Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solubility of the Pharmaceuticals in Choline Chloride- or Betaine-Based NADES
2.2. Solubility of the Pharmaceuticals in the NADES Based Organic Acid and Base
| NADES | Molar Ratio | Pharmaceuticals | ||||||
|---|---|---|---|---|---|---|---|---|
| Ch | Gri | Mp | Nf | Ra | Spi | Tmp | ||
| CA:F:W | 1:1:5 | ++ | − | ++ | − | ++ | − | ++ |
| CA:S:W | 1:1:6 | ++ | − | + | − | + | − | + |
| CA:Go:W | 1:1:2 | ++ | − | + | − | ++ | − | + |
| CA:So:W | 1:1:7 | ++ | − | ++ | − | ++ | − | + |
| CA:Xo:W | 1:1:5 | ++ | − | ++ | − | ++ | − | ++ |
| CA:Po:W | 1:1:3.7 | ++ | − | ++ | − | ++ | +/P | ++ |
| CA:Pro:W | 1:1:5 | − | − | +/P | − | ++ | − | ++ |
| MA:F:W | 1:1:7 | ++ | − | ++ | − | ++ | − | ++ |
| MA:G:W | 1:1:7 | ++ | − | +/P | − | ++ | − | +/P |
| MA:F:G:W | 1:1:1:7 | ++ | − | ++ | − | ++ | − | ++ |
| MA:S:W | 1:1:7 | ++ | − | + | − | ++ | − | + |
| MA:Xo:W | 1:1:4 | ++ | − | ++ | − | ++ | − | ++ |
| MA:Po:W | 1:1:3 | ++ | − | ++ | − | ++ | ++ | ++ |
| MA:BA:W | 1:1:3 | ++ | − | + | − | ++ | − | ++ |
| MA:Pro:W | 1:1:3.5 | ++ | − | + | − | ++ | ++ | ++ |
| LA:F | 5:1 | ++ | − | ++ | − | ++ | ++ | + |
| LA:Po | 1:1 | ++ | − | ++ | − | ++ | ++ | ++ |
| LA:BA:W | 2:1:1 | ++ | − | ++ | − | ++ | − | − |
| AA:F:W | 5:2:5 | ++ | +/P | ++ | − | +/P | ++ | ++ |
| AA:Po | 1:1 | ++ | + | ++ | − | ++ | ++ | ++ |
| AA:BA | 5:1 | ++ | + | ++ | − | ++ | ++ | ++ |
2.3. Solubility and Stability of the Pharmaceuticals in Sugar-Based NADES
| NADES | Molar Ratio | Pharmaceuticals | ||||||
|---|---|---|---|---|---|---|---|---|
| Ch | Gri | Mp | Nf | Ra | Spi | Tmp | ||
| G:F:W | 1:1:10 | ++ | − | +/P | − | ++ | − | − |
| S:F:W | 1:1:10 | ++ | − | ++ | − | ++ | − | − |
| S:F:G:W | 1:1:1:11 | ++ | − | + | − | ++ | − | − |

2.4. Solubility and Stability of the Pharmaceuticals in Selected NADES, Lactic Acid, Acetic Acid, and Propylene Glycol in a Range of Concentrations
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Deep Eutectic Solvents
3.3. Solubility and Stability Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Choi, Y.H.; Van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef] [PubMed]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents for the “green”extraction of vanillin from vanilla pods. Flavour Fragr. J. 2017, 33, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria baicalensis as a Replacement for Conventional Organic Solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic Liquids as Active Pharmaceutical Ingredients. Chem. Med. Chem. 2011, 6, 975–985. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D.; Mjalli, F.S. Formation of type III Deep Eutectic Solvents and effect of water on their intermolecular interactions. Fluid Phase Equilibria 2017, 441, 43–48. [Google Scholar] [CrossRef]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Anti-microbial activities of ionic liquids. Green Chem. 2002, 5, 52–56. [Google Scholar] [CrossRef]
- Pernak, J.; Goc, I.; Mirska, I. Anti-microbial activities of protic ionic liquids with lactate anion. Green Chem. 2004, 6, 323–329. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [Green Version]
- Mizuuchi, H.; Jaitely, V.; Murdan, S.; Florence, A. Room temperature ionic liquids and their mixtures: Potential pharmaceutical solvents. Eur. J. Pharm. Sci. 2008, 33, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Jaitely, V.; Mizuuchi, H.; Florence, A.T. Current-stimulated release of solutes solubilized in water-immiscible room temperature ionic liquids (RTILs). J. Drug Target. 2010, 18, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parmar, V.S.; Malhotra, S.V. Enhanced solubility and selective benzoylation of nucleosides in novel ionic liquid. Tetrahedron Lett. 2007, 48, 809–812. [Google Scholar] [CrossRef]
- Smith, K.B.; Bridson, R.H.; Leeke, G.A. Solubilities of Pharmaceutical Compounds in Ionic Liquids. J. Chem. Eng. Data 2011, 56, 2039–2043. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Stoimenovski, J.; Macfarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. Ionic Liquid Salt Forms of Active Pharmaceutical Ingredients: A Position Paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef]
- McCrary, P.D.; Beasley, P.A.; Gurau, G.; Narita, A.; Barber, P.S.; Cojocaru, O.A.; Rogers, R.D. Drug specific, tuning of an ionic liquid’s hydrophilic–lipophilic balance to improve water solubility of poorly soluble active pharmaceutical ingredients. New J. Chem. 2013, 37, 2196–2202. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Rogers, R.D. Overcoming the problems of solid state drug formulations with ionic liquids. Ther. Deliv. 2014, 5, 489–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulekuhn, G.S.; Dressman, J.B.; Saal, C. Trends in Active Pharmaceutical Ingredient Salt Selection based on Analysis of the Orange Book Database. J. Med. Chem. 2007, 50, 6665–6672. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Tahara, Y.; Tamura, M.; Kamiya, N.; Goto, M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem. Commun. 2010, 46, 1452–1454. [Google Scholar] [CrossRef]
- Williams, H.D.; Sahbaz, Y.; Ford, L.; Nguyen, T.-H.; Scammells, P.J.; Porter, C.J.H. Ionic liquids provide unique opportunities for oral drug delivery: Structure optimization and in vivo evidence of utility. Chem. Commun. 2014, 50, 1688–1690. [Google Scholar] [CrossRef]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef]
- Rozema, E.; Sips, H.C.M.; Verpoorte, R.; Meijer, O.C.; Kooijman, S.; Van Dam, A.D.; Choi, Y.H. Extending pharmacological dose-response curves for salsalate with natural deep eutectic solvents. RSC Adv. 2015, 5, 61398–61401. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules 2017, 22, 1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamseddin, A.; Crauste, C.; Durand, E.; Villeneuve, P.; Dubois, G.; Durand, T.; Vercauteren, J.; Veas, F. Resveratrol formulated with a natural deep eutectic solvent inhibits active matrix metalloprotease-9 in hormetic conditions. Eur. J. Lipid Sci. Technol. 2017, 119, 1700171. [Google Scholar] [CrossRef] [Green Version]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Kelley, S.P.; Narita, A.; Holbrey, J.D.; Green, K.D.; Reichert, W.M.; Rogers, R.D. Understanding the Effects of Ionicity in Salts, Solvates, Co-Crystals, Ionic Co-Crystals, and Ionic Liquids, Rather than Nomenclature, Is Critical to Understanding Their Behavior. Cryst. Growth Des. 2013, 13, 965–975. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Kunz, W. Properties of sugar-based low-melting mixtures. Mol. Phys. 2014, 112, 1241–1245. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Budavari, S. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals, 13th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Goho, A. The crystal form of a drug can be the secret to its success. Sci. News 2004, 166, 122–124. [Google Scholar] [CrossRef]
- Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of Functional Therapeutic Deep Eutectic Solvents Based on Choline Chloride and Ascorbic Acid. ACS Sustain. Chem. Eng. 2018, 6, 10355–10363. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents from choline chloride and betaine—Physicochemical properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Nangia, A. Eutectics as improved pharmaceutical materials: Design, properties and characterization. Chem. Commun. 2014, 50, 906–923. [Google Scholar] [CrossRef] [PubMed]
- Jalil, R.; Nixon, J.R. Biodegradable poly(lactic acid) and poly(lactide-co-glycolide) microcapsules: Problems associated with preparative techniques and release properties. J. Microencapsul. 1990, 7, 297–325. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with d,l-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. J. Control Release 1993, 25, 89–98. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nat. Cell Biol. 1997, 388, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Feng, S.-S. Preparation and characterization of poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA-PEG-PLA) microspheres for controlled release of paclitaxel. Biomaterials 2003, 24, 5037–5044. [Google Scholar] [CrossRef]

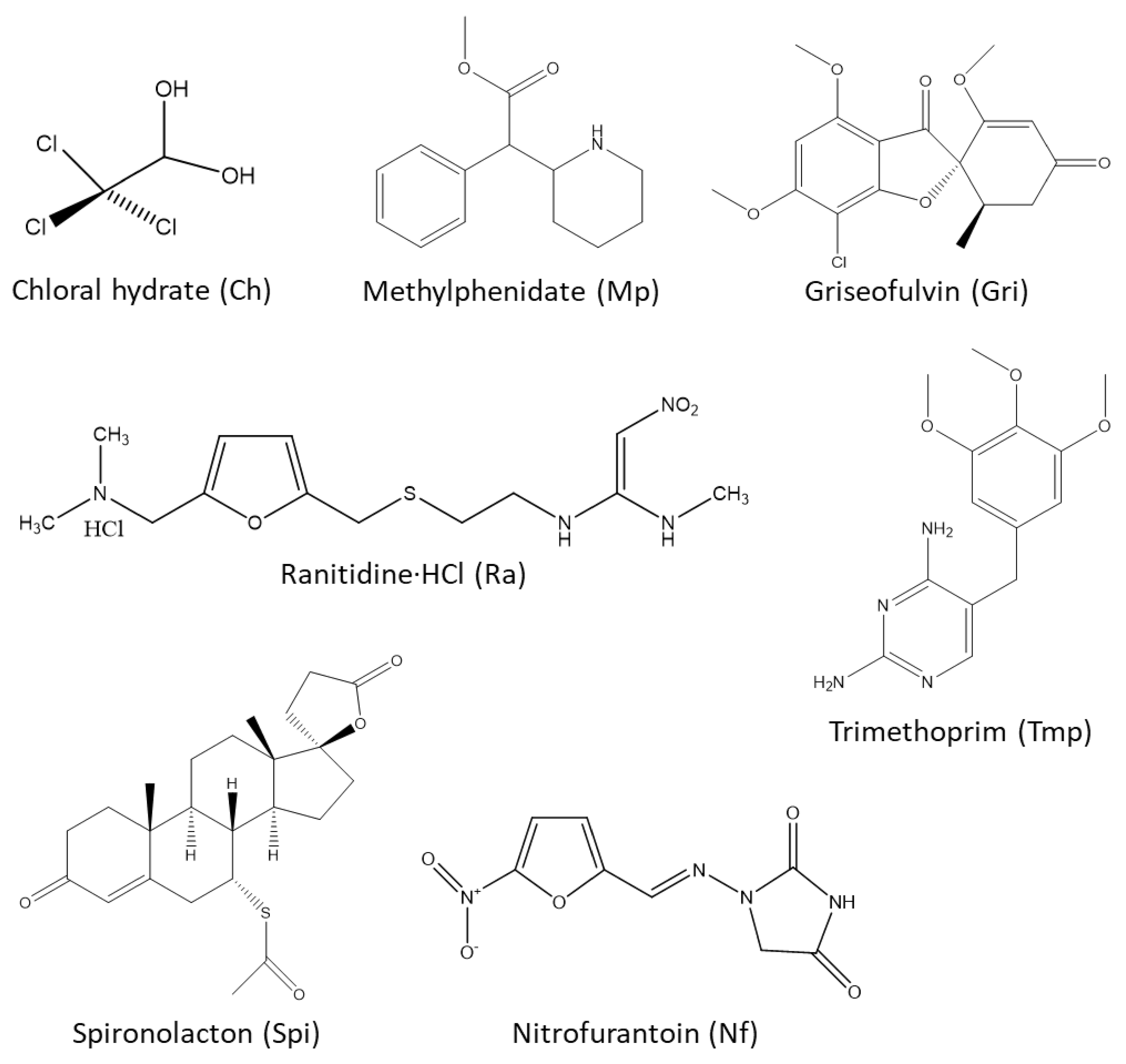

| Compound | pKa/PH | Solubility (in g/mL), Uses, and Some Other Information |
|---|---|---|
| Chloral Hydrate (Ch) | - | Freely soluble in water (2.4, 0 °C), (8.3, 25 °C), (14.3, 40 °C), but undergoes dissociation. Soluble in ethanol (0.77), chloroform (0.50), ether (0.66), glycerol (2.0), and olive oil (0.71). Freely soluble in acetone, methyl ethyl ketone. Moderately or sparingly soluble in turpentine, petroleum ether, carbon tetrachloride, benzene, and toluene. Therapeutic use—sedative |
| Griseofulvin (Gri) | - | Practically insoluble in water, petroleum ether. Slightly soluble in ethanol, methanol, acetone, benzene, chloroform, ethyl acetate, and acetic acid. Soluble in DMF (0.12–0.14). Therapeutic use—antifungal |
| Methylphenidate (Mp) | pKa 8.9 (of HCl crystals) | Practically insoluble in water and petroleum ether. Soluble in ethanol, ethyl acetate, and ether. Methylphenidate∙HCl is soluble in water, ethanol, and chloroform. Therapeutic use—CNS stimulant |
| Nitrofurantoin (Nf) | pKa 7.2 | Soluble in water pH 7 (0.00019), 95% ethanol (0.00051), acetone (0.0051), DMF (0.08), peanut oil (0.000021), glycerol (0.0006), and polyethylene glycol (0.015). Therapeutic use—antibacterial |
| Ranitidine∙HCl (Ra) | - | Freely soluble in water and acetic acid. Soluble in methanol and sparingly soluble in ethanol. Practically insoluble in chloroform. Therapeutic use—antiulcer |
| Spironolactone (Spi) | - | Soluble in most organic solvents and practically insoluble in water. Therapeutic use—diuretic |
| Trimethoprim (Tmp) | pKa 6.6 | Soluble (at 25 °C) in DMAC (0.139), benzyl alcohol (0.073), propylene glycol (0.026), chloroform (0.018), methanol (0.012), water (0.0004), ether (0.00003), And benzene (0.00002). Therapeutic use—antibacterial |
| Choline chloride (CC) | - | Very soluble in water (neutral) and ethanol. |
| Betaine (Be) | - | Soluble in water (1.60), methanol (0.43), and ethanol (0.07). Sparingly soluble in ether. The pH of saturated solution of betaine monohydrate is about 8.0. Solubility of Betaine∙HCl (at 25 °C) in water, 0.65; ethanol, 0.04. Practically insoluble in chloroform and ether. The pH of 5% (w/v) aqueous solution = 1.0. Therapeutic use—in treatment of homocystinuria |
| Beta-Alanine (BA) | pK1 3.60 pK2 10.19 | Freely soluble in water (pH of 5% aqueous solution: 6.0–7.3). Slightly soluble in ethanol; practically insoluble in ether and acetone. |
| l-Proline (Pro) | pI 6.30 pK1 1.99 pK2 10.60 | Soluble in water (1.27, 0 °C), (1.62, 25 °C), (2.07, 50 °C), (2.39, 65 °C), and ethanol (0.012, 35 °C). Insoluble in ether, butanol, and isopropanol. |
| Acetic acid (AA) | pKa 4.74 | An excellent solvent for many organic compounds. Miscible with water, ethanol, glycerol, ether, and carbon tetrachloride. pH of aqueous solution—2.4 (1.0 M); 2.9 (0.1 M); 3.4 (0.01 M). Used in pharmaceutical and food industries as acidifier and preservative. LD50 orally in rats—3.73 g/kg. |
| Lactic acid (LA) | pKa 3.86 | Soluble in water, ethanol, furfurol; less soluble in ether. Practically insoluble in chloroform, petroleum ether, and carbon disulfide. There are a wide range of applications of LA in pharmaceutical-, food- and cosmetics industries. LD50 orally in rats—3.73 g/kg. |
| dl-Malic acid (MA) | - | Solubility (at 20 °C) in: water, 0.56; methanol, 0.65; ethanol, 0.36; acetone, 0.14; dioxane, 0.23; and diethyl ether, 0.006. Practically insoluble in benzene. Used in pharmaceutical and food industries as flavoring agent, flavor enhancer, and acidulant. |
| Citric acid (CA) | pK1 3.128 pK2 4.761 pK3 6.396 | Solubility of the anhydrate form in water increases with higher temperatures, e.g., 0.59 at 20 °C; 0.71 at 50 °C; and 0.84 at 100 °C. pH of 0.1 N solution = 2.2. Solubility of the monohydrate crystals in ether, 0.015; chloroform, 0.0001; amyl alcohol, 0.125; amyl acetate, 0.052; and ethyl acetate, 0.048; methanol, 1.56 (at 19 °C); propanol, 0.49 (at 19 °C). Pharmaceutical incompatibilities—K-tartrate, alkali- and alkaline earth carbonates and bicarbonates, acetates, sulfides. Used in pharmaceutical, food and beverage industries as acidulant, effervescent, pH adjuster, antioxidant. LD50 i.p. in rats—975 mg/kg. |
| Propylene glycol (Po) | - | Hygroscopic, viscous liquid. Miscible with water, acetone, chloroform. Soluble in ether; dissolves essential oils; and immiscible with fixed oils. It’s stable at room temperature, but tends to oxidize at high temperatures to produce propionaldehyde, lactic acid, pyruvic acid, and acetic acid. Used in pharmaceutical and food industries as solvent, emulsifier, and humectant. LD50 orally in rats—25 mL/kg. |
| Glycerol (Go) | - | Hygroscopic syrupy liquid with sweet warm taste (about 0.6 times as sweet as cane sugar), neutral to litmus. Miscible with water, ethanol. Soluble in ethyl acetate (1:11) and ethyl ether (1:500). Insoluble in benzene, chloroform, carbon tetrachloride, carbon disulfide, petroleum ether, and oils. Used in pharmaceutical, food & cosmetics industries as solvent, humectant, emollient, and sweetener. LD50 in rats—orally, >20 mL/kg; i.v. 4.4 mL. |
| Xylitol (Xo) | - | Solubility of the stable form (orthorhombic needles or prisms) in methanol, 0.047; ethanol, 0.0095; and water, 0.642. Use: as oral nutrient (sweetness equal to sucrose), i.v. nutrient, and in anticaries preparation. LD50 orally in mice: approx. 22 g/kg. |
| Sorbitol (So) | pH 7.0 | Freely soluble in water (up to 0.83), a concentrated solution provides a higher viscosity than its corresponding glycerol solution. Sparingly soluble in cold ethanol, but solubility increases with increased temperature. Soluble in methanol, isopropanol, butanol, cyclohexanol, phenol, acetone, acetic acid, DMF, pyridine, and acet-amide solutions. Uses in food industries—sugar substitute for diabetics, humectant, softener in peanut/coconut butter, sequestrant in soft drinks and wines, to reduce undesirable aftertaste of saccharin. Uses in pharmaceutical industry—flavor agent, excipient in tablet to increase absorption of vitamins, and laxative. |
| Mannitol (Mo) | pKa 13.5 (18 °C) | Soluble (at 25 °C) in water (0.18), ethanol (0.012), and glycerol (0.055). More soluble in hot water. Soluble in pyridine, aniline, aqueous solution of alkalis. Insoluble in ether. Used in pharmaceutical and food industries as excipient, diluent, lubricant, anticaking agent, stabilizer, thickener, sweetener, and flavoring agent. Used in therapy as diuretic and diagnostic aid of renal function. |
| d-Fructose (F) | pKa 12.06 (18 °C) | Occurs in both furanose and pyranose forms. Freely soluble in water. Soluble in ethanol (0.066) and methanol (0.071). Slightly soluble in cold, but freely soluble in hot acetone. Soluble in pyridine, ethylamine, and methylamine. |
| α-d-Glucose (G) | pH 5.9 (0.5 M aqueous solution) | The α-form-monohydrate is soluble in water (1.0) and ethanol (0.017). The α-form-anhydrate is soluble in water (0.91, 25 °C); (1.25, 30 °C); (2.44, 50 °C); (3.57, 70 °C); (5.55, 90 °C), and in methanol (0.008, 20 °C). Very sparingly soluble in ethanol, ether, and acetone. Soluble in hot glacial acetic acid, pyridine, and aniline. |
| NADES | Molar Ratio | Pharmaceuticals | ||||||
|---|---|---|---|---|---|---|---|---|
| Ch | Gri | Mp | Nf | Ra | Spi | Tmp | ||
| CC:LA | 1:1 | ++ | − | ++ | − | ++ | − | − |
| CC:MA:W | 1:1:4 | ++ | − | ++ | − | ++ | − | − |
| CC:MeA:W | 1:1:4 | ++ | − | ++ | − | ++ | − | − |
| CC:CA:W | 1:1:6 | ++ | − | ++ | − | ++ | − | − |
| CC:Po:W | 1:1:1 | ++ | − | ++ | − | ++ | − | − |
| CC:Go:W | 1:1:1 | ++ | − | +/P | − | + | − | − |
| CC:So:W | 3:1:6 | ++ | − | − | − | + | − | − |
| CC:G:W | 5:2:5 | ++ | − | − | − | + | − | − |
| CC:F:W | 1:1:3 | ++ | − | − | − | + | − | − |
| CC:S:W | 4:1:7 | ++ | − | − | − | − | − | − |
| CC:Man:W | 5:2:5 | ++ | − | − | − | + | − | − |
| CC:Tre:W | 4:1:5 | ++ | − | − | − | + | − | − |
| CC:X:W | 2:1:2 | ++ | − | − | − | + | − | − |
| CC:MA:Xo:W | 1:1:1:4 | ++ | − | +/P | − | ++ | − | − |
| CC:MA:Pro:W | 1:1:1:4 | ++ | − | +/P | − | ++ | − | − |
| CC:AA:Pro:W | 1:1:1:5 | ++ | − | ++ | + | ++ | +/P | − |
| Be:S:W | 2:1:8 | ++ | − | ++ | − | ++ | − | − |
| Be:MA:W | 1:1:7 | ++ | − | ++ | − | ++ | − | ++ |
| Be:MA:G:W | 1:1:1:7 | ++ | − | +/P | − | ++ | − | − |
| Be:MA:Pro:W | 1:1:1:7 | ++ | − | ++ | − | ++ | − | ++ |
| Be:AA:Pro:W | 1:1:1:5 | +/P | − | +/P | − | +/P | − | − |
| NADES | Molar Ratio of NADES Components | wt.% of Water | Drug Tested | Observation of Solubility of Drug (mg/mL) in NADES | Molar Ratio of NADES and Drug (a) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 50 | 70 | 100 | 150 | 200 | 250 | |||||
| CC:AA:Pro:W | 1:1:1:5 | 22 | Ch | + | + | + | + | + | + | + | +/P | +/P | 3:3:3:15:1 |
| Ra | + | + | + | + | + | + | + | + | + | 4:4:4:20:1 | |||
| Mp | + | − | np | np | np | np | np | np | np | np | |||
| Nf | +P | − | np | np | np | np | np | np | np | np | |||
| CA:F:W | 1:1:5 | 19 | Mp | + | + | + | + | + | + | − | np | np | 6:6:38:1 |
| Tmp | + | + | + | + | + | − | np | np | np | 11:11:68:1 | |||
| CA:S:W | 1:1:6 | 16 | Mp | + | − | np | np | np | np | np | np | np | np |
| Tmp | + | + | − | np | np | np | np | np | np | np | |||
| CA:Po:W | 1:1:4 | 21 | Ch | + | + | + | + | + | + | + | + | + | 5:5:20:2 |
| Ra | + | + | + | + | + | + | + | + | + | 5:5:21:1 | |||
| Mp | + | + | + | + | + | + | + | + | + | 7:7:28:2 | |||
| Tmp | + | + | + | + | + | + | + | + | + | 4:4:17.5:1 | |||
| Spi | − | − | np | np | np | np | np | np | np | np | |||
| MA:F:W | 1:1:7 | 28 | Ch | + | + | + | + | + | + | + | + | + | 2:2:14:1 |
| Ra | + | + | + | + | + | + | + | + | + | 4:4:29:1 | |||
| Mp | + | + | + | + | + | + | +/P | np | np | 7:7:52:1 | |||
| Tmp | + | + | + | + | + | +/P | np | np | np | 13:13:93:1 | |||
| MA:Po:W | 1:1:3 | 20 | Ch | + | + | + | + | + | + | + | + | + | 3:3:9:1 |
| Ra | + | + | + | + | + | + | + | + | + | 6:6:19:1 | |||
| Mp | + | + | + | + | + | + | + | + | + | 4:4:13:1 | |||
| Tmp | + | + | + | + | + | + | + | + | + | 5:5:16:1 | |||
| Spi | + | + | − | np | np | np | np | np | np | np | |||
| LA:F | 5:1 | 0 | Ch | + | + | + | + | + | + | + | + | + | 6:1:1 |
| Ra | + | + | + | + | + | + | + | + | + | 13:3:1 | |||
| Mp | + | + | + | + | + | + | + | + | + | 9:2:1 | |||
| Tmp | + | − | np | np | np | np | np | np | np | np | |||
| Spi | + | − | np | np | np | np | np | np | np | np | |||
| LA:Po | 1:1 | 0 | Ch | + | + | + | + | + | + | + | + | + | 9:9:2 |
| Ra | + | + | + | + | + | + | + | + | + | 10:10:1 | |||
| Mp | + | + | + | + | + | + | + | + | − | 8:8:1 | |||
| Tmp | + | + | + | + | + | + | − | np | np | 19:19:1 | |||
| Spi | + | + | + | + | − | np | np | np | np | 56:56:1 | |||
| AA:F:W | 5:2:5 | 27 | Mp | + | + | + | + | + | + | +/P | np | np | 19:8:19:1 |
| Tmp | + | + | + | + | + | + | +/P | np | np | 24:10:24:1 | |||
| Spi | + | + | + | + | + | + | + | +/P | np | 23:9:23:1 | |||
| AA:Po | 1:1 | 0 | Mp | + | + | + | + | + | + | +/P | np | np | 17:17:1 |
| Tmp | + | + | + | + | +/P | np | np | np | np | 42:42:1 | |||
| Spi | + | + | + | + | + | + | + | +/P | +/P | 20:20:1 | |||
| Gri | +/P | − | np | np | np | np | np | np | np | np | |||
| AA:BA | 5:1 | 0 | Mp | + | + | + | + | + | + | + | + | +/P | 17:3:1 |
| Tmp | + | + | + | + | +/P | np | np | np | np | 85:17:1 | |||
| Spi | + | + | + | + | + | + | + | + | + | 24:5:1 | |||
| Gri | + | − | np | np | np | np | np | np | np | np | |||
| S:F:W | 1:1:10 | 25 | Ch | + | + | + | + | + | + | + | + | + | 4:4:40:3 |
| Ra | + | + | + | + | + | + | + | + | + | 3:3:28:1 | |||
| Mp | + | + | − | np | np | np | np | np | np | np | |||
| S:F:G:W | 1:1:1:11 | 22 | Ra | + | + | + | + | + | + | + | − | np | 4:4:4:44:1 |
| Tmp | − | Np | np | np | np | np | np | np | np | np | |||
| Solvent | Drug Tested | Solubility (mg/mL) | Molar Ratio (a) Solvent:Drug | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 50 | 80 | 100 | 150 | 200 | 250 | |||
| Acetic acid | Ch | + | + | + | + | + | + | + | + | + | 11, 6:1 |
| Ra | + | + | + | + | + | + | + | + | + | 24, 5:1 | |
| Mp | + | + | + | + | + | +/P | np | np | np | 51:1 | |
| Tmp | + | + | + | + | + | +/P | +/P | np | np | 63, 4:1 | |
| Spi | + | + | + | + | + | + | + | + | + | 29:1 | |
| Gri | + | + | + | +/P | +/P | np | np | np | np | np | |
| Nf | − | − | np | np | np | np | np | np | np | np | |
| l−Lactic acid | Ch | + | + | + | + | + | + | + | + | + | 9:1 |
| Ra | + | + | + | + | + | + | + | + | + | 18, 8:1 | |
| Mp | + | + | + | + | + | + | + | + | + | 12, 5:1 | |
| Tmp | + | + | + | + | + | + | + | + | − | 19, 4:1 | |
| Spi | + | + | + | + | + | + | + | + | + | 22:1 | |
| Gri | + | + | − | np | np | np | np | np | np | np | |
| Nf | − | − | np | np | np | np | np | np | np | np | |
| Propylene glycol | Ch | + | + | + | + | + | + | + | + | + | 9:1 |
| Ra | + | + | + | + | + | + | + | + | + | 19:1 | |
| Mp | + | + | + | + | + | − | np | np | np | 40:1 | |
| Tmp | + | + | + | − | np | np | np | np | np | 198:1 | |
| Spi | + | + | − | np | np | np | np | np | np | 569:1 | |
| Gri | − | − | np | np | np | np | np | np | np | np | |
| Nf | − | − | np | np | np | np | np | np | np | np | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, N.R.; Spelbos, V.S.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations. Molecules 2021, 26, 2645. https://doi.org/10.3390/molecules26092645
Mustafa NR, Spelbos VS, Witkamp G-J, Verpoorte R, Choi YH. Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations. Molecules. 2021; 26(9):2645. https://doi.org/10.3390/molecules26092645
Chicago/Turabian StyleMustafa, Natali Rianika, Vincent Simon Spelbos, Geert-Jan Witkamp, Robert Verpoorte, and Young Hae Choi. 2021. "Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations" Molecules 26, no. 9: 2645. https://doi.org/10.3390/molecules26092645
APA StyleMustafa, N. R., Spelbos, V. S., Witkamp, G.-J., Verpoorte, R., & Choi, Y. H. (2021). Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations. Molecules, 26(9), 2645. https://doi.org/10.3390/molecules26092645









