Gaseous Plastron on Natural and Biomimetic Surfaces for Resisting Marine Biofouling
Abstract
:1. Introduction
2. Factors Influencing the Formation and Maintenance of Gaseous Plastron
2.1. Surface Wettability
2.2. Buoyancy
2.3. Micro/nano-Morphologies
3. Antifouling Strategy Leveraged by Natural Organisms Bearing Gaseous Plastron
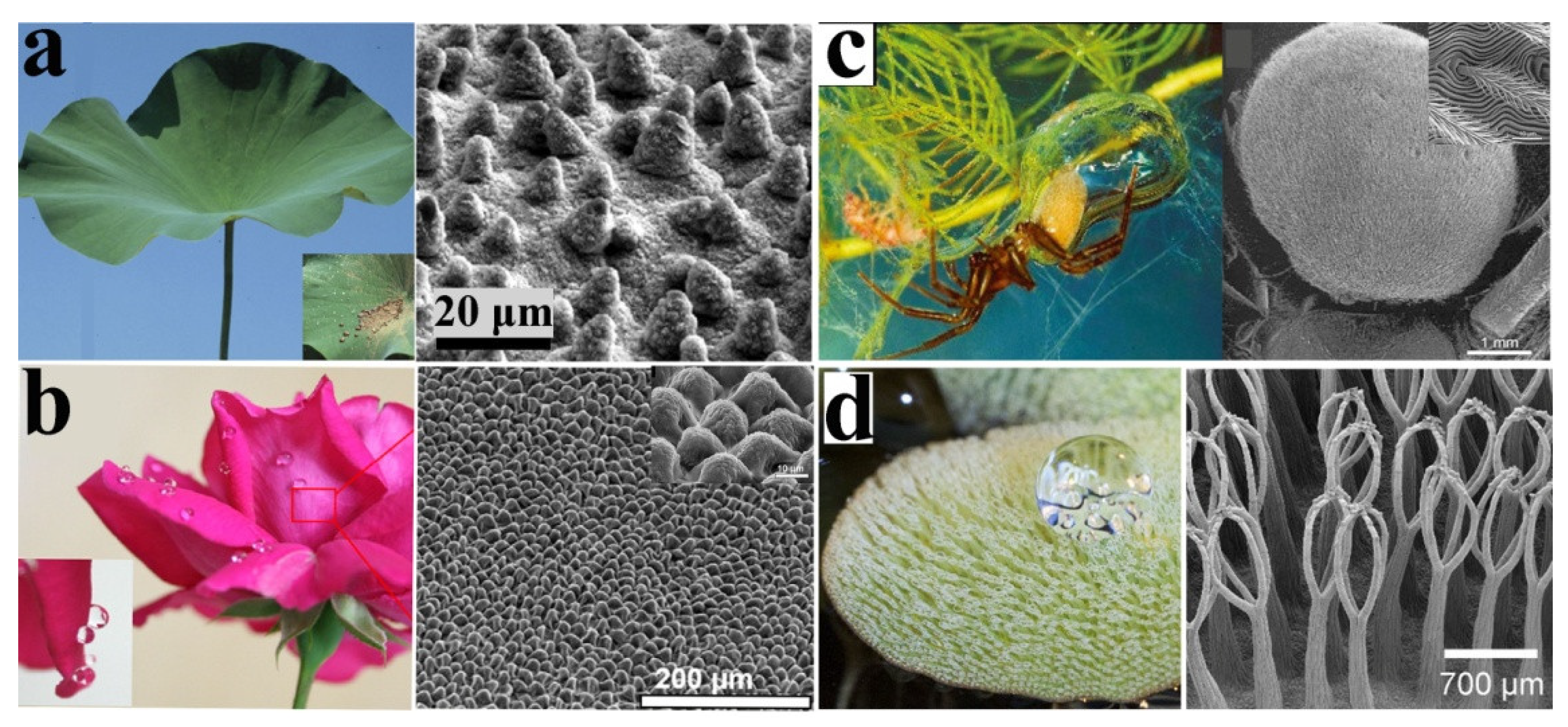
3.1. Lotus Leaf
3.2. Flower Petals
3.3. Water Spider
3.4. Salvinia
4. Application of Gaseous Plastron for Anti-Fouling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vinagre, P.; Simas, T.; Cruz, E.; Pinori, E.; Svenson, J. Marine biofouling: A european database for the marine renewable energy sector. J. Mar. Sci. Eng. 2020, 8, 495. [Google Scholar] [CrossRef]
- Faria, S.I.; Teixeira-Santos, R.; Gomes, L.C.; Silva, E.R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J.M. Experimental assessment of the performance of two marine coatings to curb biofilm formation of microfoulers. Coatings 2020, 10, 893. [Google Scholar] [CrossRef]
- Nalini, S.; Sandy Richard, D.; Mohammed Riyaz, S.U.; Kavitha, G.; Inbakandan, D. Antibacterial macro molecules from marine organisms. Int. J. Biol. Macromol. 2018, 115, 696–710. [Google Scholar] [CrossRef]
- Roubeix, V. Epidiatomic diatoms: An insight into interaction specificity. Phycol. Res. 2020, 68, 249–253. [Google Scholar] [CrossRef]
- Li, Q.; Sun, C.; Wang, Y.; Cai, H.; Li, L.; Li, J.; Shi, H. Fusion of microplastics into the mussel byssus. Environ. Pollut. 2019, 252, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.M.; van de Merwe, J.P.; Connolly, R.M. Global oxygen isoscapes for barnacle shells: Application for tracing movement in oceans. Sci. Total Environ. 2020, 705, 135782. [Google Scholar] [CrossRef]
- Monty, J.P.; Dogan, E.; Hanson, R.; Scardino, A.J.; Ganapathisubramani, B.; Hutchins, N. An assessment of the ship drag penalty arising from light calcareous tubeworm fouling. Biofouling 2016, 32, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Townsin, R. The ship hull fouling penalty. Biofouling 2003, 19, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Champ, M.A. A review of organotin regulatory strategies, pending actions, related costs and benefits. Sci. Total Environ. 2000, 258, 21–71. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Lewis, J.; Coutts, A. Biofouling invasions. Biofouling 2010, 24, 348–365. [Google Scholar]
- Vladkova, T. Surface engineering for non-toxic biofouling control. J. Univ. Chem. Technol. Metall. 2007, 42, 239–256. [Google Scholar]
- Vladkova, T. Surface modificationapproach to control biofouling. Marine Indust. Biofouling 2009, 4, 135–163. [Google Scholar]
- Vladkova, T.; Akuzov, D.; Koeppel, A.; Bruemmer, F. Current approaches to reduction marine biofilm formation. J. Chem. Technol. Metall. 2014, 49, 345–355. [Google Scholar]
- Ditsche-Kuru, P.; Schneider, E.S.; Melskotte, J.E.; Brede, M.; Leder, A.; Barthlott, W. Superhydrophobic surfaces of the water bug notonecta glauca: A model for friction reduction and air retention. Beilstein J. Nanotechnol. 2011, 2, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmert, A.; Florian Bohn, H.; Ditsche-Kuru, P.; Barthlott, W. Dry under water: Comparative morphology and functional aspects of air-retaining insect surfaces. J. Morphol. 2011, 272, 442–451. [Google Scholar] [CrossRef]
- Cui, X.; Liu, J.; Xie, L.; Huang, J.; Liu, Q.; Israelachvili, J.N.; Zeng, H. Modulation of hydrophobic interaction by mediating surface nanoscale structure and chemistry, not monotonically by hydrophobicity. Angew Chem. Int. Ed. Engl. 2018, 57, 11903–11908. [Google Scholar] [CrossRef]
- Yousefi, S.Z.; Tabatabaei-Panah, P.S.; Seyfi, J. Emphasizing the role of surface chemistry on hydrophobicity and cell adhesion behavior of polydimethylsiloxane/TiO2 nanocomposite films. Colloid. Surface. B 2018, 167, 492–498. [Google Scholar] [CrossRef]
- Zheng, D.; Jiang, Y.; Yu, W.; Jiang, X.; Zhao, X.; Choi, C.-H.; Sun, G. Salvinia-effect-inspired “sticky” superhydrophobic surfaces by meniscus-confined electrodeposition. Langmuir 2017, 33, 13640–13648. [Google Scholar] [CrossRef]
- Scardino, A.J.; Harvey, E.; De Nys, R. Testing attachment point theory: Diatom attachment on microtextured polyimide biomimics. Biofouling 2006, 22, 55–60. [Google Scholar] [CrossRef]
- Scardino, A.J.; Guenther, J.; de Nys, R. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef]
- Wu, A.H.F.; Nakanishi, K.; Cho, K.L.; Lamb, R. Diatom attachment inhibition: Limiting surface accessibility through air entrapment. Biointerphases 2013, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branner, F.G. Means and method for protection from marine parasites. U.S. Patent No. 123,021, 29 January 1937. [Google Scholar]
- Wang, B.; Zhang, Y.; Shi, L.; Jing, L.; Guo, Z. Advances in the theory of superhydrophobic surfaces. J. Mater. Chem. 2012, 22, 20112–20127. [Google Scholar] [CrossRef]
- Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. Wetting and spreading. Rev. Mod. Phys. 2009, 81, 739–805. [Google Scholar] [CrossRef]
- Ishida, N.; Sakamoto, M.; Miyahara, M.; Higashitani, K. Optical observation of gas bridging between hydrophobic surfaces in water. J. Colloid Interf. Sci. 2002, 253, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces. J. Phys. Condens. Matter. 2008, 20, 225010–225033. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.S.; Watson, G.S.; Cribb, B.W.; Watson, J.A. Non-wetting wings and legs of the cranefly aided by fine structures of the cuticle. J. Exper. Biol. 2010, 214, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Bush, J.W.M.; Hu, D.L.; Prakash, M. The integument of water-walking arthropods: Form and function. Adv. Insect Physiol. 2008, 34, 117–192. [Google Scholar]
- Du, P.; Hu, H.; Ren, F.; Song, D. Bubble characterizations on hydrophobic surface using lattice boltzmann simulation with large density ratios. Int. J. Numer. Meth. H. 2017, 27, 1311–1322. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L. Definition of superhydrophobic states. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Hashimoto, K.; Watanabe, T. Recent studies on super-hydrophobic films. Monatsh. Chem. 2001, 132, 31–41. [Google Scholar] [CrossRef]
- Patankar, N.A. On the modeling of hydrophobic contact angles on rough surfaces. Langmuir 2003, 19, 1249–1253. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. The “lotus effect” explained: Two reasons why two length scales of topography are important. Langmuir 2006, 22, 2966–2967. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, S.H.; Li, Y.; Li, H.J.; Zhang, L.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2003, 14, 1857–1860. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Nie, F.Q.; Zhai, J.; Jiang, L. Air bubble bursting effect of lotus leaf. Langmuir 2009, 25, 14129–14134. [Google Scholar] [CrossRef]
- Tsori, Y. Discontinuous liquid rise in capillaries with varying cross-sections. Langmuir 2006, 22, 8860–8863. [Google Scholar] [CrossRef]
- Ling, W.Y.L.; Lu, G.; Ng, T.W. Increased stability and size of a bubble on a superhydrophobic surface. Langmuir 2011, 27, 3233–3237. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Varhue, W.; McDevitt, R.; Hitt, D. Effect of wetting on the ability of nanomaterials to act as effective catalysts. Adv. Chem. Eng. Sci. 2016, 6, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Marmur, A. Underwater superhydrophobicity: Theoretical feasibility. Langmuir 2006, 22, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Schimmel, T.; Wiersch, S.; Koch, K.; Brede, M.; Barczewski, M.; Walheim, S.; Weis, A.; Kaltenmaier, A.; Leder, A.; et al. The salvinia paradox: Superhydrophobic surfaces with hydrophilic pins for air retention under water. Adv. Mater. 2010, 22, 2325–2328. [Google Scholar] [CrossRef]
- Mail, M.; Moosmann, M.; Häger, P.; Barthlott, W. Air retaining grids-a novel technology to maintain stable air layers under water for drag reduction. Phil. Trans. R. Soc. 2019, 377, 20190126. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W. Biomimic from the superhydrophobic plant leaves in nature: Binary structure and unitary structure. Plant Sci. 2007, 172, 1103–1112. [Google Scholar] [CrossRef]
- Ishida, N.; Sakamoto, M.; Miyahara, M.; Higashitani, K. Attraction between hydrophobic surfaces with and without gas phase. Langmuir 2000, 16, 5681–5687. [Google Scholar] [CrossRef]
- Tyrrell, J.W.G.; Attard, P. Atomic force microscope images of nanobubbles on a hydrophobic surface and corresponding force−separation data. Langmuir 2002, 18, 160–167. [Google Scholar] [CrossRef]
- Tyrrell, J.W.; Attard, P. Images of nanobubbles on hydrophobic surfaces and their interactions. Phys. Rev. Lett. 2001, 87, 176104. [Google Scholar] [CrossRef]
- Nguyen, A.; Stechemesser, H. Influence of dewetting kinetics on bubble-particle interaction. Phys. Chem. Chem. Phys. 2004, 6, 429–433. [Google Scholar] [CrossRef]
- Carrier, V.; Colin, A. Coalescence in draining foams. Langmuir 2003, 19, 4535–4538. [Google Scholar] [CrossRef]
- Bormashenko, E.; Stein, T.; Pogreb, R.; Aurbach, D. “Petal effect” on surfaces based on lycopodium: High-stick surfaces demonstrating high apparent contact angles. J. Phys. Chem. C 2009, 113, 5568–5572. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X. Nanobubble stability induced by contact line pinning. J. Chem. Phys. 2013, 138, 014706. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.-Q.; Zhang, D.; Feng, C.; Jiang, L. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Webb, H.K.; Fadeeva, E.; Chichkov, B.N.; Wu, A.H.F.; Lamb, R.; Wang, J.Y.; Crawford, R.J.; Ivanova, E.P. Air-directed attachment of coccoid bacteria to the surface of superhydrophobic lotus-like titanium. Biofouling 2012, 28, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.S.; Hetz, S.K. The diving bell and the spider: The physical gill of argyroneta aquatica. J. Exp. Biol. 2011, 214, 2175–2181. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Bai, H.; Huang, Z.; Tian, X.; Nie, F.-Q.; Zhao, Y.; Zhai, J.; Jiang, L. Directional water collection on wetted spider silk. Nature 2010, 463, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Pro. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Park, Y.M.; Gang, M.; Seo, Y.H.; Kim, B.H. Artificial petal surface based on hierarchical micro- and nanostructures. Thin Solid Films 2011, 520, 362–367. [Google Scholar] [CrossRef]
- Huang, C.; Guo, Z. The wettability of gas bubbles: From macro behaviors to nano structures to applications. Nanoscale 2018, 10, 19659–19672. [Google Scholar] [CrossRef]
- Mayser, M.; Bohn, H.; Reker, M.; Barthlott, W. Measuring air layer volumes retained by submerged floating-ferns salvinia and biomimetic superhydrophobic surfaces. Beilstein J. Nanotechnol. 2014, 5, 812–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant surfaces: Structures and functions for biomimetic innovations. Nano-Micro. Lett. 2017, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Philos. Trans. R. Soc. A Sci. 2016, 374, 20160191. [Google Scholar] [CrossRef] [Green Version]
- Barthlott, W.; Wiersch, S.; Čolić, Z.; Koch, K. Classification of trichome types within species of the water fern salvinia, and ontogeny of the egg-beater trichomes. Botany 2009, 87, 830–836. [Google Scholar] [CrossRef]
- Barthlott, W.; Riede, K. Mimicry and ultrastructural analogy between the semi-aquatic grasshopper paulinia acuminata (orthoptera) and its foodplant, the water-fern salvinia auriculata (filicatae). Amazoniana 1994, 13, 47–58. [Google Scholar]
- Amabili, M.; Giacomello, A.; Meloni, S.; Casciola, C.M. Unraveling the salvinia paradox: Design principles for submerged superhydrophobicity. Adv. Mater. Interfaces 2015, 2, 1500248. [Google Scholar] [CrossRef] [Green Version]
- Babu, D.; Mail, M.; Barthlott, W.; Schneider, J. Superhydrophobic vertically aligned carbon nanotubes for biomimetic air retention under water (salvinia effect). Adv. Mater. Interfaces 2017, 4, 1700273. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, S.; Huang, T.-Y.; Dong, A.; Cao, D.; Li, H.; Xue, Y.; Lv, P.; Duan, H. Superrepellency of underwater hierarchical structures on Salvinia leaf. Proc. Natl. Acad. Sci. USA 2020, 117, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.F.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.B. Engineered antifouling microtopographies–effect of feature size, geometry, and roughness on settlement of zoospores of the green alga ulva. Biofouling 2007, 23, 55–62. [Google Scholar] [CrossRef]
- Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Schumacher, J.F.; Wilkerson, W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered antifouling microtopographies–correlating wettability with cell attachment. Biofouling 2006, 22, 11–21. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, L.; Hu, H.; Bing, W.; Tian, L.; Zhao, J. A synergistic antibacterial platform: Combining mechanical and photothermal effects based on van-mos2–au nanocomposites. Nanotechnology 2021, 32, 085102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme mimicry for combating bacteria and biofilms. Acc. Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Bers, A.V.; Wahl, M. The influence of natural surface microtopographies on fouling. Biofouling 2004, 20, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bing, W.; Tian, L.; Wang, Y.; Jin, H.; Ren, L.; Dong, S. Biofouling: Bio-inspired non-bactericidal coating used for antibiofouling. Adv. Mater. Technol. 2019, 4, 1800480. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244–254. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Scardino, A.J.; de Nys, R. Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 2011, 27, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Pettitt, M.; Wode, F.; Sancet, M.; Fu, J.; Ji, J.; Callow, M.; Callow, J.; Rosenhahn, A.; Grunze, M. Interaction of zoospores of the green alga ulva with bioinspired micro- and nanostructured surfaces prepared by polyelectrolyte layer-by-layer self-assembly. Adv. Funct. Mater. 2010, 20, 1984–1993. [Google Scholar] [CrossRef]
- Hwang, G.B.; Page, K.; Patir, A.; Nair, S.P.; Allan, E.; Parkin, I.P. The anti-biofouling properties of superhydrophobic surfaces are short-lived. ACS Nano 2018, 12, 6050–6058. [Google Scholar] [CrossRef]
- Scardino, A.J.; Fletcherm, L.E.; Lewis, J.A. Fouling control using air bubble curtains: Protection for stationary vessels. J. Marine Eng. Technol. 2009, 8, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Esmeryan, K.D.; Castano, C.E.; Chaushev, T.A.; Mohammadi, R.; Vladkova, T.G. Silver-doped superhydrophobic carbon soot coatings with enhanced wear resistance and anti-microbial performance. Colloid. Surface. A 2019, 582, 123880. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Bing, W.; Chen, C.; Chen, Z. Gaseous Plastron on Natural and Biomimetic Surfaces for Resisting Marine Biofouling. Molecules 2021, 26, 2592. https://doi.org/10.3390/molecules26092592
Cai Y, Bing W, Chen C, Chen Z. Gaseous Plastron on Natural and Biomimetic Surfaces for Resisting Marine Biofouling. Molecules. 2021; 26(9):2592. https://doi.org/10.3390/molecules26092592
Chicago/Turabian StyleCai, Yujie, Wei Bing, Chen Chen, and Zhaowei Chen. 2021. "Gaseous Plastron on Natural and Biomimetic Surfaces for Resisting Marine Biofouling" Molecules 26, no. 9: 2592. https://doi.org/10.3390/molecules26092592






