Graphene-Based Nanomaterials as the Cathode for Lithium-Sulfur Batteries
Abstract
1. Introduction
- The insulation of sulfur reduces the electron transfer rate (conductivity: 5 × 10−30 S·cm−1 at 25 °C) [14].
- The volume expansion of the sulfur cathode material after multiple electrode reactions destroys the electrode structure.
- The dissolution of soluble lithium polysulfide triggers a “shuttle effect”, which causes energy loss and low battery life.
2. Graphene as the Positive Electrode Skeleton
2.1. The Interactions between Sulfur and Graphene
2.2. Configurations of Pure Graphene and Sulfur
3. Heteroatom Doped Graphene
3.1. Single-Atom Doped Graphene
3.1.1. Nitrogen Doping
3.1.2. Boron Doping
3.2. Dual Doped Graphene
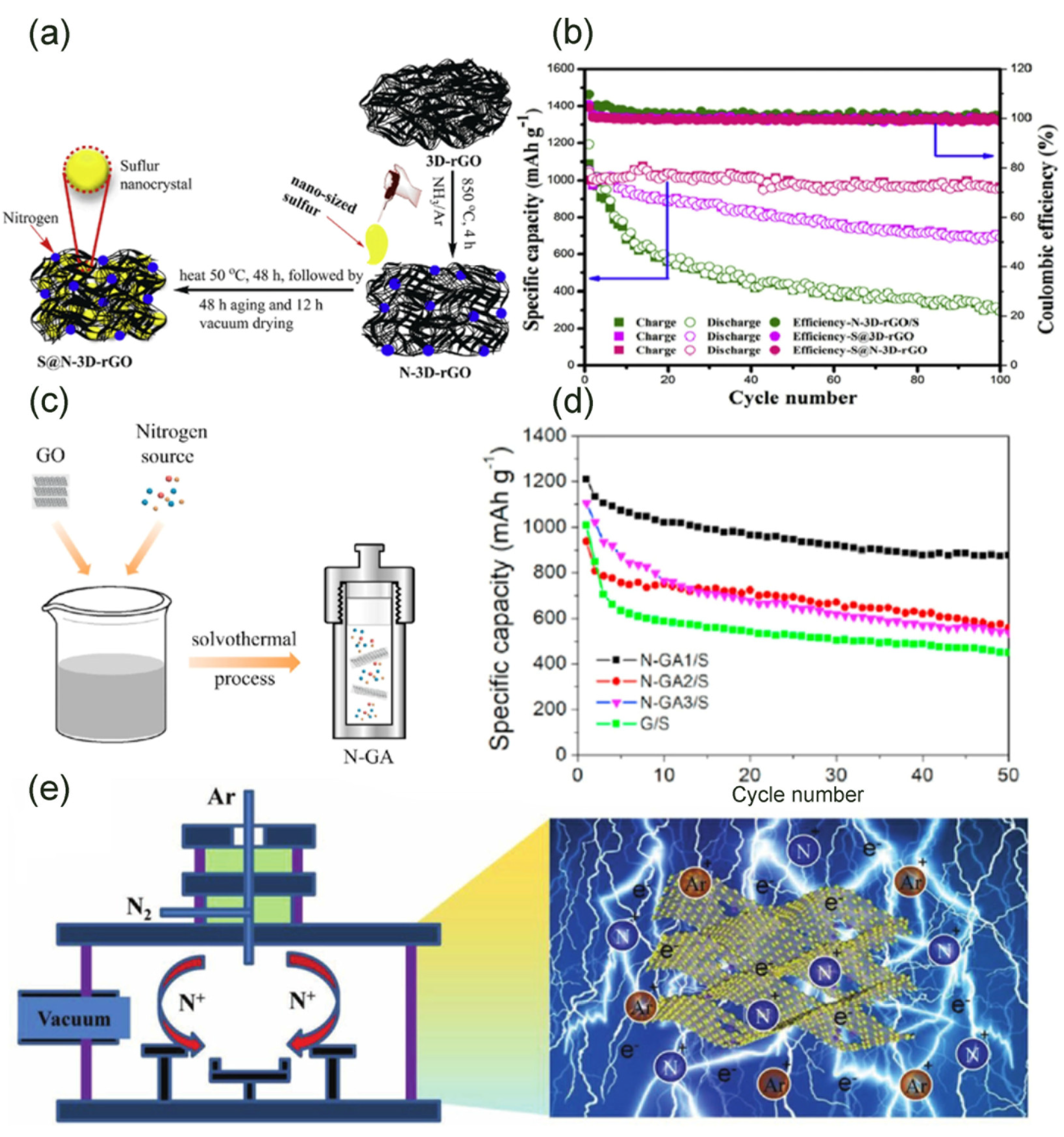
3.2.1. N/S Dual Doped
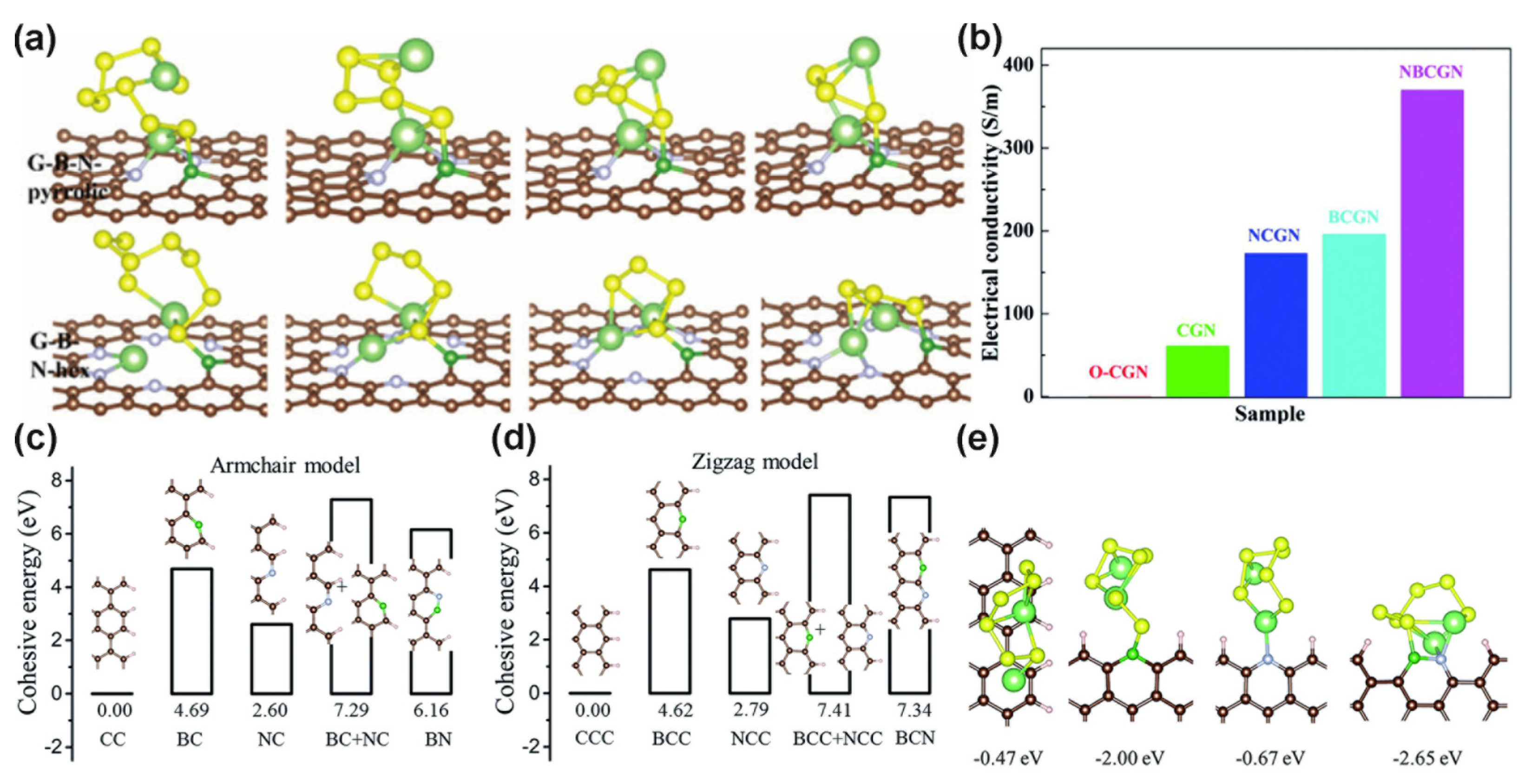
3.2.2. N/B Dual Doped
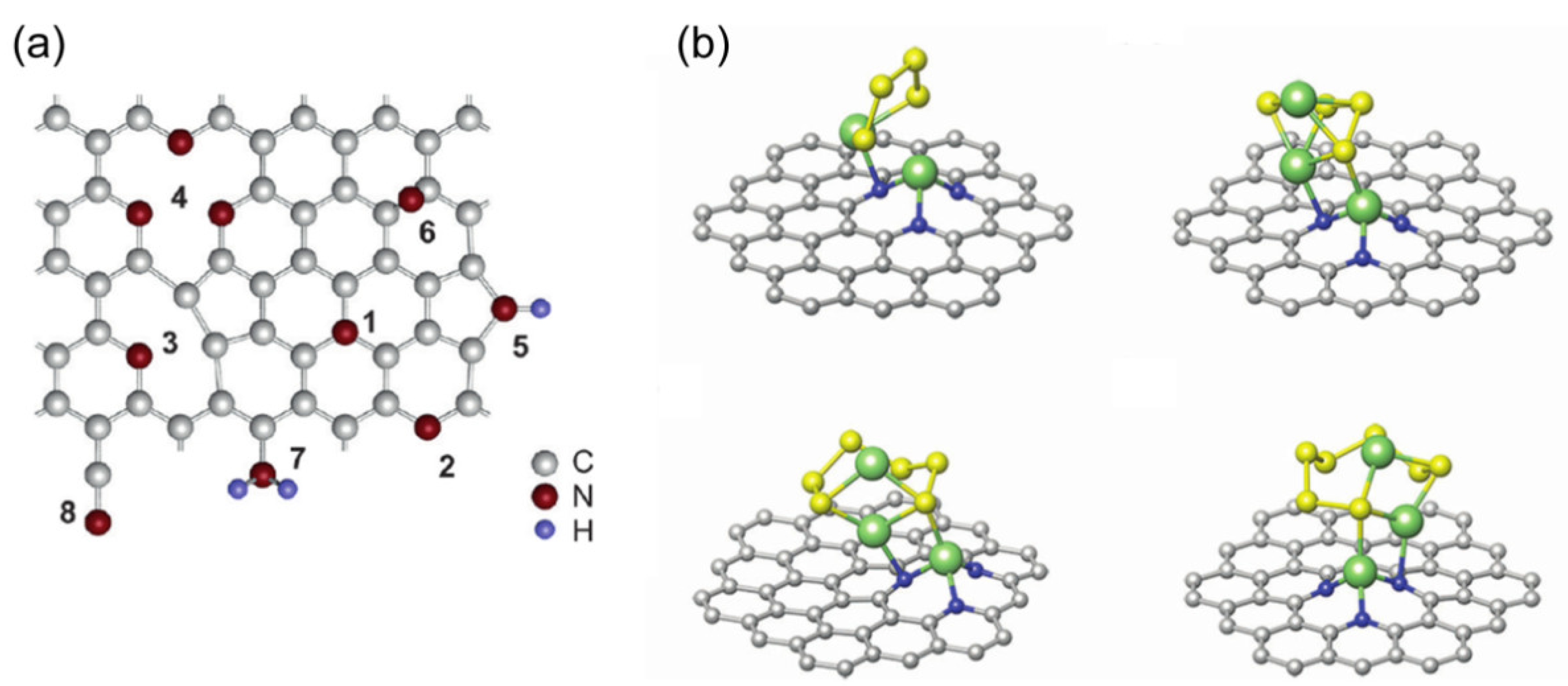
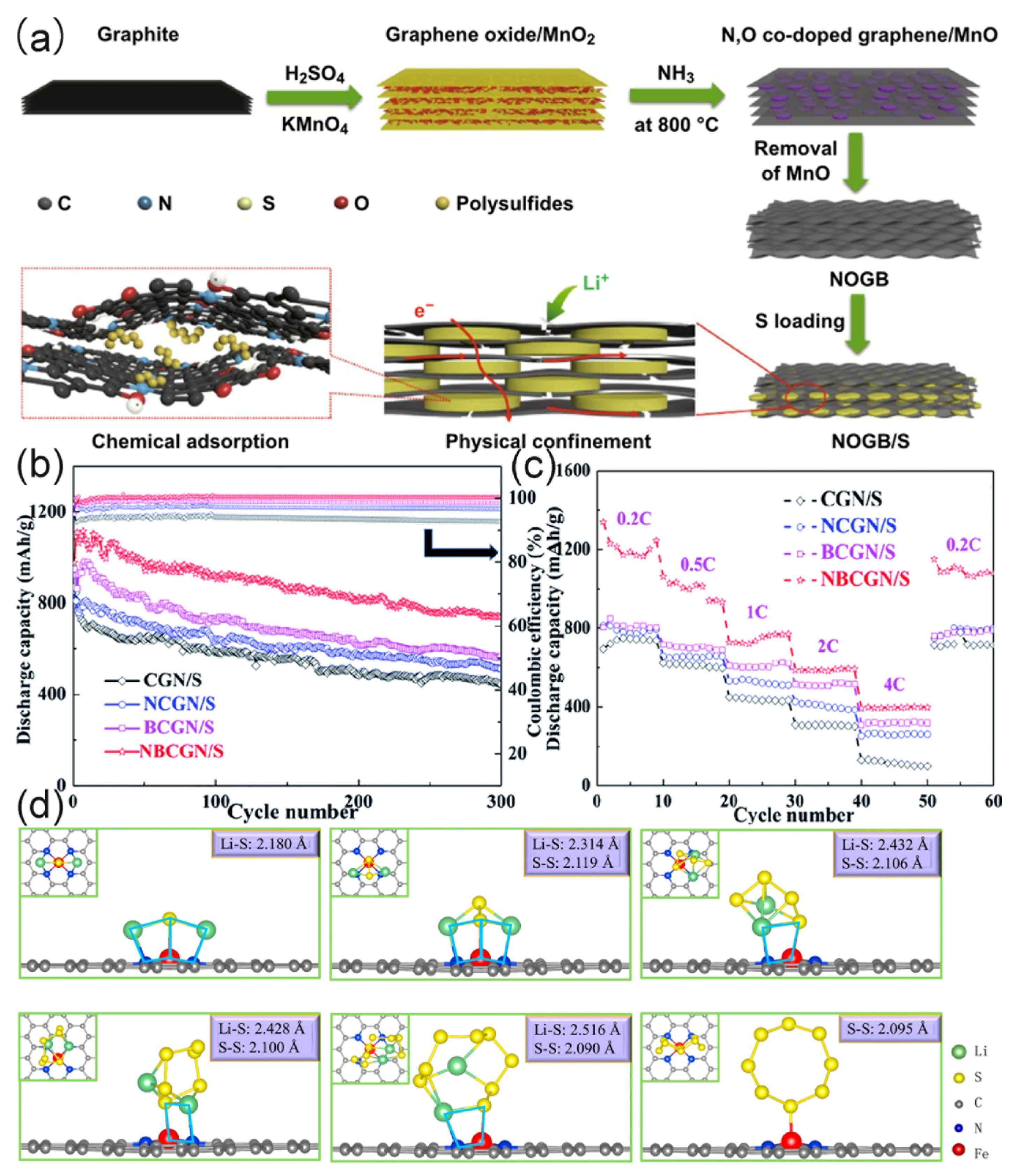
3.2.3. N/O Dual Doped
3.2.4. Graphene Co-Doped with Single-Atom Metal and N
4. Graphene-Based Composite Materials
4.1. Metal Compound/Graphene Composite
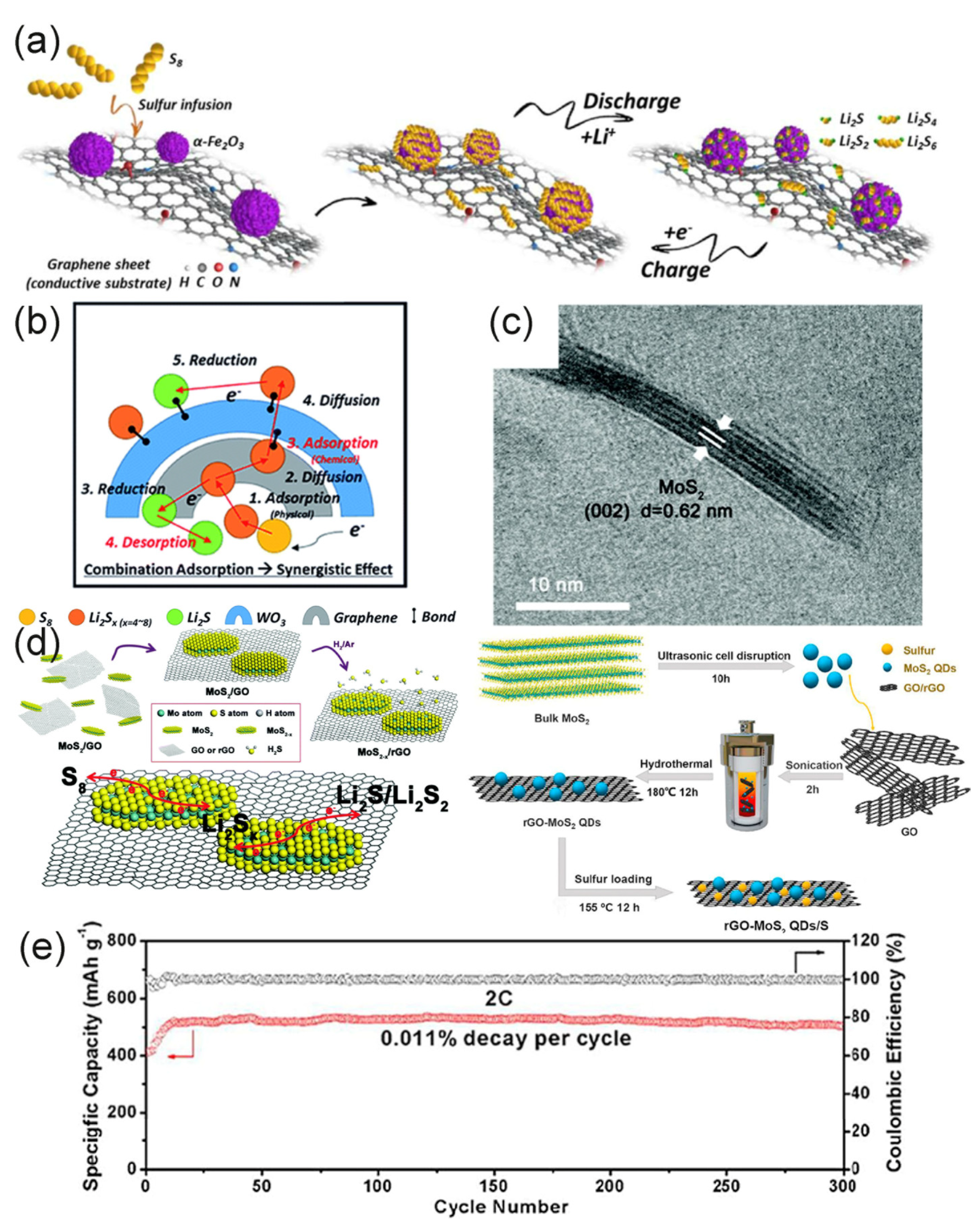
4.1.1. Metal Oxide
4.1.2. Metal Sulfide
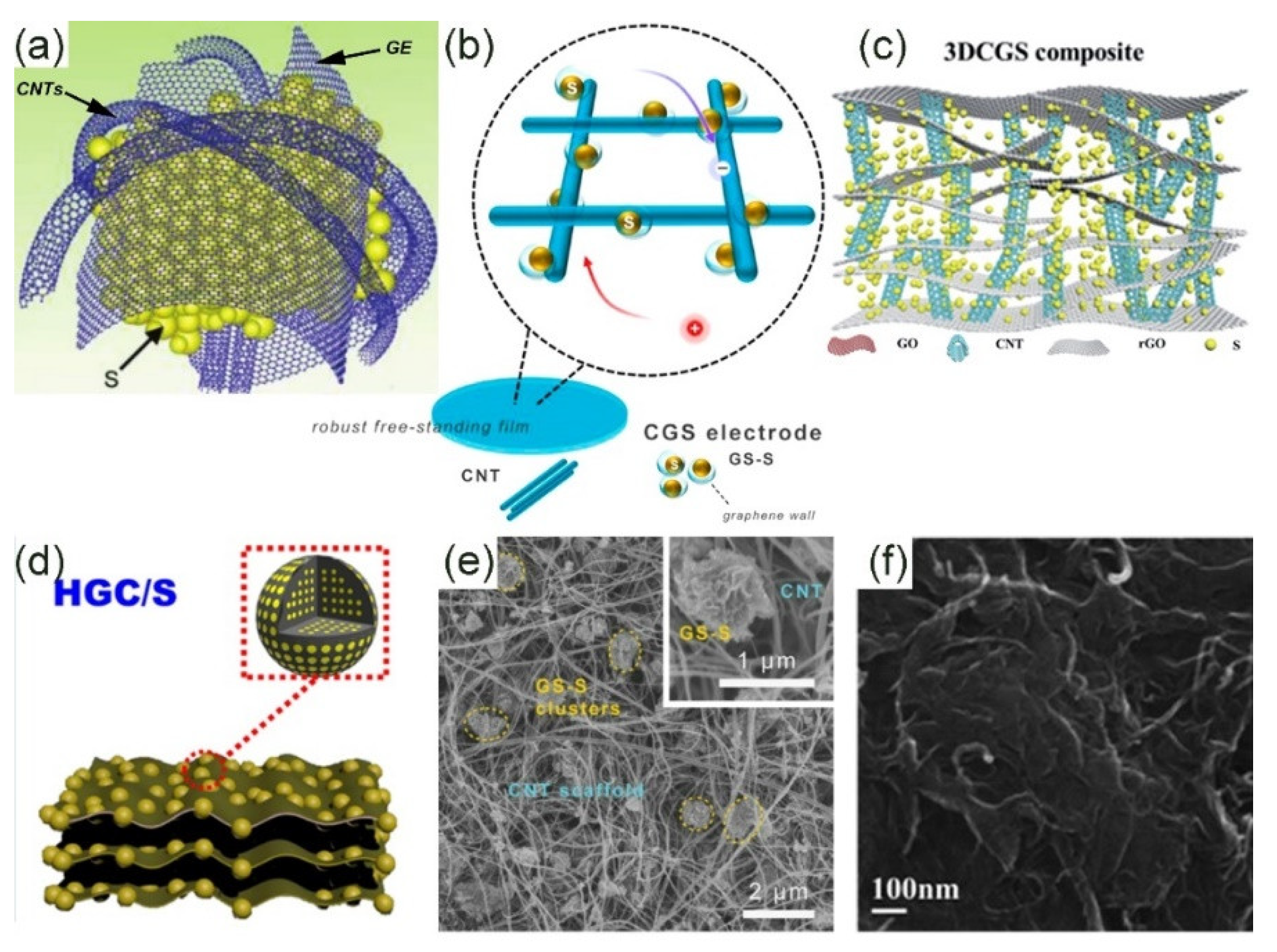
4.2. Other Carbon Materials/Graphene Composite Materials
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The Path Towards Sustainable Energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Erratum: Li–O2 and Li–S Batteries with High Energy Storage. Nat. Mater. 2011, 11, 172. [Google Scholar] [CrossRef]
- Song, M.K.; Cairns, E.J.; Zhang, Y. Lithium/Sulfur Batteries with High Specific Energy: Old Challenges and New Opportunities. Nanoscale 2013, 5, 2186–2204. [Google Scholar] [CrossRef]
- Wang, D.W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.M.; Gentle, I.R.; Lu, G.Q.M. Carbon–Sulfur Composites for Li–S Batteries: Status and Prospects. J. Mater. Chem. A 2013, 1. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid Electrolyte Lithium/Sulfur Battery: Fundamental Chemistry, Problems, and Solutions. J. Power Sour. 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, K.; Chen, F.; Chen, W.; Wei, X.; Liu, X.W.; Liu, J. Natural Integrated Carbon Architecture for Rechargeable Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2016, 4, 666–670. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhuang, T.Z.; Zhang, Q.; Peng, H.J.; Chen, C.M.; Wei, F. Permselective Graphene Oxide Membrane for Highly Stable and Anti-Self-Discharge Lithium–Sulfur Batteries. ACS Nano 2015, 9, 3002–3011. [Google Scholar] [CrossRef]
- Jin, F.; Xiao, S.; Lu, L.; Wang, Y. Efficient Activation of High-Loading Sulfur by Small CNTs Confined Inside a Large CNT for High-Capacity and High-Rate Lithium-Sulfur Batteries. Nano Lett. 2016, 16, 440–447. [Google Scholar] [CrossRef]
- Li, B.; Li, S.; Xu, J.; Yang, S. A New Configured Lithiated Silicon–Sulfur Battery Built on 3D Graphene with Superior Electrochemical Performances. Energy Environ. Sci. 2016, 9, 2025–2030. [Google Scholar] [CrossRef]
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically Structured Sulfur/Carbon Nanocomposite Material for High-Energy Lithium Battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. Engl. 2011, 50, 5904–5908. [Google Scholar] [CrossRef]
- Fang, R.; Li, G.; Zhao, S.; Yin, L.; Du, K.; Hou, P.; Wang, S.; Cheng, H.M.; Liu, C.; Li, F. Single-Wall Carbon Nanotube Network Enabled Ultrahigh Sulfur-Content Electrodes for High-Performance Lithium-Sulfur Batteries. Nano Energy 2017, 42, 205–214. [Google Scholar] [CrossRef]
- Xu, J.; Shui, J.; Wang, J.; Wang, M.; Liu, H.K.; Dou, S.X.; Jeon, I.Y.; Seo, J.M.; Baek, J.B.; Dai, L. Sulfur-Graphene Nanostructured Cathodes via Ball-Milling for High-Performance Lithium-Sulfur Batteries. ACS Nano 2014, 8, 10920–10930. [Google Scholar] [CrossRef]
- Chong, W.G.; Huang, J.Q.; Xu, Z.L.; Qin, X.; Wang, X.; Kim, J.K. Lithium-Sulfur Battery Cable Made from Ultralight, Flexible Graphene/Carbon Nanotube/Sulfur Composite Fibers. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Hwa, Y.; Seo, H.K.; Yuk, J.M.; Cairns, E.J. Freeze-Dried Sulfur-Graphene Oxide-Carbon Nanotube Nanocomposite for High Sulfur-Loading Lithium/Sulfur Cells. Nano Lett. 2017, 17, 7086–7094. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lee, K.T.; Nazar, L.F. A Highly Ordered Nanostructured Carbon-Sulphur Cathode for Lithium-Sulphur Batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. In Nanoscience and Technology; Macmillan Publishers Ltd.: Basingstoke, UK, 2009; pp. 11–19. [Google Scholar]
- Chen, F.; Tao, N.J. Electron Transport in Single Molecules: From Benzene to Graphene. Acc. Chem. Res. 2009, 42, 429–438. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh Electron Mobility in Suspended Graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in Lithium–Sulfur Batteries Based on Multifunctional Cathodes and Electrolytes. Nat. Energy 2016, 1. [Google Scholar] [CrossRef]
- Yang, L.; Li, Q.; Wang, Y.; Chen, Y.; Guo, X.; Wu, Z.; Chen, G.; Zhong, B.; Xiang, W.; Zhong, Y. A Review of Cathode Materials in Lithium-Sulfur Batteries. Ionics 2020, 26, 5299–5318. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Hu, J.; Feng, Y.; He, P.; Ma, J. Recent Advances in Cathode Materials for Rechargeable Lithium-Sulfur Batteries. Nanoscale 2019, 11, 15418–15439. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Wu, Z.S.; Chen, J. Two-Dimensional Materials for Advanced Li-S Batteries. Energy Storage Mater. 2019, 22, 284–310. [Google Scholar] [CrossRef]
- Dai, C.; Sun, G.; Hu, L.; Xiao, Y.; Zhang, Z.; Qu, L. Recent Progress in Graphene-Based Electrodes for Flexible Batteries. InfoMat 2019, 2, 509–526. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.M. The Regulating Role of Carbon Nanotubes and Graphene in Lithium-Ion and Lithium-Sulfur Batteries. Adv. Mater. 2019, 31, e1800863. [Google Scholar] [CrossRef]
- Wu, S.; Ge, R.; Lu, M.; Xu, R.; Zhang, Z. Graphene-Based Nano-Materials for Lithium–Sulfur Battery and Sodium-ion Battery. Nano Energy 2015, 15, 379–405. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Y.; Sheng, J.; Huang, Q.; Lv, W.; Zhou, G.; Cheng, H.M. Status and Prospects of Porous Graphene Networks for Lithium–Sulfur Batteries. Mater. Horiz. 2020, 7, 2487–2518. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, J.; Liu, X.; Wang, F.; Wang, L.; Shi, C.; Huang, L.; Feng, X.; Chen, X.; Xu, L.; et al. Self-Adaptive Strain-Relaxation Optimization for High-Energy Lithium Storage Material through Crumpling of Graphene. Nat. Commun. 2014, 5, 4565. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Tang, Y.; Wang, Y.; Bi, H.; Liu, Z.; Huang, F.; Xie, X.; Jiang, M. Scotch-Tape-Like Exfoliation of Graphite Assisted with Elemental Sulfur and Graphene–Sulfur Composites for High-Performance Lithium-Sulfur Batteries. Energy Environ. Sci. 2013, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Song, N.; He, J.; Li, X. Graphene and Its Derivatives in Lithium–Sulfur Batteries. Mater. Today Energy 2018, 9, 319–335. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, J.; Kim, J.M.; Seong, C.Y.; Seong, K.D.; Piao, Y. Well-Dispersed Sulfur Wrapped in Reduced Graphene Oxide Nanoscroll as Cathode Material for Lithium–Sulfur Battery. J. Electroanal. Chem. 2016, 780, 19–25. [Google Scholar] [CrossRef]
- Huang, J.Q.; Liu, X.F.; Zhang, Q.; Chen, C.M.; Zhao, M.Q.; Zhang, S.M.; Zhu, W.; Qian, W.Z.; Wei, F. Entrapment of Sulfur in Hierarchical Porous Graphene for Lithium–Sulfur Batteries with High Rate Performance from −40 to 60°C. Nano Energy 2013, 2, 314–321. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Dong, Y.; Tang, Y.; Wang, L.; Jia, D.; Zhao, Z.; Qiu, J. Self-Assembled Sulfur/Reduced Graphene Oxide Nanoribbon Paper as a Free-Standing Electrode for High Performance Lithium-Sulfur Batteries. Chem. Commun. 2016, 52, 12825–12828. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Dai, Y.; Qiu, S.; Yang, J.; Lu, W.; Chen, L. Rational Design of Cathode Structure for High Rate Performance Lithium-Sulfur Batteries. Nano Lett. 2015, 15, 5443–5448. [Google Scholar] [CrossRef]
- Yang, J.; Shan, X.; Guo, Z.; Duan, L.; Zhang, X.; Lü, W. A Facile Synthetic Strategy of Free-Standing Holey Graphene Paper as Sulfur Host for High-Performance Flexible Lithium Sulfur Batteries. J. Electroanal. Chem. 2020, 876. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Wang, Y.; Chen, J.; Zhou, H.; Huang, Y. Macroporous Free-Standing Nano-Sulfur/Reduced Graphene Oxide Paper as Stable Cathode for Lithium-Sulfur Battery. Nano Energy 2015, 11, 678–686. [Google Scholar] [CrossRef]
- Cao, J.; Chen, C.; Zhao, Q.; Zhang, N.; Lu, Q.; Wang, X.; Niu, Z.; Chen, J. A Flexible Nanostructured Paper of a Reduced Graphene Oxide-Sulfur Composite for High-Performance Lithium-Sulfur Batteries with Unconventional Configurations. Adv. Mater. 2016, 28, 9629–9636. [Google Scholar] [CrossRef]
- Zhou, G.; Pei, S.; Li, L.; Wang, D.W.; Wang, S.; Huang, K.; Yin, L.C.; Li, F.; Cheng, H.M. A Graphene-Pure-Sulfur Sandwich Structure for Ultrafast, Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2014, 26, 625–631, 664. [Google Scholar] [CrossRef]
- Li, Y.; Guan, Q.; Cheng, J.; Wang, B. Ultrafine Nanosulfur Particles Sandwiched in Little Oxygen-Functionalized Graphene Layers as Cathodes for High Rate and Long-Life Lithium-Sulfur Batteries. Nanotechnology 2020, 31, 245404. [Google Scholar] [CrossRef]
- Papandrea, B.; Xu, X.; Xu, Y.; Chen, C.Y.; Lin, Z.; Wang, G.; Luo, Y.; Liu, M.; Huang, Y.; Mai, L.; et al. Three-Dimensional Graphene Framework with Ultra-High Sulfur Content for a Robust Lithium–Sulfur Battery. Nano Res. 2016, 9, 240–248. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Pei, S.; Qian, X.; Hou, P.X.; Cheng, H.M.; Liu, C.; Li, F. Toward More Reliable Lithium-Sulfur Batteries: An All-Graphene Cathode Structure. ACS Nano 2016, 10, 8676–8682. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yuan, J.; Geng, X.; Dou, H.; Chen, L.; Yan, X.; Zhu, H. Caterpillar-Like Graphene Confining Sulfur by Restacking Effect for High Performance Lithium Sulfur Batteries. Chem. Eng. J. 2017, 322, 454–462. [Google Scholar] [CrossRef]
- Xu, H.; Deng, Y.; Shi, Z.; Qian, Y.; Meng, Y.; Chen, G. Graphene-Encapsulated Sulfur (GES) Composites with a Core–Shell Structure as Superior Cathode Materials for Lithium-Sulfur Batteries. J. Mater. Chem. A 2013, 1. [Google Scholar] [CrossRef]
- Yeon, J.S.; Yun, S.; Park, J.M.; Park, H.S. Surface-Modified Sulfur Nanorods Immobilized on Radially Assembled Open-Porous Graphene Microspheres for Lithium-Sulfur Batteries. ACS Nano 2019, 13, 5163–5171. [Google Scholar] [CrossRef]
- He, Y.; Bai, S.; Chang, Z.; Li, Q.; Qiao, Y.; Zhou, H. Porous Hybrid Aerogels with Ultrahigh Sulfur Loading for Lithium–Sulfur Batteries. J. Mater. Chem. A 2018, 6, 9032–9040. [Google Scholar] [CrossRef]
- Cavallo, C.; Agostini, M.; Genders, J.P.; Abdelhamid, M.E.; Matic, A. A Free-Standing Reduced Graphene Oxide Aerogel as Supporting Electrode in a Fluorine-Free Li2S8 Catholyte Li-S Battery. J. Power Sour. 2019, 416, 111–117. [Google Scholar] [CrossRef]
- Zegeye, T.A.; Tsai, M.C.; Cheng, J.H.; Lin, M.H.; Chen, H.M.; Rick, J.; Su, W.N.; Kuo, C.F.J.; Hwang, B.J. Controllable Embedding of Sulfur in High Surface Area Nitrogen Doped Three Dimensional Reduced Graphene Oxide by Solution Drop Impregnation Method for High Performance Lithium-Sulfur Batteries. J. Power Sour. 2017, 353, 298–311. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, L.; Cong, H.; Zhang, X.; Lu, W.; Xue, C. Plasma Treatment for Nitrogen-Doped 3D Graphene Framework by a Conductive Matrix with Sulfur for High-Performance Li-S Batteries. Small 2019, 15, e1804347. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Mei, T.; Yao, J.; Hou, B.; Zhu, X.; Liu, X.; Wang, X. Cabbage-Like Nitrogen-Doped Graphene/Sulfur Composite for Lithium-Sulfur Batteries with Enhanced Rate Performance. J. Alloys Compd. 2018, 753, 622–629. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, Y.S.; Yang, X.X.; Ren, M.X.; Wang, Y.Q.; Lei, B.Y.; Zhao, D.L. Sulfur Encapsulated in Nitrogen-Doped Graphene Aerogel as a Cathode Material for High Performance Lithium-Sulfur Batteries. Int. J. Hydrogen Energy 2021, 46, 7642–7652. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, Z.; Cai, T.; Han, W.Q. Effect of Boron-Doping on the Graphene Aerogel Used as Cathode for the Lithium-Sulfur Battery. ACS Appl. Mater. Interfaces 2015, 7, 25202–25210. [Google Scholar] [CrossRef]
- Shi, P.; Wang, Y.; Liang, X.; Sun, Y.; Cheng, S.; Chen, C.; Xiang, H. Simultaneously Exfoliated Boron-Doped Graphene Sheets to Encapsulate Sulfur for Applications in Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2018, 6, 9661–9670. [Google Scholar] [CrossRef]
- Chen, D.; Yang, R.; Chen, L.; Zou, Y.; Ren, B.; Li, L.; Li, S.; Yan, Y.; Xu, Y. One-Pot Fabrication of Nitrogen and Sulfur Dual-Doped Graphene/Sulfur Cathode via Microwave Assisted Method for Long Cycle-Life Lithium-Sulfur Batteries. J. Alloys Compd. 2018, 746, 116–124. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. Long-Life Li/Polysulphide Batteries with High Sulphur Loading Enabled by Lightweight Three-Dimensional Nitrogen/Sulphur-Codoped Graphene Sponge. Nat. Commun. 2015, 6, 7760. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, B.; Hou, H.; Zeinu, K.; He, Y.; Yang, X.; Xue, W.; He, X.; Huang, L.; Zhu, X.; et al. Facile Synthesis of Mesoporous Graphene Platelets with in Situ Nitrogen and Sulfur Doping for Lithium-Sulfur Batteries. RSC Adv. 2017, 7, 22567–22577. [Google Scholar] [CrossRef]
- Xu, J.; Su, D.; Zhang, W.; Bao, W.; Wang, G. A Nitrogen–Sulfur Co-Doped Porous Graphene Matrix as a Sulfur Immobilizer for High Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2016, 4, 17381–17393. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J.; Zhou, H.; Fu, C.; Wang, G.; Yang, L.; Xu, C.; Chen, Z.; Yang, W.; Kuang, Y. Hydrothermal Preparation of Nitrogen, Boron Co-Doped Curved Graphene Nanoribbons with High Dopant Amounts for High-Performance Lithium Sulfur Battery Cathodes. J. Mater. Chem. A 2017, 5, 7403–7415. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, S.; Jiang, Y.; Jiang, Z.; Zhang, L.; Chang, J.; Wei, T.; Fan, Z. Sandwiching Sulfur into the Dents Between N, O Co-Doped Graphene Layered Blocks with Strong Physicochemical Confinements for Stable and High-Rate Li–S Batteries. Nano-Micro Lett. 2020, 12. [Google Scholar] [CrossRef]
- Ji, J.; Sha, Y.; Li, Z.; Gao, X.; Zhang, T.; Zhou, S.; Qiu, T.; Zhou, S.; Zhang, L.; Ling, M.; et al. Selective Adsorption and Electrocatalysis of Polysulfides through Hexatomic Nickel Clusters Embedded in N-Doped Graphene toward High-Performance Li-S Batteries. Research 2020, 2020, 5714349. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of Nitrogen-Doped Graphene Films for Lithium Battery Application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, R.; Wu, M.; Shi, G. Graphene Materials for Lithium–Sulfur Batteries. Energy Storage Mater. 2015, 1, 51–73. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping Carbons Beyond Nitrogen: An Overview of Advanced Heteroatom Doped Carbons with Boron, Sulphur and Phosphorus for Energy Applications. Energy Environ. Sci. 2013, 6. [Google Scholar] [CrossRef]
- Tang, H.; Yang, J.; Zhang, G.; Liu, C.; Wang, H.; Zhao, Q.; Hu, J.; Duan, Y.; Pan, F. Self-Assembled N-Graphene Nanohollows Enabling Ultrahigh Energy Density Cathode for Li-S Batteries. Nanoscale 2017, 10, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, W.; Zhao, W.; Li, G.; Hou, Y.; Liu, M.; Zhou, L.; Ye, F.; Li, H.; Wei, Z.; et al. High-Rate, Ultralong Cycle-Life Lithium/Sulfur Batteries Enabled by Nitrogen-Doped Graphene. Nano Lett. 2014, 14, 4821–4827. [Google Scholar] [CrossRef]
- Usachov, D.; Vilkov, O.; Gruneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.K.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; et al. Nitrogen-Doped Graphene: Efficient Growth, Structure, and Electronic Properties. Nano Lett. 2011, 11, 5401–5407. [Google Scholar] [CrossRef] [PubMed]
- Lota, G.; Fic, K.; Frackowiak, E. Carbon Nanotubes and Their Composites in Electrochemical Applications. Energy Environ. Sci. 2011, 4. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Yin, L.; Koratkar, N.; Li, F.; Cheng, H.-M. Stabilizing Sulfur Cathodes Using Nitrogen-Doped Graphene as a Chemical Immobilizer for Li-S Batteries. Carbon 2016, 108, 120–126. [Google Scholar] [CrossRef]
- Sun, F.; Wang, J.; Chen, H.; Li, W.; Qiao, W.; Long, D.; Ling, L. High Efficiency Immobilization of Sulfur on Nitrogen-Enriched Mesoporous Carbons for Li-S Batteries. ACS Appl. Mater. Interfaces 2013, 5, 5630–5638. [Google Scholar] [CrossRef]
- Yi, G.S.; Sim, E.S.; Chung, Y.C. Effect of Lithium-Trapping on Nitrogen-Doped Graphene as an Anchoring Material for Lithium-Sulfur Batteries: A Density Functional Theory Study. Phys. Chem. Chem. Phys. 2017, 19, 28189–28194. [Google Scholar] [CrossRef]
- Song, J.; Yu, Z.; Gordin, M.L.; Wang, D. Advanced Sulfur Cathode Enabled by Highly Crumpled Nitrogen-Doped Graphene Sheets for High-Energy-Density Lithium-Sulfur Batteries. Nano Lett. 2016, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, X.; Sun, X.; Wang, C. Nitrogen-Doped Graphene Nanosheets/Sulfur Composite as Lithium–Sulfur Batteries Cathode. Mater. Sci. Eng. B 2016, 213, 83–89. [Google Scholar] [CrossRef]
- Yanilmaz, A.; Tomak, A.; Akbali, B.; Bacaksiz, C.; Ozceri, E.; Ari, O.; Senger, R.T.; Selamet, Y.; Zareie, H.M. Nitrogen Doping for Facile and Effective Modification of Graphene Surfaces. RSC Adv. 2017, 7, 28383–28392. [Google Scholar] [CrossRef]
- Fan, X.; Sun, W.; Meng, F.; Xing, A.; Liu, J. Advanced Chemical Strategies for Lithium–Sulfur Batteries: A Review. Green Energy Environ. 2018, 3, 2–19. [Google Scholar] [CrossRef]
- Deng, C.; Wang, Z.; Wang, S.; Yu, J. Inhibition of Polysulfide Diffusion in Lithium–Sulfur Batteries: Mechanism and Improvement Strategies. J. Mater. Chem. A 2019, 7, 12381–12413. [Google Scholar] [CrossRef]
- Zhao, Y.; Bakenova, Z.; Zhang, Y.; Peng, H.; Xie, H.; Bakenov, Z. High Performance Sulfur/Nitrogen-Doped Graphene Cathode for Lithium/Sulfur Batteries. Ionics 2015, 21, 1925–1930. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, W.; Zhang, R.; Luan, C.; Zeng, Q.; Wang, C.; Li, S.; Huang, Z.; Liao, H.; Ji, X. Electrochemical Exfoliation of Graphite into Nitrogen-doped Graphene in Glycine Solution and its Energy Storage Properties. Electrochim. Acta 2016, 204, 100–107. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Functionalization of Graphene Sheets by the Diazonium Chemistry during Electrochemical Exfoliation of Graphite. Carbon 2017, 111, 83–93. [Google Scholar] [CrossRef]
- Xing, L.B.; Xi, K.; Li, Q.; Su, Z.; Lai, C.; Zhao, X.; Kumar, R.V. Nitrogen, Sulfur-Codoped Graphene Sponge as Electroactive Carbon Interlayer for High-Energy and Power Lithium-Sulfur Batteries. J. Power Sour. 2016, 303, 22–28. [Google Scholar] [CrossRef]
- Li, J.; Xue, C.; Xi, B.; Mao, H.; Qian, Y.; Xiong, S. Heteroatom Dopings and Hierarchical Pores of Graphene for Synergistic Improvement of Lithium–Sulfur Battery Performance. Inorg. Chem. Front. 2018, 5, 1053–1061. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Su, Y.; Zhao, J. Shuttle Inhibition by Chemical Adsorption of Lithium Polysulfides in B and N Co-Doped Graphene for Li-S Batteries. Phys. Chem. Chem. Phys. 2016, 18, 25241–25248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, P.; Man, X.L.; Wang, D.; Huang, J.; Shu, H.B.; Liu, Z.G.; Wang, L. Fe, N Co-Doped Graphene as a Multi-functional Anchor Material for Lithium-Sulfur Battery. J. Phys. Chem. Solids 2019, 126, 280–286. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Q.; Qian, W.; Xiao, H.; Li, Z.; Ma, L.; Tian, X. Binary Hierarchical Porous Graphene/Pyrolytic Carbon Nanocomposite Matrix Loaded with Sulfur as a High-Performance Li-S Battery Cathode. ACS Appl. Mater. Interfaces 2018, 10, 18726–18733. [Google Scholar] [CrossRef]
- Hou, T.Z.; Chen, X.; Peng, H.J.; Huang, J.Q.; Li, B.Q.; Zhang, Q.; Li, B. Design Principles for Heteroatom-Doped Nanocarbon to Achieve Strong Anchoring of Polysulfides for Lithium-Sulfur Batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef]
- Ogoke, O.; Hwang, S.; Hultman, B.; Chen, M.; Karakalos, S.; He, Y.; Ramsey, A.; Su, D.; Alexandridis, P.; Wu, G. Large-Diameter and Heteroatom-Doped Graphene Nanotubes Decorated with Transition Metals as Carbon Hosts for Lithium–Sulfur Batteries. J. Mater. Chem. A 2019, 7, 13389–13399. [Google Scholar] [CrossRef]
- Toh, R.J.; Poh, H.L.; Sofer, Z.; Pumera, M. Transition Metal (Mn, Fe, Co, Ni)-Doped Graphene Hybrids for Electrocatalysis. Chem. Asian J. 2013, 8, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhu, K.; Li, D.; Bai, F.; Wei, Y.; Zhang, P. N-Doped Graphene/Fe–Fe3C Nano-Composite Synthesized by a Fe-Based Metal Organic Framework and Its Anode Performance in Lithium ion Batteries. Chem. Eng. J. 2014, 258, 93–100. [Google Scholar] [CrossRef]
- Chen, X.; Yu, L.; Wang, S.; Deng, D.; Bao, X. Highly Active and Stable Single Iron Site Confined in Graphene Nanosheets for Oxygen Reduction Reaction. Nano Energy 2017, 32, 353–358. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, D.; Cao, D.; Zeng, X.C. A Universal Principle for a Rational Design of Single-Atom Electrocatalysts. Nat. Catal. 2018, 1, 339–348. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Lu, Z.; Wang, W. Bifunctional CoNx Embedded Graphene Electrocatalysts for OER and ORR: A Theoretical Evaluation. Carbon 2018, 130, 112–119. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, B.; Luo, M.; Ning, S.; Peng, M.; Zhao, Y.; Lu, Y.R.; Chan, T.S.; de Groot, F.M.F.; Tan, Y. Single Platinum Atoms Embedded in Nanoporous Cobalt Selenide as Electrocatalyst for Accelerating Hydrogen Evolution Reaction. Nat. Commun. 2019, 10, 1743. [Google Scholar] [CrossRef]
- Luo, R.; Luo, M.; Wang, Z.; Liu, P.; Song, S.; Wang, X.; Chen, M. The Atomic Origin of Nickel-Doping-Induced Catalytic Enhancement in MoS2 for Electrochemical Hydrogen Production. Nanoscale 2019, 11, 7123–7128. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Pu, Z.; Zhou, H.; Yu, J.; Amiinu, I.S.; Zhu, J.; Liang, Q.; Yang, J.; He, D.; Hu, Z.; et al. A Universal Synthesis Strategy for Single Atom Dispersed Cobalt/Metal Clusters Heterostructure Boosting Hydrogen Evolution Catalysis at All PH Values. Nano Energy 2019, 59, 472–480. [Google Scholar] [CrossRef]
- Luo, G.; Zhao, J.; Wang, B. First-Principles Study of Transition Metal Doped Li2S as Cathode Materials in Lithium Batteries. J. Renew. Sustain. Energy 2012, 4. [Google Scholar] [CrossRef]
- Zeng, Q.W.; Hu, R.M.; Chen, Z.B.; Shang, J.X. Single-Atom Fe and N Co-Doped Graphene for Lithium-Sulfur Batteries: A Density Functional Theory Study. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Cui, M.; Zheng, Z.; Wang, J.; Wang, Y.; Zhao, X.; Ma, R.; Liu, J. Rational Design of Lithium-Sulfur Battery Cathodes Based on Differential Atom Electronegativity. Energy Storage Mater. 2021, 35, 577–585. [Google Scholar] [CrossRef]
- Rehman, S.; Guo, S.; Hou, Y. Rational Design of Si/SiO2 @Hierarchical Porous Carbon Spheres as Efficient Polysulfide Reservoirs for High-Performance Li-S Battery. Adv. Mater. 2016, 28, 3167–3172. [Google Scholar] [CrossRef]
- Fan, F.Y.; Chiang, Y.M. Electrodeposition Kinetics in Li-S Batteries: Effects of Low Electrolyte/Sulfur Ratios and Deposition Surface Composition. J. Electrochem. Soc. 2017, 164, A917–A922. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, L.; Hao, Z.; Liu, X.; Xiang, J.; Zhang, Z.; Huang, Y.; Xie, J. Free-Standing Mn3O4@CNF/S Paper Cathodes with High Sulfur Loading for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 13406–13412. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wu, Z.; Zhao, G.; Sun, C.; Zhou, C.; Gong, X.; Diao, G. Core-Shell Structure and Interaction Mechanism of Gamma-MnO2 Coated Sulfur for Improved Lithium-Sulfur Batteries. Small 2017, 13. [Google Scholar] [CrossRef]
- Hu, B.; Mai, L.; Chen, W.; Yang, F. From MoO3 Nanobelts to MoO2 Nanorods: Structure Transformation and Electrical Transport. ACS Nano 2009, 3, 478–482. [Google Scholar] [CrossRef]
- Al Salem, H.; Babu, G.; Rao, C.V.; Arava, L.M. Electrocatalytic Polysulfide Traps for Controlling Redox Shuttle Process of Li-S Batteries. J. Am. Chem. Soc. 2015, 137, 11542–11545. [Google Scholar] [CrossRef] [PubMed]
- He, Y.B.; Liu, M.; Xu, Z.L.; Zhang, B.; Li, B.; Kang, F.; Kim, J.K. Li-ion Reaction to Improve the Rate Performance of Nanoporous Anatase TiO2 Anodes. Energy Technol. 2013, 1, 668–674. [Google Scholar] [CrossRef]
- Tang, C.; Li, B.Q.; Zhang, Q.; Zhu, L.; Wang, H.F.; Shi, J.L.; Wei, F. CaO-Templated Growth of Hierarchical Porous Graphene for High-Power Lithium-Sulfur Battery Applications. Adv. Funct. Mater. 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Zheng, C.; Niu, S.; Lv, W.; Zhou, G.; Li, J.; Fan, S.; Deng, Y.; Pan, Z.; Li, B.; Kang, F.; et al. Propelling Polysulfides Transformation for High-Rate and Long-Life Lithium–Sulfur Batteries. Nano Energy 2017, 33, 306–312. [Google Scholar] [CrossRef]
- Choi, S.; Seo, D.H.; Kaiser, M.R.; Zhang, C.; van der laan, T.; Han, Z.J.; Bendavid, A.; Guo, X.; Yick, S.; Murdock, A.T.; et al. WO3 Nanolayer Coated 3D-Graphene/Sulfur Composites for High Performance Lithium/Sulfur Batteries. J. Mater. Chem. A 2019, 7, 4596–4603. [Google Scholar] [CrossRef]
- Wei, H.; Ding, Y.; Li, H.; Zhang, Q.; Hu, N.; Wei, L.; Yang, Z. MoS2 Quantum Dots Decorated Reduced Graphene Oxide as a Sulfur Host for Advanced Lithium-Sulfur Batteries. Electrochim. Acta 2019, 327. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of Polysulfide Conversion by Sulfur-Deficient MoS2 Nanoflakes for Lithium–Sulfur Batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, H.J.; Lee, M.H.; Park, H.S. Hierarchically Structured Reduced Graphene Oxide/WO3 Frameworks for an Application into Lithium ion Battery Anodes. Chem. Eng. J. 2015, 281, 724–729. [Google Scholar] [CrossRef]
- Guan, X.H.; Zhang, Z.W.; Yang, L.; Wang, G.S. One-Pot Hydrothermal Synthesis of Hexagonal WO3 Nanorods/Graphene Composites as High-Performance Electrodes for Supercapacitors. Chempluschem 2017, 82, 1174–1181. [Google Scholar] [CrossRef]
- Wu, X.; Yao, S. Flexible Electrode Materials Based on WO3 Nanotube Bundles for High Performance Energy Storage Devices. Nano Energy 2017, 42, 143–150. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium-Sulfur Batteries. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, W.; Zhu, X.; Zhang, L.; Li, Q.; Ding, F.; Liu, Z.; Sun, J. Vanadium Dioxide-Graphene Composite with Ultrafast Anchoring Behavior of Polysulfides for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 15733–15741. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, H.; Zhao, F.; Liu, Y.; Li, J.; Liu, X. Simultaneous Defect-Engineered and Thiol Modified of MoO2 for Improved Catalytic Activity in Lithium-Sulfur Batteries: A Study of Synergistic Polysulfide Adsorption-Conversion Function. Chem. Eng. J. 2021, 409. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.H. Catalytic Effects in Lithium-Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2018, 5, 1700270. [Google Scholar] [CrossRef]
- Rout, C.S.; Kim, B.H.; Xu, X.; Yang, J.; Jeong, H.Y.; Odkhuu, D.; Park, N.; Cho, J.; Shin, H.S. Synthesis and Characterization of Patronite Form of Vanadium Sulfide on Graphitic Layer. J. Am. Chem. Soc. 2013, 135, 8720–8725. [Google Scholar] [CrossRef]
- Huo, H.; Zhao, Y.; Xu, C. 3D Ni3S2 Nanosheet Arrays Supported on Ni Foam for High-Performance Supercapacitor and Non-Enzymatic Glucose Detection. J. Mater. Chem. A 2014, 2. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, H.J.; Hou, T.Z.; Huang, J.Q.; Chen, C.M.; Wang, D.W.; Cheng, X.B.; Wei, F.; Zhang, Q. Powering Lithium-Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/Graphene Cocatalyst for Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the Surface Structure of MoS2 to Preferentially Expose Active Edge Sites for Electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kiriya, D.; Lobaccaro, P.; Nyein, H.Y.; Taheri, P.; Hettick, M.; Shiraki, H.; Sutter-Fella, C.M.; Zhao, P.; Gao, W.; Maboudian, R.; et al. General Thermal Texturization Process of MoS2 for Efficient Electrocatalytic Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 4047–4053. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Kumar, B.; Liu, C.; Phillips, P.; Yasaei, P.; Behranginia, A.; Zapol, P.; Klie, R.F.; Curtiss, L.A.; Salehi-Khojin, A. Cathode Based on Molybdenum Disulfide Nanoflakes for Lithium-Oxygen Batteries. ACS Nano 2016, 10, 2167–2175. [Google Scholar] [CrossRef]
- Tang, W.; Goh, B.M.; Hu, M.Y.; Wan, C.; Tian, B.; Deng, X.; Peng, C.; Lin, M.; Hu, J.Z.; Loh, K.P. In Situ Raman and Nuclear Magnetic Resonance Study of Trapped Lithium in the Solid Electrolyte Interface of Reduced Graphene Oxide. J. Phys. Chem. C 2016, 120, 2600–2608. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, Z.; Xi, B.; Yu, Z.; Zhou, Z.; Chen, X.a. Ni3S2 Anchored to N/S Co-Doped Reduced Graphene Oxide with Highly Pleated Structure as a Sulfur Host for Lithium–Sulfur Batteries. J. Mater. Chem. A 2020, 8, 3834–3844. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, Y.; Manthiram, A. Dual-Confined Flexible Sulfur Cathodes Encapsulated in Nitrogen-Doped Double-Shelled Hollow Carbon Spheres and Wrapped with Graphene for Li-S Batteries. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, J.; Hwang, T.; Piao, Y. Sulfur-Loaded Monodisperse Carbon Nanocapsules Anchored on Graphene Nanosheets as Cathodes for High Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2017, 5, 975–981. [Google Scholar] [CrossRef]
- Thieme, S.; Brückner, J.; Bauer, I.; Oschatz, M.; Borchardt, L.; Althues, H.; Kaskel, S. High Capacity Micro-Mesoporous Carbon–Sulfur Nanocomposite Cathodes with Enhanced Cycling Stability Prepared by a Solvent-Free Procedure. J. Mater. Chem. A 2013, 1. [Google Scholar] [CrossRef]
- Su, F.Y.; He, Y.B.; Li, B.; Chen, X.C.; You, C.H.; Wei, W.; Lv, W.; Yang, Q.H.; Kang, F. Could Graphene Construct an Effective Conducting Network in a High-Power Lithium ion Battery? Nano Energy 2012, 1, 429–439. [Google Scholar] [CrossRef]
- Wei, W.; Lv, W.; Wu, M.B.; Su, F.Y.; He, Y.B.; Li, B.; Kang, F.; Yang, Q.H. The Effect of Graphene Wrapping on the Performance of LiFePO4 for a Lithium ion Battery. Carbon 2013, 57, 530–533. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Qian, W.Z.; Zhang, Y.Y.; Wei, F. The Road for Nanomaterials Industry: A Review of Carbon Nanotube Production, Post-Treatment, and Bulk Applications for Composites and Energy Storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Zhang, Q.; Huang, J.Q.; Liu, X.F.; Zhu, W.; Zhao, M.Q.; Qian, W.Z.; Wei, F. Composite Cathodes Containing SWCNT@S Coaxial Nanocables: Facile Synthesis, Surface Modification, and Enhanced Performance for Li-Ion Storage. Part. Part. Syst. Charact. 2013, 30, 158–165. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Liu, X.F.; Zhang, Q.; Tian, G.L.; Huang, J.Q.; Zhu, W.; Wei, F. Graphene/Single-Walled Carbon Nanotube Hybrids: One-Step Catalytic Growth and Applications for High-Rate Li–S Batteries. ACS Nano 2012, 6, 10759–10769. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.J.; Huang, J.Q.; Zhao, M.Q.; Zhang, Q.; Cheng, X.B.; Liu, X.Y.; Qian, W.Z.; Wei, F. Nanoarchitectured Graphene/CNT@Porous Carbon with Extraordinary Electrical Conductivity and Interconnected Micro/Mesopores for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 2772–2781. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, H.J.; Liang, J.; Huang, J.Q.; Chen, C.M.; Guo, X.; Zhu, W.; Li, P.; Zhang, Q. Interconnected Carbon Nanotube/Graphene Nanosphere Scaffolds as Free-Standing Paper Electrode for High-Rate and Ultra-Stable Lithium–Sulfur Batteries. Nano Energy 2015, 11, 746–755. [Google Scholar] [CrossRef]
- Su, D.; Cortie, M.; Wang, G. Fabrication of N-Doped Graphene-Carbon Nanotube Hybrids from Prussian Blue for Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Li, P.; Fu, F.; Wang, Z.; Zhang, W. Three-Dimensional CNT/Graphene–Sulfur Hybrid Sponges with High Sulfur Loading as Superior-Capacity Cathodes for Lithium–Sulfur Batteries. J. Mater. Chem. A 2015, 3, 18605–18610. [Google Scholar] [CrossRef]
- Jia, J.; Wang, K.; Zhang, X.; Sun, X.; Zhao, H.; Ma, Y. Graphene-Based Hierarchically Micro/Mesoporous Nanocomposites as Sulfur Immobilizers for High-Performance Lithium–Sulfur Batteries. Chem. Mater. 2016, 28, 7864–7871. [Google Scholar] [CrossRef]
- Gómez-Urbano, J.L.; Gómez-Cámer, J.L.; Botas, C.; Rojo, T.; Carriazo, D. Graphene Oxide-Carbon Nanotubes Aerogels with High Sulfur Loadings Suitable as Binder-Free Cathodes for High Performance Lithium Sulfur Batteries. J. Power Sour. 2019, 412, 408–415. [Google Scholar] [CrossRef]
- Wen, X.; Xiang, K.; Zhu, Y.; Xiao, L.; Liao, H.; Chen, W.; Chen, X.; Chen, H. 3D Hierarchical Nitrogen-Doped Graphene/CNTs Microspheres as a Sulfur Host for High-Performance Lithium-Sulfur Batteries. J. Alloys Compd. 2020, 815. [Google Scholar] [CrossRef]
- Wu, H.; Xia, L.; Ren, J.; Zheng, Q.; Xu, C.; Lin, D. A High-Efficiency N/P Co-Doped Graphene/CNT@Porous Carbon Hybrid Matrix as a Cathode Host for High Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2017, 5, 20458–20472. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Q.; Zhang, B.; Chen, C.; Lin, Z. Integrating Conductivity, Immobility, and Catalytic Ability into High-N Carbon/Graphene Sheets as an Effective Sulfur Host. Adv. Mater. 2020, 32, e1906357. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.K.; Piao, Y. N-doped Carbon Framework/Reduced Graphene Oxide Nanocomposite as a Sulfur Reservoir for Lithium-Sulfur Batteries. Electrochim. Acta 2016, 222, 1345–1353. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Du, H.; He, S.; Liu, L.; Fu, Z.; Xie, L.; Ai, W.; Huang, W. Molecularly Designed N, S Co-Doped Carbon Nanowalls Decorated on Graphene as a Highly Efficient Sulfur Reservoir for Li–S Batteries: A Supramolecular Strategy. J. Mater. Chem. A 2020, 8, 5449–5457. [Google Scholar] [CrossRef]



| Dopant Atom | Sulfur Host | Sulfur Content | Main Bonds | Doping Content (at.%) | Initial Capacity/Rate (mAh·g−1/C) | Retain Capacity/Cycles | Ref. |
|---|---|---|---|---|---|---|---|
| N | S@N-3D-rGO | 80 wt% | C–C/C=C/C–O/C=O/C–N | - | 1042/0.2 | 94.8%/100 | [54] |
| 3D RNGO/S | 90 wt% | - | 1186/0.1 | 96%/200 | [55] | ||
| NG/S | 74 wt% | 6.89 | 1309/1 | 663 mAh·g−1/300 | [56] | ||
| N-GA1/S | 75.5 wt% | 9.23 | 1210.7/0.1 | 72.4%/50 | [57] | ||
| B | BGA-S | 59 wt% | −BC2O, −BCO2, −BC3 | 1.76 | 1290/0.2 | 994 mAh·g−1/100 | [58] |
| S@BEEG | 72.5 wt% | 1.86 | 1476/0.1 | 82%/130/1 C | [59] | ||
| N, S | NSG-4/S | 68 wt% | C–N/C–S/S–O/S–S | N: 2.47 S: 6.31 | 1583/0.1 | 819 mAh·g−1/100 | [60] |
| 3D N, S-GP/S | 8.5 mg·cm−2 | - | 1200/0.2 | 63%/500/0.5 C | [61] | ||
| NSG/S | 43.3 wt% | N: 5.99 S: 5.89 | 1433/2 | 684 mAh·g−1/200 | [62] | ||
| A-NSG@S | 72.4 wt% | –C=S–/C–N/C–S/C=N | N: 4.18 S: 0.85 | 1178/0.2 | 780 mAh·g−1/600 | [63] | |
| N, B | NBCGN/S | 65 wt% | −BC2O/−BCO2/ −BC3/B2O3/B-N | N: 6.6 B: 7.0 | 1200/0.2 | 76%/300 | [64] |
| N, O | NOGB/S | 76 wt% | –COOC–/C–OH/C=O/ | N: 3.0 O: 18.1 | 1413/0.1 | 526 mAh·g−1/1000/1 C | [65] |
| Ni, N | S@Ni-N/G | 2.0 mg·cm–2 | Ni–N/Ni–C/Ni–Ni/Ni–S | - | 1103.6/0.2 | 953.5 mAh·g−1/100 | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Xing, F.; Gao, Q. Graphene-Based Nanomaterials as the Cathode for Lithium-Sulfur Batteries. Molecules 2021, 26, 2507. https://doi.org/10.3390/molecules26092507
Tian J, Xing F, Gao Q. Graphene-Based Nanomaterials as the Cathode for Lithium-Sulfur Batteries. Molecules. 2021; 26(9):2507. https://doi.org/10.3390/molecules26092507
Chicago/Turabian StyleTian, Jingkun, Fei Xing, and Qiqian Gao. 2021. "Graphene-Based Nanomaterials as the Cathode for Lithium-Sulfur Batteries" Molecules 26, no. 9: 2507. https://doi.org/10.3390/molecules26092507
APA StyleTian, J., Xing, F., & Gao, Q. (2021). Graphene-Based Nanomaterials as the Cathode for Lithium-Sulfur Batteries. Molecules, 26(9), 2507. https://doi.org/10.3390/molecules26092507






