Direct and Indirect Bactericidal Effects of Cold Atmospheric-Pressure Microplasma and Plasma Jet
Abstract
:1. Introduction
2. Results
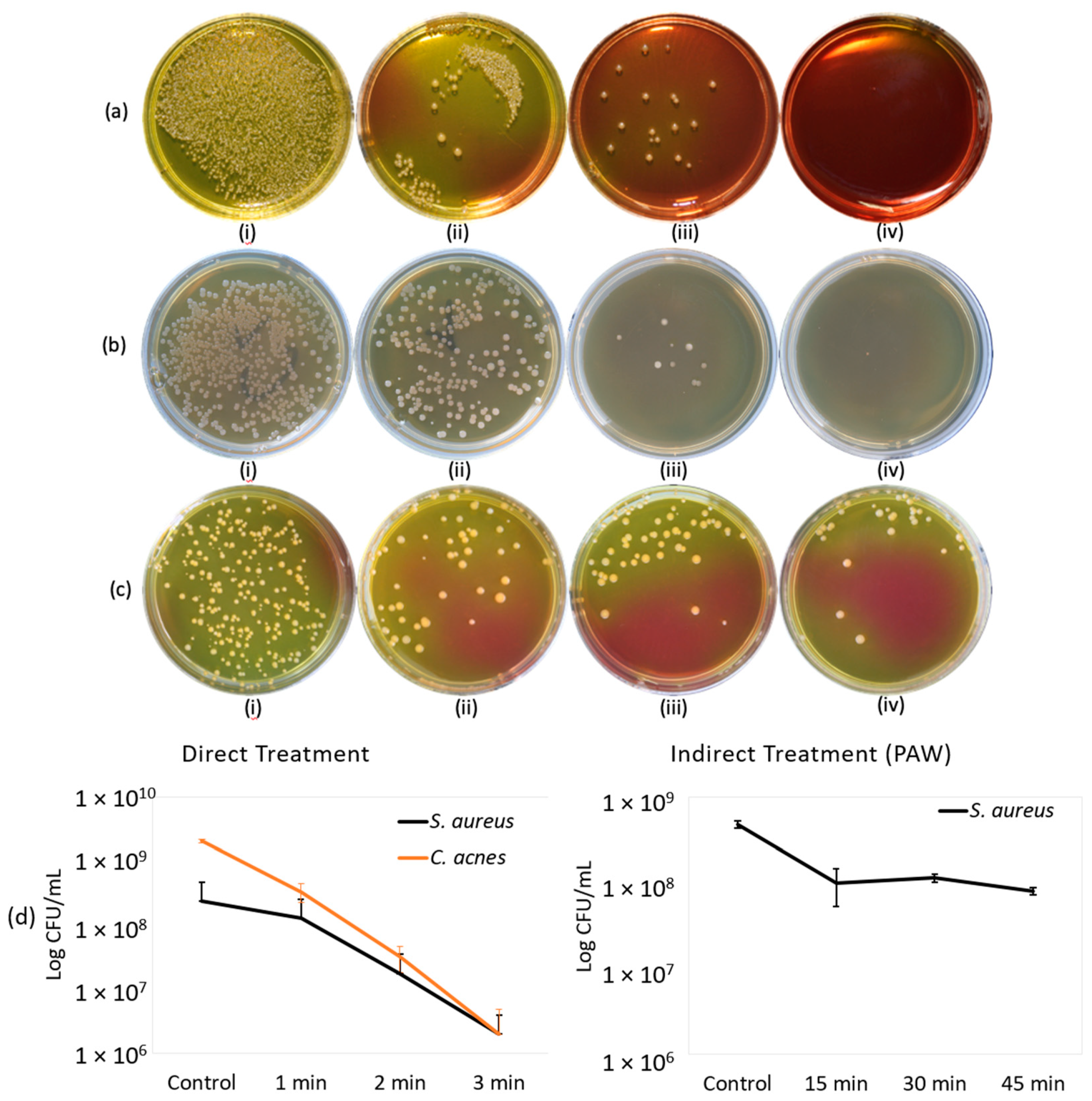
2.1. Direct Treatment
2.2. Indirect Treatment
2.3. Ozone Generated by Direct and Indirect Treatment
2.4. Power and Streamer Discharge for Direct and Indirect Treatment
2.5. UV-Vis Spectroscopy of Treated Water
2.6. Effect of Ozone on RONS
3. Materials and Methods
3.1. Direct Treatment
3.2. Indirect Treatment
3.3. Preparation of Bacterial Liquid
3.3.1. Staphylococcus aureus
3.3.2. Cutibacterium acnes
3.4. Samples Preparation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.-F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.-U. Cold Atmospheric Air Plasma Sterilization against Spores and Other Microorganisms of Clinical Interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanasiou, K. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/ polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Lerouge, S.; Tabrizian, M.; Wertheimer, M.R.; Marchand, R.; Yahia, L. Safety of plasma-based sterilization: Surface modifications of polymeric medical devices induced by Sterrad and Plazlyte processes. Biol. Med. Mater. Eng. 2002, 12, 3–13. [Google Scholar]
- E Holy, C.; Cheng, C.; E Davies, J.; Shoichet, M.S. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials 2000, 22, 25–31. [Google Scholar] [CrossRef]
- Matthews, I.P.; Gibson, C.; Samuel, A.H. Sterilisation of implantable devices. Clin. Mater. 1994, 15, 191–215. [Google Scholar] [CrossRef]
- Laroussi, M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188–1191. [Google Scholar] [CrossRef]
- Kayes, M.M.; Critzer, F.J.; Kelly-Wintenberg, K.; Roth, J.R.; Montie, T.C.; Golden, D.A. Inactivation of Foodborne Pathogens Using A One Atmosphere Uniform Glow Discharge Plasma. Foodborne Pathog. Dis. 2007, 4, 50–59. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Von Woedtke, T.; Brandenburg, R.; Weltmann, K.D.; Polak, M.; Winter, J.; Von dem Hagen, T. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Appl. Phys. 2011, 44, 18. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold Atmospheric Plasma: Charged Species and Their Interactions with Cells and Tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Yahaya, A.G.; Tomomichi, A.; Mustafa, F.; Jaroslav, K.; Blajan, M.; Shimizu, K. Skin treatment: Sterilization and Drug Delivery by Microplasma. In Proceedings of the 6th Internarional Symposium toward the Future of Advanced Researches in Shizuoka University 2020(ISFAR-SU2020), Hamamatsu, Japan, 5 March 2020; p. 54. [Google Scholar]
- Suschek, C.V.; Opländer, C. The application of cold atmospheric plasma in medicine: The potential role of nitric oxide in plasma-induced effects. Clin. Plasma Med. 2016, 4. [Google Scholar] [CrossRef]
- Park, S.; Choe, W.; Moon, S.Y.; Yoo, S.J. Electron characterization in weakly ionized collisional plasmas: From principles to techniques. Adv. Phys. X 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Kolb, J.F.; Mohamed, A.-A.H.; Price, R.O.; Swanson, R.J.; Bowman, A.; Chiavarini, R.L.; Stacey, M.; Schoenbach, K.H. Cold atmospheric pressure air plasma jet for medical applications. Appl. Phys. Lett. 2008, 92, 241501. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Wu, S.; Gou, J.; Pan, Y. An atmospheric-pressure, high-aspect-ratio, cold micro-plasma. Sci. Rep. 2014, 4, 7488. [Google Scholar] [CrossRef] [Green Version]
- Recek, N.; Andjelic, S.; Hojnik, N.; Filipič, G.; Lazović, S.; Vesel, A.; Primc, G.; Mozetič, M.; Hawlina, M.; Petrovski, G.; et al. Microplasma Induced Cell Morphological Changes and Apoptosis of Ex Vivo Cultured Human Anterior Lens Epithelial Cells—Relevance to Capsular Opacification. PLoS ONE 2016, 11, e0165883. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, R.; Shimizu, K.; Numabe, Y. Effects of microplasma irradiation on human gingival fibroblasts. Odontology 2015, 103, 194–202. [Google Scholar] [CrossRef]
- Shimizu, K.; A Tran, N.; Hayashida, K.; Blajan, M. Comparison of atmospheric microplasma and plasma jet irradiation for increasing of skin permeability. J. Phys. D Appl. Phys. 2016, 49, 315201. [Google Scholar] [CrossRef]
- Pai, K.; Timmons, C.; Roehm, K.D.; Ngo, A.; Narayanan, S.S.; Ramachandran, A.; Jacob, J.D.; Ma, L.M.; Madihally, S.V. Investigation of the Roles of Plasma Species Generated by Surface Dielectric Barrier Discharge. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nicol, M.J.; Brubaker, T.R.; Ii, B.J.H.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Blajan, M.; Tatematsu, S. Basic Study of Remote Disinfection and Sterilization Effect by Using Atmospheric Microplasma. IEEE Trans. Ind. Appl. 2012, 48, 1182–1188. [Google Scholar] [CrossRef]
- Shimizu, K.; Komuro, Y.; Tatematsu, S.; Blajan, M. Study of Sterilization and Disinfection in Room Air by Using Atmospheric Microplasma. Pharm. Anal. Acta 2011, S1. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef] [Green Version]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef] [Green Version]
- Bogaerts, A.; Yusupov, M.; Razzokov, J.; Van der Paal, J. Plasma for cancer treatment: How can RONS penetrate through the cell membrane? Answers from computer modeling. Front. Chem. Sci. 2019, 13, 253–263. [Google Scholar] [CrossRef]
- Hwang, I.; Jeong, J.; You, T.; Jung, J. Water electrode plasma discharge to enhance the bacterial inactivation in water. Biotechnol. Biotechnol. Equip. 2018, 32, 530–534. [Google Scholar] [CrossRef] [Green Version]
- De Backer, J.; Razzokov, J.; Hammerschmid, D.; Mensch, C.; Hafideddine, Z.; Kumar, N.; Van Raemdonck, G.; Yusupov, M.; Van Doorslaer, S.; Johannessen, C.; et al. The effect of reactive oxygen and nitrogen species on the structure of cytoglobin: A potential tumor suppressor. Redox Biol. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; Von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef]
- Yun, S.; Yoon, S.-Y.; Hong, E.J.; Giri, S.S.; Kim, S.G.; Han, S.J.; Kwon, J.; Oh, W.T.; Bin Lee, S.; Park, S.C. Effect of plasma-activated water, used as a disinfectant, on the hatch rate of dormant cysts of the Artemia salina. Aquaculture 2020, 523, 735232. [Google Scholar] [CrossRef]
- Lerouge, S.; Wertheimer, M.R.; Yahia, L. Plasma Sterilization: A Review of Parameters, Mechanisms, and Limitations. Plasmas Polym. 2001, 6, 175–188. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureusInfections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. MMWR Morb. Mortal Wkly. Rep. 2003, 52, 88. [Google Scholar]
- Boucher, H.W.; Corey, G.R. Epidemiology of Methicillin-ResistantStaphylococcus aureus. Clin. Infect. Dis. 2008, 46, S344–S349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; Calfee, R.P.; Plante, M.; Fischer, S.A.; Green, A. Propionibacterium acnes colonization of the human shoulder. J. Shoulder Elb. Surg. 2009, 18, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.Y.; Fenollar, F.; Stein, A.; Borrione, F.; Cohen, E.; LeBail, B.; Raoult, D. Propionibacterium acnesPostoperative Shoulder Arthritis: An Emerging Clinical Entity. Clin. Infect. Dis. 2008, 46, 1884–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangiamore, S.J.; Saleh, A.; Grosso, M.J.; Alolabi, B.; Bauer, T.W.; Iannotti, J.P.; Ricchetti, E.T. Early Versus Late Culture Growth of Propionibacterium acnes in Revision Shoulder Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2015, 97, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Del Greco, F.P.; Kaufman, F. Lifetime and reactions of OH radicals in discharge-flow systems. Discuss. Faraday Soc. 1962, 33, 128–138. [Google Scholar] [CrossRef]
- Szili, E.J.; Oh, J.-S.; Hong, S.-H.; Hatta, A.; Short, R.D. Probing the transport of plasma-generated RONS in an agarose target as surrogate for real tissue: Dependency on time, distance and material composition. J. Phys. D Appl. Phys. 2015, 48, 202001. [Google Scholar] [CrossRef]
- Oh, J.-S.; Szili, E.J.; Hatta, A.; Ito, M.; Shirafuji, T.; Oh, I. Ito Tailoring the Chemistry of Plasma-Activated Water Using a DC-Pulse-Driven Non-Thermal Atmospheric-Pressure Helium Plasma Jet. Plasma 2019, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.-S.; Szili, E.J.; Gaur, N.; Hong, S.-H.; Furuta, H.; Short, R.D.; Hatta, A. In-situ UV Absorption Spectroscopy for Monitoring Transport of Plasma Reactive Species through Agarose as Surrogate for Tissue. J. Photopolym. Sci. Technol. 2015, 28, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Gómez, M. Effect of dissolved oxygen concentration on nitrate removal from groundwater using a denitrifying submerged filter. J. Hazard. Mater. 2002, 90, 267–278. [Google Scholar] [CrossRef]
- Nakajima, M.; Hayamizu, T.; Nishimura, H. Effect of oxygen concentration on the rates of denitratification and denitrification in the sediments of an eutrophic lake. Water Res. 1984, 18, 335–338. [Google Scholar] [CrossRef]
- Schroeder, J.; Croot, P.; Von Dewitz, B.; Waller, U.; Hanel, R. Potential and limitations of ozone for the removal of ammonia, nitrite, and yellow substances in marine recirculating aquaculture systems. Aquac. Eng. 2011, 45, 35–41. [Google Scholar] [CrossRef]
- Rahmadi, P.; Kim, Y.R. Effects of different levels of ozone on ammonia, nitrite, nitrate, and dissolved organic carbon in sterilization of seawater. Desalin. Water Treat. 2013, 52, 4413–4422. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahaya, A.G.; Okuyama, T.; Kristof, J.; Blajan, M.G.; Shimizu, K. Direct and Indirect Bactericidal Effects of Cold Atmospheric-Pressure Microplasma and Plasma Jet. Molecules 2021, 26, 2523. https://doi.org/10.3390/molecules26092523
Yahaya AG, Okuyama T, Kristof J, Blajan MG, Shimizu K. Direct and Indirect Bactericidal Effects of Cold Atmospheric-Pressure Microplasma and Plasma Jet. Molecules. 2021; 26(9):2523. https://doi.org/10.3390/molecules26092523
Chicago/Turabian StyleYahaya, Ahmad Guji, Tomohiro Okuyama, Jaroslav Kristof, Marius Gabriel Blajan, and Kazuo Shimizu. 2021. "Direct and Indirect Bactericidal Effects of Cold Atmospheric-Pressure Microplasma and Plasma Jet" Molecules 26, no. 9: 2523. https://doi.org/10.3390/molecules26092523





