bartsch, R.A. Influence of structural variations in 1-alkyl (aralkyl)-3-methylimidazolium hexafluorophosphates and bis (trifluoromethylsulfonyl) imides on physical properties of the ionic liquids. ChemPhysChem 2002, 3, 161–166. [Google Scholar] [CrossRef]
- Hofmann, A.; Migeot, M.; Hanemann, T. Investigation of binary mixtures containing 1-ethyl-3-methylimidazolium bis (trifluoromethanesulfonyl) azanide and ethylene carbonate. J. Chem. Eng. Data 2016, 61, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Ohno, H.; Yoshizawa, M. Ion conductive characteristics of ionic liquids prepared by neutralization of alkylimidazoles. Solid State Ion. 2002, 154, 303–309. [Google Scholar] [CrossRef]
- Gordon, C.M.; Holbrey, J.D.; Kennedy, A.R.; Seddon, K.R. Ionic liquid crystals: Hexafluorophosphate salts. J. Mater. Chem. 1998, 8, 2627–2636. [Google Scholar] [CrossRef]
- Yang, C.; Sun, Q.; Qiao, J.; Li, Y. Ionic liquid doped polymer light-emitting electrochemical cells. J. Phys. Chem. B 2003, 107, 12981–12988. [Google Scholar] [CrossRef]
- Annat, G.; Forsyth, M.; MacFarlane, D.R. Ionic Liquid Mixtures Variations in Physical Properties and Their Origins in Molecular Structure. J. Phys. Chem. B 2012, 116, 8251–8258. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Choi, S.; Demberelnyamba, D.; Lee, H.; Oh, J.; Lee, B.-B.; Mun, S.-J. Ionic liquids based on N-alkyl-N-methylmorpholinium salts as potential electrolytes. Chem. Commun. 2004, 828–829. [Google Scholar] [CrossRef]
- Vieira, M.O.; Monteiro, W.F.; Ligabue, R.; Seferin, M.; Chaban, V.V.; Andreeva, N.A.; do Nascimento, J.F.; Einloft, S. Ionic liquids composed of linear amphiphilic anions: Synthesis, physicochemical characterization, hydrophilicity and interaction with carbon dioxide. J. Mol. Liq. 2017, 241, 64–73. [Google Scholar] [CrossRef]
- Lee, J.S.; Quan, N.D.; Hwang, J.M.; Bae, J.Y.; Kim, H.; Cho, B.W.; Kim, H.S.; Lee, H. Ionic liquids containing an ester group as potential electrolytes. Electrochem. Commun. 2006, 8, 460–464. [Google Scholar] [CrossRef]
- De Gaetano, Y.; Mohamadou, A.; Boudesocque, S.; Hubert, J.; Plantier-Royon, R.; Dupont, L. Ionic liquids derived from esters of Glycine Betaine: Synthesis and characterization. J. Mol. Liq. 2015, 207, 60–66. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Ionic liquids in separation techniques. J. Chromatogr. A 2008, 1184, 6–18. [Google Scholar] [CrossRef]
- Xu, W.; Wang, L.-M.; Nieman, R.A.; Angell, C.A. Ionic liquids of chelated orthoborates as model ionic glassformers. J. Phys. Chem. B 2003, 107, 11749–11756. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L. Ionic liquids: Perspectives for organic and catalytic reactions. J. Mol. Catal. A Chem. 2002, 182, 419–437. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X.; Zhou, Q.; Li, X.; Zhang, X.; Li, S. Ionic Liquids: Physicochemical Properties; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 0-08-095907-5. [Google Scholar]
- Emel’yanenko, V.N.; Verevkin, S.P.; Heintz, A.; Schick, C. Ionic liquids. Combination of combustion calorimetry with high-level quantum chemical calculations for deriving vaporization enthalpies. J. Phys. Chem. B 2008, 112, 8095–8098. [Google Scholar] [CrossRef]
- Morais, A.R.; da Costa Lopes, A.M.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquids’ cation and anion influence on aromatic amine solubility. Ind. Eng. Chem. Res. 2013, 52, 14722–14726. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.-X.; Wei, J.; Guan, W.; Pan, Y.; Zheng, L.; Wu, Y.; Yang, J.-Z. Ionic parachor and its application to pyridinium-based ionic liquids of {[Cnpy][DCA](n = 2, 3, 4, 5, 6)}. J. Chem. Thermodyn. 2015, 89, 51–59. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ohte, Y.; Yamamura, Y.; Saito, K. Is the liquid or the solid phase responsible for the low melting points of ionic liquids? Alkyl-chain-length dependence of thermodynamic properties of [Cnmim][Tf2N]. Chem. Phys. Lett. 2009, 470, 295–299. [Google Scholar] [CrossRef]
- Kurzin, A.V.; Evdokimov, A.N.; Feofanova, M.A.; Baranova, N.V. Isothermal Vapor–Liquid Equilibrium Data for the Toluene+ Methanol+ N-Butylpyridinium Bromide System. J. Chem. Eng. Data 2017, 62, 889–892. [Google Scholar] [CrossRef]
- Elias, H.; Strecker, H. Kinetik des homogenen Isotopenaustausches zwischen Isopropylchlorid und Chlorid-Ionen in Dimethylformamid. Chem. Ber. 1966, 99, 1019–1025. [Google Scholar] [CrossRef]
- Kissinger, P.; Heineman, W.R. Laboratory Techniques in Electroanalytical Chemistry, Revised and Expanded, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018; ISBN 978-1-351-99071-4. [Google Scholar]
- MacFarlane, D.R.; Pringle, J.M.; Johansson, K.M.; Forsyth, S.A.; Forsyth, M. Lewis base ionic liquids. Chem. Commun. 2006, 1905–1917. [Google Scholar] [CrossRef]
- Schneider, S.; Hawkins, T.; Rosander, M.; Mills, J.; Brand, A.; Hudgens, L.; Warmoth, G.; Vij, A. Liquid azide salts. Inorg. Chem. 2008, 47, 3617–3624. [Google Scholar] [CrossRef]
- Le, M.L.P.; Tran, N.A.; Ngo, H.P.K.; Nguyen, T.G. Liquid electrolytes based on ionic liquids for lithium-ion batteries. J. Solut. Chem. 2015, 44, 2332–2343. [Google Scholar] [CrossRef]
- Villanueva, M.; Parajó, J.J.; Sánchez, P.B.; García, J.; Salgado, J. Liquid range temperature of ionic liquids as potential working fluids for absorption heat pumps. J. Chem. Thermodyn. 2015, 91, 127–135. [Google Scholar] [CrossRef]
- Parajó, J.J.; Villanueva, M.; Sánchez, P.B.; Salgado, J. Liquid window of some biologically-active ionic liquids. J. Chem. Thermodyn. 2018, 126, 1–10. [Google Scholar] [CrossRef]
- Castro, M.C.; Arce, A.; Soto, A.; Rodríguez, H. Liquid-liquid equilibria of mutually immiscible ionic liquids with a common anion of basic character. J. Chem. Thermodyn. 2016, 102, 12–21. [Google Scholar] [CrossRef]
- Domańska, U.; Pobudkowska, A.; Eckert, F. Liquid–liquid equilibria in the binary systems (1,3-dimethylimidazolium, or 1-butyl-3-methylimidazolium methylsulfate+ hydrocarbons). Green Chem. 2006, 8, 268–276. [Google Scholar] [CrossRef]
- Paduszyński, K.; Chiyen, J.; Ramjugernath, D.; Letcher, T.M.; Domańska, U. Liquid–liquid phase equilibrium of (piperidinium-based ionic liquid+ an alcohol) binary systems and modelling with NRHB and PCP-SAFT. Fluid Phase Equilibria 2011, 305, 43–52. [Google Scholar] [CrossRef]
- Vila, J.; Fernández-Castro, B.; Rilo, E.; Carrete, J.; Domínguez-Pérez, M.; Rodríguez, J.R.; García, M.; Varela, L.M.; Cabeza, O. Liquid–solid–liquid phase transition hysteresis loops in the ionic conductivity of ten imidazolium-based ionic liquids. Fluid Phase Equilibria 2012, 320, 1–10. [Google Scholar] [CrossRef]
- Sahandzhieva, K.; Naydenov, D.; Pérez-Salado Kamps, Á.; Bart, H.-J.; Maurer, G. Liquid−Liquid Equilibrium in Systems with an Ionic Liquid: Experimental Data for an Example of the Biphasic Acid Scavenging Utilizing Ionic Liquids Process. J. Chem. Eng. Data 2010, 55, 4903–4906. [Google Scholar] [CrossRef]
- Trindade, C.A.; Visak, Z.P.; Bogel-Łukasik, R.; Bogel-Łukasik, E.; da Ponte, M.N. Liquid-liquid equilibrium of mixtures of imidazolium-based ionic liquids with propanediols or glycerol. Ind. Eng. Chem. Res. 2010, 49, 4850–4857. [Google Scholar] [CrossRef]
- Pernak, J.; Smiglak, M.; Griffin, S.T.; Hough, W.L.; Wilson, T.B.; Pernak, A.; Zabielska-Matejuk, J.; Fojutowski, A.; Kita, K.; Rogers, R.D. Long alkyl chain quaternary ammonium-based ionic liquids and potential applications. Green Chem. 2006, 8, 798–806. [Google Scholar] [CrossRef]
- Kim, J.; Singh, R.P.; Shreeve, J.M. Low melting inorganic salts of alkyl-, fluoroalkyl-, alkyl ether-, and fluoroalkyl ether-substituted oxazolidine and morpholine. Inorg. Chem. 2004, 43, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, Y.R.; Xue, H.; Shreeve, J.M. Low melting N-4-functionalized-1-alkyl or polyfluoroalkyl-1,2,4-triazolium salts. Inorg. Chem. 2004, 43, 361–367. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Golding, J.; Forsyth, S.; Forsyth, M.; Deacon, G.B. Low viscosity ionic liquids based on organic salts of the dicyanamide anion. Chem. Commun. 2001, 16, 1430–1431. [Google Scholar] [CrossRef]
- Zhou, Z.-B.; Matsumoto, H.; Tatsumi, K. Low-melting, low-viscous, hydrophobic ionic liquids: 1-alkyl (alkyl ether)-3-methylimidazolium perfluoroalkyltrifluoroborate. Chem. A Eur. J. 2004, 10, 6581–6591. [Google Scholar] [CrossRef]
- Zhou, Z.-B.; Matsumoto, H.; Tatsumi, K. Low-melting, low-viscous, hydrophobic ionic liquids: Aliphatic quaternary ammonium salts with perfluoroalkyltrifluoroborates. Chem. A Eur. J. 2005, 11, 752–766. [Google Scholar] [CrossRef]
- Fang, D.; Luo, J.; Xin-Li, Z.; Zu-Liang, L. Mannich reaction in water using acidic ionic liquid as recoverable and reusable catalyst. Catal. Lett. 2007, 116, 76–80. [Google Scholar]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Drummond, C.J. Many protic ionic liquids mediate hydrocarbon-solvent interactions and promote amphiphile self-assembly. Langmuir 2007, 23, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Pontes, P.V.; Crespo, E.A.; Martins, M.A.; Silva, L.P.; Neves, C.M.; Maximo, G.J.; Hubinger, M.D.; Batista, E.A.; Pinho, S.P.; Coutinho, J.A. Measurement and PC-SAFT modeling of solid-liquid equilibrium of deep eutectic solvents of quaternary ammonium chlorides and carboxylic acids. Fluid Phase Equilibria 2017, 448, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Domańska, U.; Bogel-Łukasik, E. Measurements and Correlation of the (Solid + Liquid) Equilibria of [1-Decyl-3-methylimidazolium Chloride + Alcohols (C2−C12)]. Ind. Eng. Chem. Res. 2003, 42, 6986–6992. [Google Scholar] [CrossRef]
- Nishikawa, K.; Wang, S.; Katayanagi, H.; Hayashi, S.; Hamaguchi, H.; Koga, Y.; Tozaki, K. Melting and freezing behaviors of prototype ionic liquids, 1-butyl-3-methylimidazolium bromide and its chloride, studied by using a nano-watt differential scanning calorimeter. J. Phys. Chem. B 2007, 111, 4894–4900. [Google Scholar] [CrossRef]
- Weiss, H.; Mars, J.; Li, H.; Kircher, G.; Ivanova, O.; Feoktystov, A.; Soltwedel, O.; Bier, M.; Mezger, M. Mesoscopic correlation functions in heterogeneous ionic liquids. J. Phys. Chem. B 2017, 121, 620–629. [Google Scholar] [CrossRef]
- Golding, J.; Forsyth, S.; Macfarlane, D.R.; Forsyth, M.; Deacon, G.B. Methanesulfonate and p-toluenesulfonate salts of the N-methyl-N-alkylpyrrolidinium and quaternary ammonium cations: Novel low cost ionic liquids. Green Chem. 2002, 4, 223–229. [Google Scholar] [CrossRef]
- Hill, A.J.; Huang, J.; Efthimiadis, J.; Meakin, P.; Forsyth, M.; MacFarlane, D.R. Microstructural and molecular level characterisation of plastic crystal phases of pyrrolidinium trifluoromethanesulfonyl salts. Solid State Ion. 2002, 154–155, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Le, L.T.; Vo, T.D.; Ngo, K.H.; Okada, S.; Alloin, F.; Garg, A.; Le, P.M. Mixing ionic liquids and ethylene carbonate as safe electrolytes for lithium-ion batteries. J. Mol. Liq. 2018, 271, 769–777. [Google Scholar] [CrossRef]
- Hu, H.-C.; Soriano, A.N.; Leron, R.B.; Li, M.-H. Molar heat capacity of four aqueous ionic liquid mixtures. Thermochim. Acta 2011, 519, 44–49. [Google Scholar] [CrossRef]
- Forsyth, S.A.; Fraser, K.J.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. N-methyl-N-alkylpyrrolidinium nonafluoro-1-butanesulfonate salts: Ionic liquid properties and plastic crystal behaviour. Green Chem. 2006, 8, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Sakaebe, H.; Matsumoto, H. N-Methyl-N-propylpiperidinium bis (trifluoromethanesulfonyl) imide (PP13–TFSI)–novel electrolyte base for Li battery. Electrochem. Commun. 2003, 5, 594–598. [Google Scholar] [CrossRef]
- Xue, H.; Gao, Y.; Twamley, B.; Shreeve, J.M. New energetic salts based on nitrogen-containing heterocycles. Chem. Mater. 2005, 17, 191–198. [Google Scholar] [CrossRef]
- Tao, G.; He, L.; Sun, N.; Kou, Y. New generation ionic liquids: Cations derived from amino acids. Chem. Commun. 2005, 3562–3564. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Czepukowicz, A.; Poźniak, R. New ionic liquids and their antielectrostatic properties. Ind. Eng. Chem. Res. 2001, 40, 2379–2383. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Turner, M.B.; Reichert, W.M.; Rogers, R.D. New ionic liquids containing an appended hydroxyl functionality from the atom-efficient, one-pot reaction of 1-methylimidazole and acid with propylene oxide. Green Chem. 2003, 5, 731–736. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Welz-Biermann, U.; Kucheryna, A.; Bissky, G.; Willner, H. New ionic liquids with tris (perfluoroalkyl) trifluorophosphate (FAP) anions. J. Fluor. Chem. 2005, 126, 1150–1159. [Google Scholar] [CrossRef]
- Wasserscheid, P.; van Hal, R.; Bösmann, A. New, Halogen-Free Ionic Liquids—Synthesis, Properties and Applications. ECS Proc. Vol. 2002, 2002, 146. [Google Scholar] [CrossRef]
- Zhao, D.; Fei, Z.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Nitrile-functionalized pyridinium ionic liquids: Synthesis, characterization, and their application in carbon− carbon coupling reactions. J. Am. Chem. Soc. 2004, 126, 15876–15882. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, T.; Zhou, Z.; Dai, Y. Novel Brønsted-acidic ionic liquids for esterifications. Ind. Eng. Chem. Res. 2005, 44, 4147–4150. [Google Scholar] [CrossRef]
- Wu, B.; Reddy, R.G.; Rogers, R.D. Novel ionic liquid thermal storage for solar thermal electric power systems. In Proceedings of the International Solar Energy Conference, Washington, DC, USA, 21–25 April 2001; Volume 16702, pp. 445–451. [Google Scholar]
- Yagupolskii, Y.L.; Sokolenko, T.M.; Petko, K.I.; Yagupolskii, L.M. Novel ionic liquids—Imidazolium salts with a difluoromethylene fragment directly bonded to the nitrogen atom. J. Fluor. Chem. 2005, 126, 667–670. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Pastoriza-Gallego, M.J.; Shimizu, K.; Marrucho, I.M.; Lopes, J.N.C.; Piñeiro, M.M.; Rebelo, L.P.N. On the formation of a third, nanostructured domain in ionic liquids. J. Phys. Chem. B 2013, 117, 10826–10833. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Jun, L.; Xinli, Z.; Zhiwen, Y.; Zuliang, L. One-pot green procedure for Biginelli reaction catalyzed by novel task-specific room-temperature ionic liquids. J. Mol. Catal. A Chem. 2007, 274, 208–211. [Google Scholar] [CrossRef]
- Fei, Z.; Zhao, D.; Scopelliti, R.; Dyson, P.J. Organometallic complexes derived from alkyne-functionalized imidazolium salts. Organometallics 2004, 23, 1622–1628. [Google Scholar] [CrossRef]
- Lapidus, A.; Eliseev, O.; Bondarenko, T.; Stepin, N. Palladium catalysed hydroxycarbonylation of 1-phenylethanol in molten salt media. J. Mol. Catal. A Chem. 2006, 252, 245–251. [Google Scholar] [CrossRef]
- Henderson, W.A.; Passerini, S. Phase behavior of ionic liquid− LiX mixtures: Pyrrolidinium cations and TFSI-anions. Chem. Mater. 2004, 16, 2881–2885. [Google Scholar] [CrossRef]
- Štejfa, V.; Rohlíček, J.; Červinka, C. Phase behaviour and heat capacities of selected 1-ethyl-3-methylimidazolium-based ionic liquids. J. Chem. Thermodyn. 2020, 142, 106020. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowska, M. Phase behaviour of 1-butyl-1-methylpyrrolidinium thiocyanate ionic liquid. Fluid Phase Equilibria 2011, 308, 55–63. [Google Scholar] [CrossRef]
- Chen, H.; Kwait, D.C.; Gönen, Z.S.; Weslowski, B.T.; Abdallah, D.J.; Weiss, R.G. Phase characterization and properties of completely saturated quaternary phosphonium salts. Ordered, room-temperature ionic liquids. Chem. Mater. 2002, 14, 4063–4072. [Google Scholar] [CrossRef]
- Ma, G.; Zhou, Y.; Su, T.; Wei, W.; Gong, Y.; Hu, X.; Hong, Y.; Su, Y.; Wang, H.; Li, J. Phase equilibria and diffusion behavior of high pressure CO2 in tetra-n-heptyl ammonium bromide. J. Supercrit. Fluids 2016, 107, 370–376. [Google Scholar] [CrossRef]
- Domańska, U.; Marciniak, A.; Krolikowski, M. Phase equilibria and modeling of ammonium ionic liquid, C2NTf2, solutions. J. Phys. Chem. B 2008, 112, 1218–1225. [Google Scholar] [CrossRef]
- Domanska, U.; Królikowski, M.; Ramjugernath, D.; Letcher, T.M.; Tumba, K. Phase equilibria and modeling of pyridinium-based ionic liquid solutions. J. Phys. Chem. B 2010, 114, 15011–15017. [Google Scholar] [CrossRef]
- Vataščin, E.; Dohnal, V. Phase equilibria of (water + [EMIM] bromide) and (water + [EMIM] tosylate). Fluid Phase Equilibria 2019, 483, 84–91. [Google Scholar] [CrossRef]
- Domańska, U.; Ługowska, K.; Pernak, J. Phase equilibria of didecyldimethylammonium nitrate ionic liquid with water and organic solvents. J. Chem. Thermodyn. 2007, 39, 729–736. [Google Scholar] [CrossRef]
- Arce, A.; Earle, M.J.; Katdare, S.P.; Rodríguez, H.; Seddon, K.R. Phase equilibria of mixtures of mutually immiscible ionic liquids. Fluid Phase Equilibria 2007, 261, 427–433. [Google Scholar] [CrossRef]
- Wong, D.S.H.; Chen, J.P.; Chang, J.M.; Chou, C.H. Phase equilibria of water and ionic liquids [emim][PF6] and [bmim][PF6]. Fluid Phase Equilibria 2002, 194, 1089–1095. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowski, M.; Ślesińska, K. Phase equilibria study of the binary systems (ionic liquid+thiophene): Desulphurization process. J. Chem. Thermodyn. 2009, 41, 1303–1311. [Google Scholar] [CrossRef]
- Domanska, U.; Królikowski, M.; Pobudkowska, A.; Letcher, T.M. Phase equilibria study of the binary systems (n-butyl-4-methylpyridinium tosylate ionic liquid+ organic solvent, or water). J. Chem. Eng. Data 2009, 54, 1435–1441. [Google Scholar] [CrossRef]
- Królikowski, M.; Królikowska, M.; Lipińska, A. Phase equilibria study on bromide-based ionic liquids with glycols and sulfolane. Experimental data and correlation. J. Chem. Thermodyn. 2018, 122, 142–153. [Google Scholar] [CrossRef]
- Crosthwaite, J.M.; Muldoon, M.J.; Dixon, J.K.; Anderson, J.L.; Brennecke, J.F. Phase transition and decomposition temperatures, heat capacities and viscosities of pyridinium ionic liquids. J. Chem. Thermodyn. 2005, 37, 559–568. [Google Scholar] [CrossRef]
- Ishida, H.; Iwachido, T.; Ikeda, R. Phase Transitions in Dimethylammonium Tetrafluoroborate and Molecular Motions in Its Ionic Plastic Phase Studied by 1H and 19F NMR, Thermal Measurements, and X-Ray Powder Diffraction Techniques. Ber. Der Bunsenges. Für Phys. Chem. 1992, 96, 1468–1470. [Google Scholar] [CrossRef]
- Fillion, J.J.; Xia, H.; Desilva, M.A.; Quiroz-Guzman, M.; Brennecke, J.F. Phase transitions, decomposition temperatures, viscosities, and densities of phosphonium, ammonium, and imidazolium ionic liquids with aprotic heterocyclic anions. J. Chem. Eng. Data 2016, 61, 2897–2914. [Google Scholar] [CrossRef]
- Yeon, S.-H.; Kim, K.-S.; Choi, S.; Lee, H.; Kim, H.S.; Kim, H. Physical and electrochemical properties of 1-(2-hydroxyethyl)-3-methyl imidazolium and N-(2-hydroxyethyl)-N-methyl morpholinium ionic liquids. Electrochim. Acta 2005, 50, 5399–5407. [Google Scholar] [CrossRef]
- Nishida, T.; Tashiro, Y.; Yamamoto, M. Physical and electrochemical properties of 1-alkyl-3-methylimidazolium tetrafluoroborate for electrolyte. J. Fluor. Chem. 2003, 120, 135–141. [Google Scholar] [CrossRef]
- Tsunashima, K.; Sugiya, M. Physical and electrochemical properties of low-viscosity phosphonium ionic liquids as potential electrolytes. Electrochem. Commun. 2007, 9, 2353–2358. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kageyama, H.; Miyazaki, Y. Physical and electrochemical properties of room temperature molten salt based on aliphatic onium cations and asymmetric amide anion. ECS Proc. Vol. 2002, 2002, 1057. [Google Scholar] [CrossRef]
- Cabeza, O.; Vila, J.; Rilo, E.; Domínguez-Pérez, M.; Otero-Cernadas, L.; López-Lago, E.; Méndez-Morales, T.; Varela, L.M. Physical properties of aqueous mixtures of the ionic 1-ethl-3-methyl imidazolium octyl sulfate: A new ionic rigid gel. J. Chem. Thermodyn. 2014, 75, 52–57. [Google Scholar] [CrossRef]
- Fletcher, S.I.; Sillars, F.B.; Hudson, N.E.; Hall, P.J. Physical properties of selected ionic liquids for use as electrolytes and other industrial applications. J. Chem. Eng. Data 2010, 55, 778–782. [Google Scholar] [CrossRef]
- González, B.; González, E.J. Physical properties of the pure 1-methyl-1-propylpyrrolidinium bis (trifluoromethylsulfonyl) imide ionic liquid and its binary mixtures with alcohols. J. Chem. Thermodyn. 2014, 68, 109–116. [Google Scholar] [CrossRef]
- Neale, A.R.; Schütter, C.; Wilde, P.; Goodrich, P.; Hardacre, C.; Passerini, S.; Balducci, A.; Jacquemin, J. Physical–chemical characterization of binary mixtures of 1-Butyl-1-methylpyrrolidinium bis {(trifluoromethyl) sulfonyl} imide and aliphatic nitrile solvents as potential electrolytes for electrochemical energy storage applications. J. Chem. Eng. Data 2017, 62, 376–390. [Google Scholar] [CrossRef]
- Domańska, U.; Krolikowska, M.; Paduszyński, K. Physico-chemical properties and phase behaviour of piperidinium-based ionic liquids. Fluid Phase Equilibria 2011, 303, 1–9. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsumoto, K.; Hagiwara, R.; Nohira, T. Physicochemical and electrochemical properties of K [N (SO2F) 2]–[N-methyl-N-propylpyrrolidinium][N (SO2F) 2] ionic liquids for potassium-ion batteries. J. Phys. Chem. C 2017, 121, 18450–18458. [Google Scholar] [CrossRef]
- De La Hoz, A.T.; Brauer, U.G.; Miller, K.M. Physicochemical and Thermal Properties for a Series of 1-Alkyl-4-methyl-1, 2, 4-triazolium Bis (trifluoromethylsulfonyl) imide Ionic Liquids. J. Phys. Chem. B 2014, 118, 9944–9951. [Google Scholar] [CrossRef]
- Zawadzki, M.; Królikowska, M.; Antonowicz, J.; Lipiński, P.; Karpińska, M. Physicochemical and thermodynamic properties of the {1-alkyl-1-methylmorpholinium bromide,[C1Cn = 3, 4, 5MOR] Br, or 1-methyl-1-pentylpiperidinium bromide,[C1C5PIP] Br+ water} binary systems. J. Chem. Thermodyn. 2016, 98, 324–337. [Google Scholar] [CrossRef]
- Królikowska, M.; Zawadzki, M.; Kuna, T. Physicochemical and thermodynamic properties of the {1-alkyl-1-methylpiperidinium bromide [C1Cn = 2, 4PIP][Br], or 1-butylpyridinium bromide,[C4Py][Br], or tri (ethyl) butylammonium bromide [N2, 2, 2, 4][Br]+ water} binary systems. Thermochim. Acta 2019, 671, 220–231. [Google Scholar] [CrossRef]
- Ferrara, C.; Dall’Asta, V.; Berbenni, V.; Quartarone, E.; Mustarelli, P. Physicochemical characterization of AlCl3–1-Ethyl-3-methylimidazolium chloride ionic liquid electrolytes for aluminum rechargeable batteries. J. Phys. Chem. C 2017, 121, 26607–26614. [Google Scholar] [CrossRef]
- Li, H.; Zhao, G.; Liu, F.; Zhang, S. Physicochemical Characterization of MF m–-Based Ammonium Ionic Liquids. J. Chem. Eng. Data 2013, 58, 1505–1515. [Google Scholar] [CrossRef]
- González, B.; Gómez, E.; Domínguez, Á.; Vilas, M.; Tojo, E. Physicochemical characterization of new sulfate ionic liquids. J. Chem. Eng. Data 2011, 56, 14–20. [Google Scholar] [CrossRef]
- Domańska, U.; Bogel-Łukasik, R. Physicochemical properties and solubility of alkyl-(2-hydroxyethyl)-dimethylammonium bromide. J. Phys. Chem. B 2005, 109, 12124–12132. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef]
- Tokuda, H.; Ishii, K.; Susan, M.A.B.H.; Tsuzuki, S.; Hayamizu, K.; Watanabe, M. Physicochemical properties and structures of room-temperature ionic liquids. 3. Variation of cationic structures. J. Phys. Chem. B 2006, 110, 2833–2839. [Google Scholar] [CrossRef]
- Salminen, J.; Papaiconomou, N.; Kumar, R.A.; Lee, J.-M.; Kerr, J.; Newman, J.; Prausnitz, J.M. Physicochemical properties and toxicities of hydrophobic piperidinium and pyrrolidinium ionic liquids. Fluid Phase Equilibria 2007, 261, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.A.; van den Bruinhorst, A.; Schröer, W.; Rathke, B.; Kroon, M.C. Physicochemical properties of fatty acid based ionic liquids. J. Chem. Thermodyn. 2016, 100, 156–164. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Yang, M.; Li, P.-P.; Sun, S.-S.; Welz-Biermann, U.; Tan, Z.-C.; Zhang, Q.-G. Physicochemical properties of ionic liquids [C3py][NTf2] and [C6py][NTf2]. J. Chem. Eng. Data 2011, 56, 4094–4101. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Zhu, X.; Wang, X.; Jia, H.; Xiao, X.; Yu, H.; Yang, X. Physicochemical properties of ionic liquids based on imidazolium/pyrrolidinium cations and maleate/phthalate anions. Solid State Ion. 2008, 179, 516–521. [Google Scholar] [CrossRef]
- Hazrati, N.; Abdouss, M.; Miran Beigi, A.A.; Pasban, A.A.; Rezaei, M. Physicochemical properties of long chain alkylated imidazolium based chloride and bis (trifluoromethanesulfonyl) imide ionic liquids. J. Chem. Eng. Data 2017, 62, 3084–3094. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Zhang, J.; Zhang, S.; Zhu, L.; Yang, J.; Zhang, X.; Deng, Y. Physicochemical properties of nitrile-functionalized ionic liquids. J. Phys. Chem. B 2007, 111, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, Q.-G.; Liu, Y.; Li, X.; Lian, S.; Kang, Z. Physicochemical property estimation of an ionic liquid based on glutamic acid− BMIGlu. J. Chem. Eng. Data 2010, 55, 2616–2619. [Google Scholar] [CrossRef]
- Che, Q.; Sun, B.; He, R. Preparation and characterization of new anhydrous, conducting membranes based on composites of ionic liquid trifluoroacetic propylamine and polymers of sulfonated poly (ether ether) ketone or polyvinylidenefluoride. Electrochim. Acta 2008, 53, 4428–4434. [Google Scholar] [CrossRef]
- Mu, Z.; Zhou, F.; Zhang, S.; Liang, Y.; Liu, W. Preparation and Characterization of New Phosphonyl-Substituted Imidazolium Ionic Liquids. Helv. Chim. Acta 2004, 87, 2549–2555. [Google Scholar] [CrossRef]
- Branco, L.C.; Rosa, J.N.; Moura Ramos, J.J.; Afonso, C.A. Preparation and characterization of new room temperature ionic liquids. Chem. A Eur. J. 2002, 8, 3671–3677. [Google Scholar] [CrossRef]
- Tsukada, Y.; Iwamoto, K.; Furutani, H.; Matsushita, Y.; Abe, Y.; Matsumoto, K.; Monda, K.; Hayase, S.; Kawatsura, M.; Itoh, T. Preparation of novel hydrophobic fluorine-substituted-alkyl sulfate ionic liquids and application as an efficient reaction medium for lipase-catalyzed reaction. Tetrahedron Lett. 2006, 47, 1801–1804. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakaebe, H.; Tatsumi, K. Preparation of room temperature ionic liquids based on aliphatic onium cations and asymmetric amide anions and their electrochemical properties as a lithium battery electrolyte. J. Power Sources 2005, 146, 45–50. [Google Scholar] [CrossRef]
- Fannin, A.A., Jr.; Floreani, D.A.; King, L.A.; Landers, J.S.; Piersma, B.J.; Stech, D.J.; Vaughn, R.L.; Wilkes, J.S.; Williams, J.L. Properties of 1, 3-dialkylimidazolium chloride-aluminum chloride ionic liquids. 2. Phase transitions, densities, electrical conductivities, and viscosities. J. Phys. Chem. 1984, 88, 2614–2621. [Google Scholar] [CrossRef]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M. Protein solubilising and stabilising ionic liquids. Chem. Commun. 2005, 4804–4806. [Google Scholar] [CrossRef]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Krodkiewska, I.; Drummond, C.J. Protic ionic liquids: Solvents with tunable phase behavior and physicochemical properties. J. Phys. Chem. B 2006, 110, 22479–22487. [Google Scholar] [CrossRef]
- Canongia Lopes, J.N.; Esperança, J.M.; de Ferro, A.M.; Pereiro, A.B.; Plechkova, N.V.; Rebelo, L.P.; Seddon, K.R.; Vázquez-Fernández, I. Protonic ammonium nitrate ionic liquids and their mixtures: Insights into their thermophysical behavior. J. Phys. Chem. B 2016, 120, 2397–2406. [Google Scholar] [CrossRef]
- Noda, A.; Hayamizu, K.; Watanabe, M. Pulsed-gradient spin− echo 1H and 19F NMR ionic diffusion coefficient, viscosity, and ionic conductivity of non-chloroaluminate room-temperature ionic liquids. J. Phys. Chem. B 2001, 105, 4603–4610. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sugimoto, T.; Kikuta, M.; Ishiko, E.; Kono, M. Pure ionic liquid electrolytes compatible with a graphitized carbon negative electrode in rechargeable lithium-ion batteries. J. Power Sources 2006, 162, 658–662. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Meakin, P.; Sun, J.; Amini, N.; Forsyth, M. Pyrrolidinium imides: A new family of molten salts and conductive plastic crystal phases. J. Phys. Chem. B 1999, 103, 4164–4170. [Google Scholar] [CrossRef]
- Singh, R.P.; Winter, R.W.; Gard, G.L.; Gao, Y.; Shreeve, J.M. Quaternary salts containing the pentafluorosulfanyl (SF5) group. Inorg. Chem. 2003, 42, 6142–6146. [Google Scholar] [CrossRef] [PubMed]
- Earle, M.J.; McCormac, P.B.; Seddon, K.R. Regioselective alkylation in ionic liquids. Chem. Commun. 1998, 2245–2246. [Google Scholar] [CrossRef]
- Gu, Y.-L.; Shi, F.; Deng, Y.-Q. Room temperature ionic liquid as leaching reagent for separation of the solid mixture of taurine and sodium sulfate. Acta Chim. Sin. Chin. Ed. 2004, 62, 532–536. [Google Scholar]
- Matsumoto, K.; Hagiwara, R.; Ito, Y. Room temperature molten fluorometallates: 1-ethyl-3-methylimidazolium hexafluoroniobate(V) and hexafluorotantalate(V). J. Fluor. Chem. 2002, 115, 133–135. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kageyama, H.; Miyazaki, Y. Room temperature molten salts based on tetraalkylammonium cations and bis (trifluoromethylsulfonyl) imide. Chem. Lett. 2001, 30, 182–183. [Google Scholar] [CrossRef]
- Matsumoto, H.; Matsuda, T.; Miyazaki, Y. Room temperature molten salts based on trialkylsulfonium cations and bis (trifluoromethylsulfonyl) imide. Chem. Lett. 2000, 29, 1430–1431. [Google Scholar] [CrossRef]
- Liu, Q.; Janssen, M.H.; van Rantwijk, F.; Sheldon, R.A. Room-temperature ionic liquids that dissolve carbohydrates in high concentrations. Green Chem. 2005, 7, 39–42. [Google Scholar] [CrossRef]
- Sun, J.; Forsyth, M.; Macfarlane, D.R. Room-temperature molten salts based on the quaternary ammonium ion. J. Phys. Chem. B 1998, 102, 8858–8864. [Google Scholar] [CrossRef]
- McMichael, K.; Clement, R. Salt effects in the solvolysis of benzhydryl chloride. J. Org. Chem. 1961, 26, 620–621. [Google Scholar] [CrossRef]
- Wlazło, M.; Zawadzki, M.; Domańska, U. Separation of water/butan-1-ol based on activity coefficients at infinite dilution in 1, 3-didecyl-2-methylimidazolium dicyanamide ionic liquid. J. Chem. Thermodyn. 2018, 116, 316–322. [Google Scholar] [CrossRef]
- Bradley, A.E.; Hardacre, C.; Holbrey, J.D.; Johnston, S.; McMath, S.E.J.; Nieuwenhuyzen, M. Small-angle X-ray scattering studies of liquid crystalline 1-alkyl-3-methylimidazolium salts. Chem. Mater. 2002, 14, 629–635. [Google Scholar] [CrossRef]
- Sifaoui, H.; Ait-Kaci, A.; Modarressi, A.; Rogalski, M. Solid–liquid equilibria of three binary systems:{1-Ethyl-3-methylimidazolium hexafluorophosphate+ 2-phenylimidazole, or 4,5-diphenylimidazole or 2,4,5-triphenylimidazole}. Thermochim. Acta 2007, 456, 114–119. [Google Scholar] [CrossRef]
- Kick, M.; Keil, P.; König, A. Solid–liquid phase diagram of the two Ionic Liquids EMIMCl and BMIMCl. Fluid Phase Equilibria 2013, 338, 172–178. [Google Scholar] [CrossRef]
- Shevelyova, M.P.; Zaitsau, D.H.; Paulechka, Y.U.; Blokhin, A.V.; Kabo, G.J.; Verevkin, S.P.; Heintz, A. Solid− Liquid Equilibrium and Activity Coefficients for Caprolactam+ 1-Hexyl-3-methylimidazolium Bis (trifluoromethylsulfonyl) imide and Cyclohexanone Oxime+ 1-Hexyl-3-methylimidazolium Bis (trifluoromethylsulfonyl) imide. J. Chem. Eng. Data 2007, 52, 1360–1365. [Google Scholar] [CrossRef]
- Yang, X.-Z.; Wang, J.; Li, G.-S.; Zhang, Z.-Z. Solubilities of 1-ethylpyridinium hexafluorophosphate in ethanol+ water from (278.15 to 345.15) K. J. Chem. Eng. Data 2009, 54, 75–77. [Google Scholar] [CrossRef]
- Lourenço, C.; Melo, C.I.; Bogel-Łukasik, R.; Bogel-Łukasik, E. Solubility advantage of pyrazine-2-carboxamide: Application of alternative solvents on the way to the future pharmaceutical development. J. Chem. Eng. Data 2012, 57, 1525–1533. [Google Scholar] [CrossRef]
- Domanska, U.; Rekawek, A.; Marciniak, A. Solubility of 1-alkyl-3-ethylimidazolium-based ionic liquids in water and 1-octanol. J. Chem. Eng. Data 2008, 53, 1126–1132. [Google Scholar] [CrossRef]
- Domańska, U.; Marciniak, A. Solubility of 1-alkyl-3-methylimidazolium hexafluorophosphate in hydrocarbons. J. Chem. Eng. Data 2003, 48, 451–456. [Google Scholar] [CrossRef]
- Domańska, U.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of 1-Dodecyl-3-methylimidazolium Chloride in Alcohols (C2− C12). J. Phys. Chem. B 2003, 107, 1858–1863. [Google Scholar] [CrossRef]
- Domańska, U.; Mazurowska, L. Solubility of 1, 3-dialkylimidazolium chloride or hexafluorophosphate or methylsulfonate in organic solvents: Effect of the anions on solubility. Fluid Phase Equilibria 2004, 221, 73–82. [Google Scholar] [CrossRef]
- Langham, J.V.; O’Brien, R.A.; Davis, J.H., Jr.; West, K.N. Solubility of CO2 and N2O in an imidazolium-based lipidic ionic liquid. J. Phys. Chem. B 2016, 120, 10524–10530. [Google Scholar] [CrossRef]
- Ramdin, M.; Vlugt, T.J.; de Loos, T.W. Solubility of CO2 in the ionic liquids [TBMN][MeSO4] and [TBMP][MeSO4]. J. Chem. Eng. Data 2012, 57, 2275–2280. [Google Scholar] [CrossRef]
- Okuniewska, P.; Ramjugernath, D.; Naidoo, P.; Domańska, U. Solubility of ionic liquids in 2-phenylethanol (PEA) and water. Fluid Phase Equilibria 2014, 376, 55–63. [Google Scholar] [CrossRef]
- Neves, C.M.; Rodrigues, A.R.; Kurnia, K.A.; Esperança, J.M.; Freire, M.G.; Coutinho, J.A. Solubility of non-aromatic hexafluorophosphate-based salts and ionic liquids in water determined by electrical conductivity. Fluid Phase Equilibria 2013, 358, 50–55. [Google Scholar] [CrossRef]
- Domańska, U.; Casás, L.M. Solubility of Phosphonium Ionic Liquid in Alcohols, Benzene, and Alkylbenzenes. J. Phys. Chem. B 2007, 111, 4109–4115. [Google Scholar] [CrossRef]
- Sarkar, A.; Sinha, B. Solution thermodynamics of aqueous nicotinic acid solutions in presence of tetrabutylammonium hydrogen sulphate. J. Serb. Chem. Soc. 2013, 78, 1225–1240. [Google Scholar] [CrossRef]
- Sarkar, A.; Mishra, D.K.; Sinha, B. Solution thermophysics of l-ascorbic acid in aqueous tetrabutylammonium hydrogen sulfate. J. Solut. Chem. 2016, 45, 560–573. [Google Scholar] [CrossRef]
- Katsuta, S.; Shiozawa, Y.; Imai, K.; Kudo, Y.; Takeda, Y. Stability of ion pairs of bis (trifluoromethanesulfonyl) amide-based ionic liquids in dichloromethane. J. Chem. Eng. Data 2010, 55, 1588–1593. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Meakin, P.; Amini, N.; Forsyth, M. Structural studies of ambient temperature plastic crystal ion conductors. J. Phys. Condens. Matter 2001, 13, 8257–8267. [Google Scholar] [CrossRef]
- Zhang, Q.-G.; Xue, F.; Tong, J.; Guan, W.; Wang, B. Studies on volumetric properties of concentrated aqueous solutions of the ionic liquid BMIBF 4. J. Solut. Chem. 2006, 35, 297–309. [Google Scholar] [CrossRef]
- Studzińska, S.; Sprynskyy, M.; Buszewski, B. Study of sorption kinetics of some ionic liquids on different soil types. Chemosphere 2008, 71, 2121–2128. [Google Scholar] [CrossRef]
- Yang, J.-Z.; Tong, J.; Li, J.-B. Study of the volumetric properties of the aqueous ionic liquid 1-methyl-3-pentylimidazolium tetrafluoroborate. J. Solut. Chem. 2007, 36, 573–582. [Google Scholar] [CrossRef]
- Law, G.; Watson, P.R. Surface tension measurements of N-alkylimidazolium ionic liquids. Langmuir 2001, 17, 6138–6141. [Google Scholar] [CrossRef]
- Pernak, J.; Wasiński, K.; Praczyk, T.; Nawrot, J.; Cieniecka-Rosłonkiewicz, A.; Walkiewicz, F.; Materna, K. Sweet ionic liquids-cyclamates: Synthesis, properties, and application as feeding deterrents. Sci. China Chem. 2012, 55, 1532–1541. [Google Scholar] [CrossRef]
- Carter, E.B.; Culver, S.L.; Fox, P.A.; Goode, R.D.; Ntai, I.; Tickell, M.D.; Traylor, R.K.; Hoffman, N.W.; Davis, J.H., Jr. Sweet success: Ionic liquids derived from non-nutritive sweeteners. Chem. Commun. 2004, 630–631. [Google Scholar] [CrossRef]
- Mirzaei, Y.R.; Twamley, B.; Shreeve, J.M. Syntheses of 1-alkyl-1,2,4-triazoles and the formation of quaternary 1-alkyl-4-polyfluoroalkyl-1, 2, 4-triazolium salts leading to ionic liquids. J. Org. Chem. 2002, 67, 9340–9345. [Google Scholar] [CrossRef]
- Ye, C.; Shreeve, J.M. Syntheses of very dense halogenated liquids. J. Org. Chem. 2004, 69, 6511–6513. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hagiwara, R.; Yoshida, R.; Ito, Y.; Mazej, Z.; Benkič, P.; Žemva, B.; Tamada, O.; Yoshino, H.; Matsubara, S. Syntheses, structures and properties of 1-ethyl-3-methylimidazolium salts of fluorocomplex anions. Dalton Trans. 2004, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Pedro, S.N.; Gomes, J.; Sintra, T.E.; Ventura, S.P.; Coutinho, J.A.; Freire, M.G.; Mohamadou, A. Synthesis and characterization of analogues of glycine-betaine ionic liquids and their use in the formation of aqueous biphasic systems. Fluid Phase Equilibria 2019, 494, 239–245. [Google Scholar] [CrossRef]
- Zhao, D.; Fei, Z.; Scopelliti, R.; Dyson, P.J. Synthesis and characterization of ionic liquids incorporating the nitrile functionality. Inorg. Chem. 2004, 43, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Suarez, P.A.Z.; Einloft, S.; Dullius, J.E.L.; de Souza, R.F.; Dupont, J. Synthesis and physical-chemical properties of ionic liquids based on 1-n-butyl-3-methylimidazolium cation. J. Chim. Phys. 1998, 95, 1626–1639. [Google Scholar] [CrossRef]
- Engin Özdil, S.; Akbaş, H.; Boz, M. Synthesis and physicochemical properties of double-chain cationic surfactants. J. Chem. Eng. Data 2016, 61, 142–150. [Google Scholar] [CrossRef]
- Quek, S.K.; Lyapkalo, I.M.; Huynh, H.V. Synthesis and properties of N, N′-dialkylimidazolium bis (nonafluorobutane-1-sulfonyl) imides: A new subfamily of ionic liquids. Tetrahedron 2006, 62, 3137–3145. [Google Scholar] [CrossRef]
- Papaiconomou, N.; Yakelis, N.; Salminen, J.; Bergman, R.; Prausnitz, J.M. Synthesis and properties of seven ionic liquids containing 1-methyl-3-octylimidazolium or 1-butyl-4-methylpyridinium cations. J. Chem. Eng. Data 2006, 51, 1389–1393. [Google Scholar] [CrossRef]
- Dong, L.; Zheng, D.X.; Wei, Z.; Wu, X.H. Synthesis of 1, 3-dimethylimidazolium chloride and volumetric property investigations of its aqueous solution. Int. J. Thermophys. 2009, 30, 1480. [Google Scholar] [CrossRef]
- Xiao, J.-C.; Shreeve, J.M. Synthesis of 2, 2′-biimidazolium-based ionic liquids: Use as a new reaction medium and ligand for palladium-catalyzed suzuki cross-coupling reactions. J. Org. Chem. 2005, 70, 3072–3078. [Google Scholar] [CrossRef]
- Bao, W.; Wang, Z.; Li, Y. Synthesis of chiral ionic liquids from natural amino acids. J. Org. Chem. 2003, 68, 591–593. [Google Scholar] [CrossRef]
- Tseng, M.-C.; Liang, Y.-M.; Chu, Y.-H. Synthesis of fused tetrahydro-β-carbolinequinoxalinones in 1-n-butyl-2, 3-dimethylimidazolium bis (trifluoromethylsulfonyl) imide ([bdmim][Tf2N]) and 1-n-butyl-2, 3-dimethylimidazolium perfluorobutylsulfonate ([bdmim][PFBuSO3]) ionic liquids. Tetrahedron Lett. 2005, 46, 6131–6136. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Zhang, Y.; Bao, W. Synthesis of new chiral ionic liquids from natural acids and their applications in enantioselective Michael addition. Tetrahedron Lett. 2005, 46, 4657–4660. [Google Scholar] [CrossRef]
- Dorn, S.; Pfeifer, A.; Schlufter, K.; Heinze, T. Synthesis of water-soluble cellulose esters applying carboxylic acid imidazolides. Polym. Bull. 2010, 64, 845–854. [Google Scholar] [CrossRef]
- Cieniecka-Rosłonkiewicz, A.; Pernak, J.; Kubis-Feder, J.; Ramani, A.; Robertson, A.J.; Seddon, K.R. Synthesis, anti-microbial activities and anti-electrostatic properties of phosphonium-based ionic liquids. Green Chem. 2005, 7, 855–862. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Zha, M.; Zhang, J.; Wang, S.-S. Synthesis, characterization and properties of geminal imidazolium ionic liquids. Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 201–205. [Google Scholar] [CrossRef]
- Busi, S.; Lahtinen, M.; Valkonen, J.; Rissanen, K. Synthesis, characterization and thermal behavior of nine new R2R2′ N+A−-type quaternary ammonium tetrafluoroborate or hexafluorophosphate salts prepared by metathesis from analogous halide salts. J. Mol. Struct. 2008, 875, 549–559. [Google Scholar] [CrossRef]
- Busi, S.; Lahtinen, M.; Mansikkamäki, H.; Valkonen, J.; Rissanen, K. Synthesis, characterization and thermal properties of small R2R′ 2N+ X−-type quaternary ammonium halides. J. Solid State Chem. 2005, 178, 1722–1737. [Google Scholar] [CrossRef]
- Kölle, P.; Dronskowski, R. Synthesis, crystal structures and electrical conductivities of the ionic liquid compounds butyldimethylimidazolium tetrafluoroborate, hexafluorophosphate and hexafluoroantimonate. Eur. J. Inorg. Chem. 2004, 2004, 2313–2320. [Google Scholar] [CrossRef]
- Marciniak, A.; Królikowski, M. Ternary (liquid+ liquid) equilibria of {trifluorotris (perfluoroethyl) phosphate based ionic liquids+ thiophene+ heptane}. J. Chem. Thermodyn. 2012, 49, 154–158. [Google Scholar] [CrossRef]
- Pringle, J.M.; Golding, J.; Baranyai, K.; Forsyth, C.M.; Deacon, G.B.; Scott, J.L.; MacFarlane, D.R. The effect of anion fluorination in ionic liquids—physical properties of a range of bis (methanesulfonyl) amide salts. New J. Chem. 2003, 27, 1504–1510. [Google Scholar] [CrossRef] [Green Version]
- Brauer, U.G.; Andreah, T.; Miller, K.M. The effect of counteranion on the physicochemical and thermal properties of 4-methyl-1-propyl-1, 2, 4-triazolium ionic liquids. J. Mol. Liq. 2015, 210, 286–292. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Almeida, H.F.; Freire, M.G.; Lopes-da-Silva, J.A.; Coutinho, J.A.; Santos, L.M. The effect of n vs. iso isomerization on the thermophysical properties of aromatic and non-aromatic ionic liquids. Fluid Phase Equilibria 2016, 423, 190–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagno, A.; Butts, C.; Chiappe, C.; D’Amico, F.; Lord, J.C.; Pieraccini, D.; Rastrelli, F. The effect of the anion on the physical properties of trihalide-based N, N-dialkylimidazolium ionic liquids. Org. Biomol. Chem. 2005, 3, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Krolikowska, M.; Zawadzki, M.; Skonieczny, M. The influence of bromide-based ionic liquids on solubility of {LiBr (1)+ water (2)} system. Experimental (solid+ liquid) phase equilibrium data. Part 2. J. Mol. Liq. 2018, 265, 316–326. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. J. Chem. Soc. Dalton Trans. 1999, 13, 2133–2140. [Google Scholar] [CrossRef]
- Bogel-Łukasik, R.; Matkowska, D.; Zakrzewska, M.E.; Bogel-Łukasik, E.; Hofman, T. The phase envelopes of alternative solvents (ionic liquid, CO2) and building blocks of biomass origin (lactic acid, propionic acid). Fluid Phase Equilibria 2010, 295, 177–185. [Google Scholar] [CrossRef]
- Fuller, J.; Carlin, R.T.; Osteryoung, R.A. The room temperature ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate: Electrochemical couples and physical properties. J. Electrochem. Soc. 1997, 144, 3881. [Google Scholar] [CrossRef]
- Alves, M.B.; Umpierre, A.P.; Santos, V.O., Jr.; Soares, V.C.D.; Dupont, J.; Rubim, J.C.; Suarez, P.A.Z. The use of Differential Scanning Calorimetry (DSC) to characterize phase diagrams of ionic mixtures of 1-n-butyl-3-methylimidazolium chloride and niobium chloride or zinc chloride. Thermochim. Acta 2010, 502, 20–23. [Google Scholar] [CrossRef]
- Gómez, E.; Calvar, N.; Domínguez, Á.; Macedo, E.A. Thermal analysis and heat capacities of 1-Alkyl-3-methylimidazolium ionic liquids with NTf2−, TFO−, and DCA− anions. Ind. Eng. Chem. Res. 2013, 52, 2103–2110. [Google Scholar] [CrossRef]
- Calvar, N.; Gómez, E.; Macedo, E.A.; Domínguez, Á. Thermal analysis and heat capacities of pyridinium and imidazolium ionic liquids. Thermochim. Acta 2013, 565, 178–182. [Google Scholar] [CrossRef]
- Mutch, M.L.; Wilkes, J.S. Thermal analysis of 1-ethyl-3-methylimidazolium tetrafluoroborate molten salt. ECS Proc. Vol. 1998, 1998, 254. [Google Scholar] [CrossRef]
- Nemoto, F.; Kofu, M.; Yamamuro, O. Thermal and structural studies of imidazolium-based ionic liquids with and without liquid-crystalline phases: The origin of nanostructure. J. Phys. Chem. B 2015, 119, 5028–5034. [Google Scholar] [CrossRef]
- Gómez, E.; Calvar, N.; Domínguez, Á.; Macedo, E.A. Thermal behavior and heat capacities of pyrrolidinium-based ionic liquids by DSC. Fluid Phase Equilibria 2018, 470, 51–59. [Google Scholar] [CrossRef]
- Stolarska, O.; Soto, A.; Rodríguez, H.; Smiglak, M. Thermal behaviour of mixtures of 1-alkylpyridinium halides with and without a common ion. J. Mol. Liq. 2018, 268, 781–790. [Google Scholar] [CrossRef]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Efimova, A.; Hubrig, G.; Schmidt, P. Thermal stability and crystallization behavior of imidazolium halide ionic liquids. Thermochim. Acta 2013, 573, 162–169. [Google Scholar] [CrossRef]
- Monteiro, B.; Maria, L.; Cruz, A.; Carretas, J.M.; Marçalo, J.; Leal, J.P. Thermal stability and specific heats of coordinating ionic liquids. Thermochim. Acta 2020, 684, 178482. [Google Scholar] [CrossRef]
- Guan, W.; Yang, J.-Z.; Li, L.; Wang, H.; Zhang, Q.-G. Thermo-chemical properties of aqueous solution containing ionic liquids: 1. The heat of reaction mixed 1-methyl-3-butylimidazolium chloride with InCl3. Fluid Phase Equilibria 2006, 239, 161–165. [Google Scholar] [CrossRef]
- Jagadeeswara Rao, C.; Venkata Krishnan, R.; Venkatesan, K.; Nagarajan, K.; Srinivasan, T. Thermochemical properties of some bis (trifluoromethyl-sulfonyl) imide based room temperature ionic liquids. J. Therm. Anal. Calorim. 2009, 97, 937–943. [Google Scholar] [CrossRef]
- Tong, B.; Liu, Q.-S.; Tan, Z.-C.; Welz-Biermann, U. Thermochemistry of alkyl pyridinium bromide ionic liquids: Calorimetric measurements and calculations. J. Phys. Chem. A 2010, 114, 3782–3787. [Google Scholar] [CrossRef] [PubMed]
- Van Valkenburg, M.E.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Tan, Z.; Xu, F.; Lv, X.; Zeng, J.; Sawada, Y. Thermodynamic investigation of room temperature ionic liquid: Heat capacity and thermodynamic functions of BPBF4. J. Therm. Anal. Calorim. 2007, 89, 289–294. [Google Scholar] [CrossRef]
- Domańska, U.; Żołek-Tryznowska, Z.; Królikowski, M. Thermodynamic Phase Behavior of Ionic Liquids. J. Chem. Eng. Data 2007, 52, 1872–1880. [Google Scholar] [CrossRef]
- Archer, D.G. Thermodynamic Properties of 1-hexyl-3-methylimidazolium Bis (trifluoromethylsulfony) Imide; US Department of Commerce, Technology Administration, National Institute of Standard and Technology: Gaithersburg, MD, USA, 2006.
- Allen, M.; Evans, D.F.; Lumry, R. Thermodynamic properties of the ethylammonium nitrate+ water system: Partial molar volumes, heat capacities, and expansivities. J. Solut. Chem. 1985, 14, 549–560. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowski, M.; Acree, W.E., Jr. Thermodynamics and activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-butyl-1-methylpyrrolidinium tetracyanoborate. J. Chem. Thermodyn. 2011, 43, 1810–1817. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M. Thermodynamics and limiting activity coefficients measurements for organic solutes and water in the ionic liquid 1-dodecyl-3-methylimidzolium bis (trifluoromethylsulfonyl) imide. J. Chem. Thermodyn. 2016, 103, 76–85. [Google Scholar] [CrossRef]
- Sakizadeh, K.; Olson, L.P.; Cowdery-Corvan, P.J.; Ishida, T.; Whitcomb, D.R. Thermographic Materials Containing Ionic Liquids. U.S. Patent US7163786B1, 16 January 2007. [Google Scholar]
- García-Andreu, M.; Castro, M.; Gascón, I.; Lafuente, C. Thermophysical characterization of 1-ethylpyridinium triflate and comparison with similar ionic liquids. J. Chem. Thermodyn. 2016, 103, 395–402. [Google Scholar] [CrossRef]
- Neves, C.M.; Batista, M.L.; Cláudio, A.F.M.; Santos, L.M.; Marrucho, I.M.; Freire, M.G.; Coutinho, J.A. Thermophysical properties and water saturation of [PF6]-based ionic liquids. J. Chem. Eng. Data 2010, 55, 5065–5073. [Google Scholar] [CrossRef]
- Chhotaray, P.K.; Gardas, R.L. Thermophysical properties of ammonium and hydroxylammonium protic ionic liquids. J. Chem. Thermodyn. 2014, 72, 117–124. [Google Scholar] [CrossRef]
- Parajo, J.J.; Villanueva, M.; Troncoso, J.; Salgado, J. Thermophysical properties of choline and pyridinium based ionic liquids as advanced materials for energy applications. J. Chem. Thermodyn. 2020, 141, 105947. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W.; Liu, J.; Li, M.; Yang, B. Thermophysical properties of dicationic imidazolium-based ionic compounds for thermal storage. J. Mol. Liq. 2019, 282, 474–483. [Google Scholar] [CrossRef]
- Govinda, V.; Attri, P.; Venkatesu, P.; Venkateswarlu, P. Thermophysical properties of dimethylsulfoxide with ionic liquids at various temperatures. Fluid Phase Equilibria 2011, 304, 35–43. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.; Brennecke, J.F. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Tao, R.; Tamas, G.; Xue, L.; Simon, S.L.; Quitevis, E.L. Thermophysical properties of imidazolium-based ionic liquids: The effect of aliphatic versus aromatic functionality. J. Chem. Eng. Data 2014, 59, 2717–2724. [Google Scholar] [CrossRef]
- Murray, S.M.; Zimlich, T.K.; Mirjafari, A.; O’Brien, R.A.; Davis, J.H., Jr.; West, K.N. Thermophysical properties of imidazolium-based lipidic ionic liquids. J. Chem. Eng. Data 2013, 58, 1516–1522. [Google Scholar] [CrossRef]
- Sudholter, E.J.R.; Engberts, J.B.F.N.; de Jeu, W.H. Thermotropic Liquid-Crystallhe Behavior of Some Single- and Double-Chalned PyrMinium Amphlphlles. J. Phys. Chem. 1981, 86, 1908–1913. [Google Scholar] [CrossRef]
- Zarrougui, R.; Dhahbi, M.; Lemordant, D. Transport and thermodynamic properties of ethylammonium nitrate–water binary mixtures: Effect of temperature and composition. J. Solut. Chem. 2015, 44, 686–702. [Google Scholar] [CrossRef]
- Gerhard, D.; Alpaslan, S.C.; Gores, H.J.; Uerdingen, M.; Wasserscheid, P. Trialkylsulfonium dicyanamides-a new family of ionic liquids with very low viscosities. Chem. Commun. 2005, 40, 5080–5082. [Google Scholar] [CrossRef]
- Shmukler, L.E.; Gruzdev, M.S.; Kudryakova, N.O.; Fadeeva, Y.A.; Kolker, A.M.; Safonova, L.P. Triethylammonium-based protic ionic liquids with sulfonic acids: Phase behavior and electrochemistry. J. Mol. Liq. 2018, 266, 139–146. [Google Scholar] [CrossRef]
- Grishina, E.P.; Ramenskaya, L.M.; Gruzdev, M.S.; Kraeva, O.V. Water effect on physicochemical properties of 1-butyl-3-methylimidazolium based ionic liquids with inorganic anions. J. Mol. Liq. 2013, 177, 267–272. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2019; van der Walt, S., Millman, J., Eds.; 2010; pp. 56–61. Available online: https://conference.scipy.org/proceedings/scipy2010/ (accessed on 14 March 2021).
- Waskom, M. Seaborn: Statistical Data Visualization. GitHub Repository; Zenodo. 2021. Available online: https://github.com/seaborn (accessed on 14 March 2021).
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Chollet, F. Keras; GitHub Repository. 2015. Available online: https://github.com/keras-team/keras (accessed on 14 March 2021).
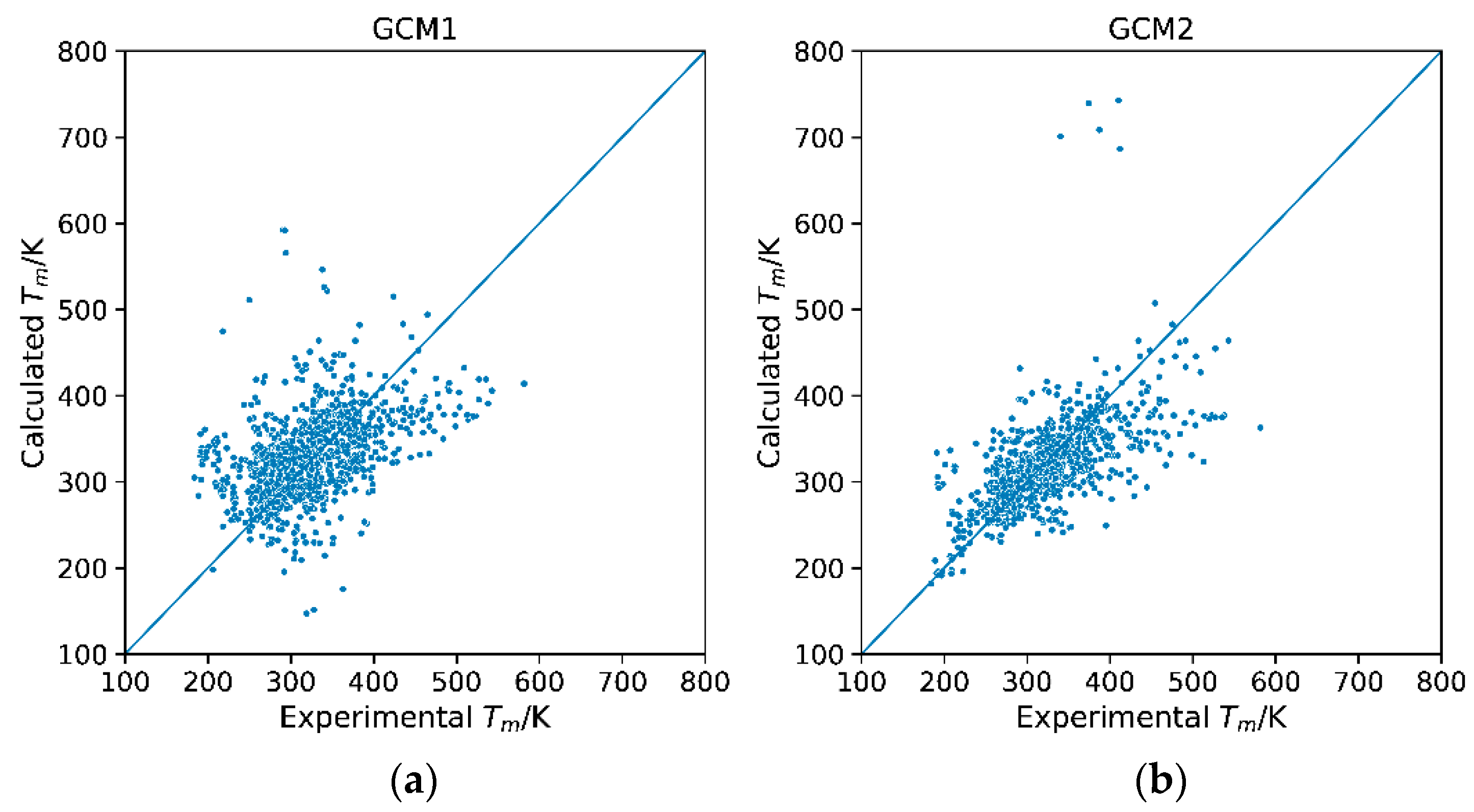








| Work | Type of Model | Applicability | Number of ILs Used | R2 | AARD |
|---|---|---|---|---|---|
| Huo et. al. [42] | GCM | Imidazolium/Benzimidazolium | 190 | 0.8984 | 5.86% |
| Aguirre et. al. [43] | GCM | Diverse | 136 | 7.8% | |
| Chen et. al. [44] | GCM | Diverse | 111 | 4.7% | |
| Katritzky et. al. [45] | QSPR | Imidazolium/Benzimidazolium bromides | 149 | 0.7442/0.7517/0.6899 | |
| Katritzky et. al. [19] | QSPR | Pyridinium bromides | 126 | 0.788/0.713 | |
| Trohalaki and Pachter [46] | QSPR | Triazolium bromides, Triazolium nitrates | 26 | 0.8730/0.8390 | |
| Farahani et. al. [47] | QSPR | Diverse | 805 | 0.721 | 7.3% |
| Bini et. al. [48] | RNN | Pyridinium bromides | 126 | 0.872 | |
| Carrera and Aires-de-Sousa [49] | QSPR | Pyridinium bromides | 126 | 0.933 | |
| Zhang et. al. [50] | QSPR | Imidazolium tetrafluoroborates, Imidazolium hexafluorophos-phates | 41 | 0.776/0.842 | |
| Eike et. al. [51] | QSPR | Ammonium Bromides | 75 | 0.790/0.775 | |
| Varnek et. al. [52] | QSPR | Diverse Bromides | 717 | 0.61 | |
| López-Martin et. al. [53] | QSPR | Imidazolium | 62 | 0.8690 | |
| Yan et. al. [54] | QSPR | Imidazolium bromides, Imidazolium chlorides | 50 | 0.8900 | |
| Lazzús [20] | GCM | Diverse | 400 | 0.8841 | 7.07% |
| Gharagheizi et. al. [21] | GCM | Diverse | 799 | 0.811 | 5.82% |
| Group, i | ΔTm,i/K | |
|---|---|---|
| T0 | 210.97 | |
| Imidazolium |  | 6.00 |
| Pyridinium |  | 59.09 |
| Pyrrolidinium |  | 50.66 |
| Pyrrolidinonium |  | 0.07 |
| Piperidinium |  | 48.26 |
| Morpholinium |  | 39.74 |
| Ammonium |  | −4.38 |
| Sulfonium |  | 5.00 |
| Phosphonium |  | −10.01 |
| Triazolium |  | 31.15 |
| Pyrazolium |  | 0.11 |
| Thiazolium |  | −0.19 |
| -H | 24.52 | |
| -CH3 | 30.98 | |
| -CH2- | −0.22 | |
| -CH< | −8.28 | |
| -OH | 44.86 | |
| -O- | −18.99 | |
| >C< | 12.71 | |
| -COO | 9.88 | |
| -CN | 40.01 | |
| -NH2 | 35.07 | |
| -F | 22.38 | |
| -Br | 22.24 | |
| -Cl | 0.11 | |
| -SO2- | 5.95 | |
| -Benzyl | 52.26 | |
| -Vinyl | 0.86 | |
| -CF2- | 10.05 | |
| -CF3 | 30.22 | |
| -SF5 | −5.79 | |
| >P = O | −36.95 | |
| Group, i | ΔTm,i/K |
|---|---|
| -CH3 | 1.55 |
| -CH2- | −2.71 |
| >CH- | 1.06 |
| >C< | −0.77 |
| >CO | −7.63 |
| -COO- | −20.94 |
| -HCOO | −24.97 |
| -OH | −0.72 |
| -O- [-O] | 2.67 |
| -CN | −16.50 |
| -N- [>N-] | −3.87 |
| -NO3 | 9.21 |
| -S- | 0.42 |
| -SO2- | −4.82 |
| -CF3 | −10.06 |
| -CF2- | 4.27 |
| -F | −4.74 |
| -Cl | 26.16 |
| -Br | 50.64 |
| -P | 4.77 |
| -B | −11.05 |
| -I | 31.77 |
| -Al | −5.92 |
| -As | 6.27 |
| -Nb | −2.74 |
| -Ta | 0.25 |
| -Sb | 12.00 |
| -Sn | 0.23 |
| -W | −0.28 |
| -CB11H11- (carborane) | 18.88 |
| -CB11H12 (carborane) | 18.19 |
| -CB11H6 (carborane) | −16.68 |
| CH- (ring) | 0.18 |
| =C< (ring) | 17.02 |
| >CO (ring) | 3.72 |
| -O- (ring) | 3.34 |
| PF6 | 28.32 |
| BF4 | 1.18 |
| B(CN)4 | −8.80 |
| Oleate | −12.14 |
| Phosphate | 18.23 |
| GCM1 | ||||
| Type | Group, i | ni | ∆Tm,i/K | ni∆Tm,i/K |
| Cation | Imidazolium | 1 | 22.837 | 22.837 |
| CH3 | 2 | −4.384 | −8.768 | |
| CH2 | 3 | −3.759 | −11.277 | |
| H | 1 | −20.98 | −20.98 | |
| Anion | –N– [>N–] | 1 | −113.15 | −113.15 |
| SO2 | 2 | 114.17 | 228.34 | |
| CF3 | 2 | −51.844 | −103.688 | |
| T0 | 288.7 | |||
| Tm/K = T0 + ∑ ni∆Tm,i | 282.014 | |||
| GCM2 | ||||
| Type | Group, i | ni | ∆Tm,i/K | ni∆Tm,i/K |
| Cation | N+ | 1 | 7.8678 | 7.8678 |
| CH2R2 | 2 | 1.6214 | 3.2428 | |
| CH3X | 1 | 12.4075 | 12.4075 | |
| CH2RX | 1 | 6.5888 | 6.5888 | |
| R-CH-X | 1 | −45.1417 | −45.1417 | |
| R-CH..X | 1 | 18.6936 | 18.6936 | |
| Ha attached to C° (sp3) no X attached to next C | 5 | −1.3355 | −6.6775 | |
| Anion | Sulfones (SO2) | 2 | −35.6122 | −71.2244 |
| Total number of Ns, Os, and Fs in the molecule, excluding N with a formal positive charge, higher oxidation states, and pyrrolyl form of N | 11 | 8.2032 | 90.2352 | |
| CX4 | 2 | −30.3021 | −60.6042 | |
| Fa attached to C2 (sp2)-C4 (sp2)/ C1 (sp)/C4 (sp3)/X | 6 | 0.7619 | 4.5714 | |
| R-SO2-R | 2 | 22.9432 | 45.8864 | |
| T0 | 264.29 | |||
| Tm/K = T0 + ∑ ni∆Tm,i | 270.136 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mital, D.K.; Nancarrow, P.; Zeinab, S.; Jabbar, N.A.; Ibrahim, T.H.; Khamis, M.I.; Taha, A. Group Contribution Estimation of Ionic Liquid Melting Points: Critical Evaluation and Refinement of Existing Models. Molecules 2021, 26, 2454. https://doi.org/10.3390/molecules26092454
Mital DK, Nancarrow P, Zeinab S, Jabbar NA, Ibrahim TH, Khamis MI, Taha A. Group Contribution Estimation of Ionic Liquid Melting Points: Critical Evaluation and Refinement of Existing Models. Molecules. 2021; 26(9):2454. https://doi.org/10.3390/molecules26092454
Chicago/Turabian StyleMital, Dhruve Kumar, Paul Nancarrow, Samira Zeinab, Nabil Abdel Jabbar, Taleb Hassan Ibrahim, Mustafa I. Khamis, and Alnoman Taha. 2021. "Group Contribution Estimation of Ionic Liquid Melting Points: Critical Evaluation and Refinement of Existing Models" Molecules 26, no. 9: 2454. https://doi.org/10.3390/molecules26092454
APA StyleMital, D. K., Nancarrow, P., Zeinab, S., Jabbar, N. A., Ibrahim, T. H., Khamis, M. I., & Taha, A. (2021). Group Contribution Estimation of Ionic Liquid Melting Points: Critical Evaluation and Refinement of Existing Models. Molecules, 26(9), 2454. https://doi.org/10.3390/molecules26092454









