Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies
Abstract
1. Introduction
2. Humic Substances as a Complex Molecular System
3. Utilization, Degradation, and Transformation of HSs by Bacteria
3.1. Bacteria Capable of HS Degradation and Utilization
3.2. Extracellular Aerobic Degradation of HSs
3.3. Anaerobic Transformations of HSs
3.4. HS Transformation in the Gut of Soil Macro- and Microfauna
4. Utilization, Degradation, and Transformation of HSs by Fungi in Soil
4.1. Fungi as HS Degraders
4.2. Structural Alteration of HSs Caused by Fungal Utilization
5. HSs as Mediators of Microbial Redox Reactions
5.1. Reduction of HSs by Microorganisms
5.2. Electron Shuttling
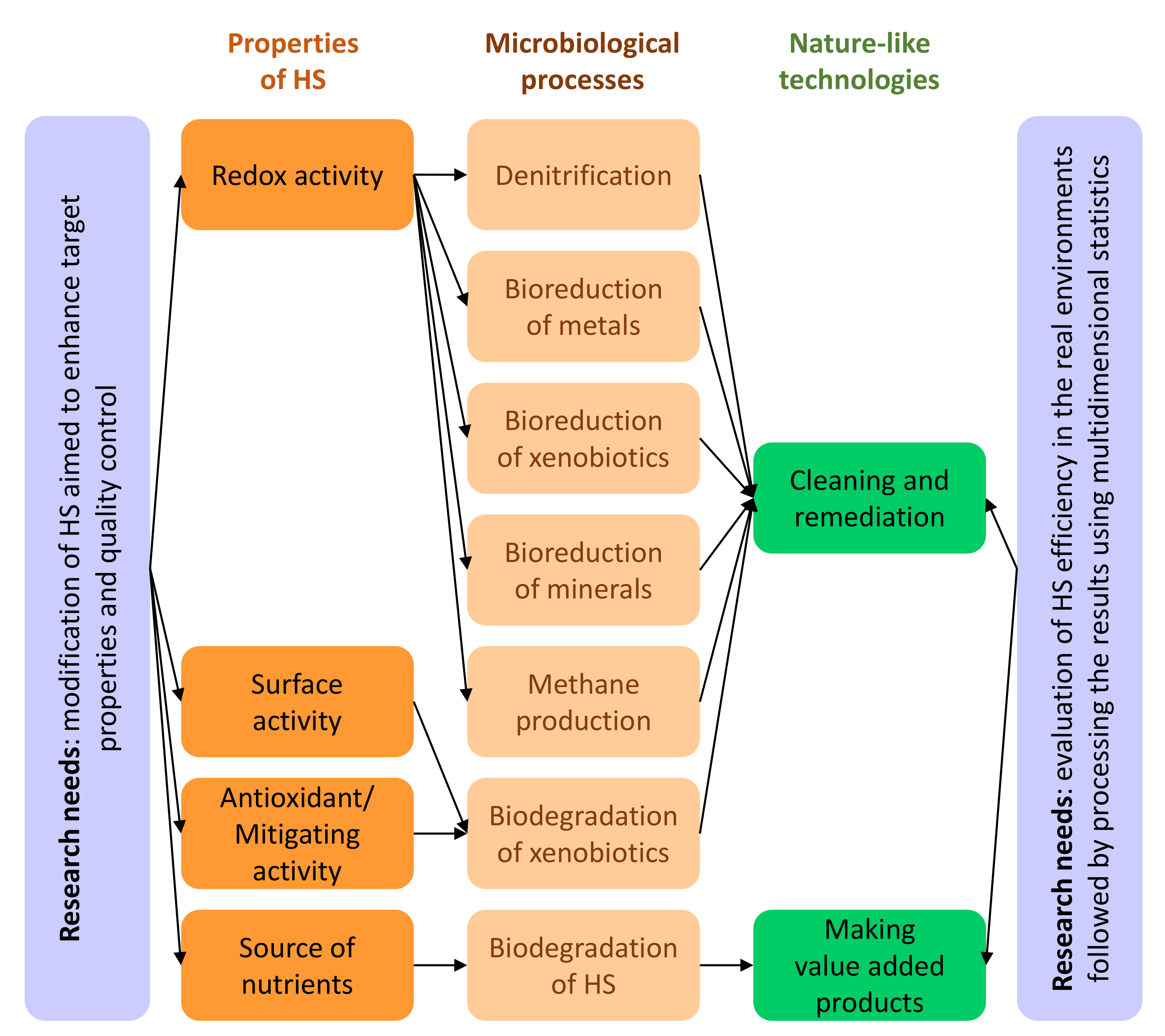
6. Nature-Like Bioremediation Technologies Based on HS–Microorganism Interactions
- The enzymes released by microorganisms to utilize HSs can catalyze oxidative binding of phenols and anilines; this approach can be applied as an alternative to the extraction of pollutants using organic solvents.
- Degradation of HSs by microorganisms can lead to the formation of low-molecular-weight compounds with high bioavailability and, as a result, biostimulating activity; this is a way to utilize low-rank coal or organic wastes to substitute traditional coal liquefaction requiring multistep treatment with chemicals.
- HSs are universal adaptogens that allow microorganisms to survive at high concentrations of toxicants; the mitigating activity of HSs can be used to increase the efficiency of bio-preparation for remediation of polluted environments.
- Participation of HSs in redox reactions can be accompanied by the transformation of organic and inorganic pollutants; degradation of chlorinated organic pollutants may be enhanced under anoxic conditions, and the reduction of some toxic metals followed by lowering their toxicity and mobility can be reached.
- Transfer of electrons from anaerobic respiration through HSs to oxygen may competitively suppress electron transfer to CO2, reducing the formation of CH4 in temporarily anoxic systems; managing methane emissions is a crucial point both for biogas production and landfill restoration.
6.1. HS-Facilitated Biodegradation of Organic Contaminants in Soil and Sediments
6.2. Reduction of Metals by HSs
6.3. Biosolubilization for Lignite Utilization
7. Research Needs
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaffney, J.S.; Marley, N.A.; Clarks, S.B. Humic and fulvic acids and organic colloidal materials in the environment. In Humic and Fulvic Acids: Isolation, Structure, and Environmental Role; Gaffney, J.S., Marley, N.A., Clarks, S.B., Eds.; American Chemical Society: Washington, DC, USA, 1996; pp. 2–16. [Google Scholar]
- Liang, Z.; Liu, J.-X.; Li, J. Decomposition and mineralization of aquatic humic substances (AHS) in treating landfill leachate using the Anammox process. Chemosphere 2009, 74, 1315–1320. [Google Scholar] [CrossRef]
- Hou, D.; He, J.; Lü, C.; Wang, W.; Zhang, F. Spatial distributions of humic substances and evaluation of sediment organic index on Lake Dalinouer, China. J. Geochem. 2014, 2014, 502597. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, R.L. The uniqueness of humic substances in each of soil, stream and marine environments. Anal. Chim. Acta 1990, 232, 19–30. [Google Scholar] [CrossRef]
- Rocker, D.; Brinkhoff, T.; Grüner, N.; Dogs, M.; Simon, M. Composition of humic acid-degrading estuarine and marine bacterial communities. FEMS Microbiol. Ecol. 2012, 80, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley and Sons, Inc.: New York, NY, USA, 1994; 521p. [Google Scholar]
- MacCarthy, P. The principles of humic substances. Soil Sci. 2001, 166, 738–751. [Google Scholar] [CrossRef]
- Filip, Z.; Tesařová, M. Microbial processing of humic substances from meadow and forest soils. In Tree Species Effects on Soils: Implications for Global Change; NATO Science Series IV: Earth and Environmental Sciences; Binkley, D., Menyailo, O., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 55, pp. 193–212. [Google Scholar]
- Grinhut, T.; Hadar, Y.; Chen, Y. Degradation and transformation of humic substances by saprotrophic fungi: Processes and mechanisms. Fungal Biol. Rev. 2007, 21, 179–189. [Google Scholar] [CrossRef]
- Piccolo, A. Humus and soil conservation. In Humic Substances in Terrestrial Ecosystems, 1st ed.; Piccolo, A., Ed.; Elsevier Science: Amsterdam, The Netherland, 1996; pp. 225–264. [Google Scholar] [CrossRef]
- Mishustin, E.N.; Nikitin, D.I. Susceptibility of humic acids to the soil microflora. Microbiology 1961, 30, 687–694. [Google Scholar]
- Filip, Z.; Pecher, W.; Berthelin, J. Microbial utilization and transformation of humic acids extracted from different soils. J. Plant Nutr. Soil. Sci. 1999, 162, 215–222. [Google Scholar] [CrossRef]
- Yanagi, Y.; Yoda, K.; Ogura, K.; Fujitake, N. Population of humic acid degrading microorganisms in Andosols under different vegetation types and grassland management regimens. Microbes Environ. 2008, 23, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Lovley, D.R.; Woodward, J.C.; Chapelle, F.H. Rapid anaerobic benzene oxidation with a variety of chelated Fe (III) forms. Appl. Environ. Microbiol. 1996, 62, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Field, J.A.; Cervantes, F.J.; van der Zee, F.P.; Lettinga, G. Role of quinones in the biodegradation of priority pollutants: A review. Water Sci. Technol. 2000, 42, 215–222. [Google Scholar] [CrossRef]
- Glasser, N.R.; Saunders, S.H.; Newman, D.K. The colorful world of extracellular electron shuttles. Annu. Rev. Microbiol. 2017, 71, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Berselli, S.; Conte, P.; Piccolo, A.; Marchetti, L. Effects of humic substances and soya lecithin on the aerobic bioremediation of a soil historically contaminated by polycyclic aromatic hydrocarbons (PAHs). Biotechnol. Bioeng. 2004, 88, 214–223. [Google Scholar] [CrossRef]
- Gao, T.-G.; Jiang, F.; Yang, J.-S.; Li, B.-Z.; Yuan, H.-L. Biodegradation of leonardite by an alkali-producing bacterial community and characterization of the degraded products. Appl. Microbiol. Biotechnol. 2012, 93, 2581–2590. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Badkoubi, A.; Mohseni-Bandpi, A.; Esrafili, A.; Jorfi, S.; Dehghanifard, E.; Baneshi, M.M. Modification of PAHs biodegradation with humic compounds. Soil Sediment. Contam. 2013, 22, 185–198. [Google Scholar] [CrossRef]
- Haider, R.; Ghauri, M.A.; Jones, E.J.; Orem, W.H.; SanFilipo, J.R. Structural degradation of Thar lignite using MW1 fungal isolate: Optimization studies. Int. Biodeterior. Biodegrad. 2015, 100, 149–154. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Stepanova, E.V.; Koroleva, O.V. Mitigating activity of humic substances: Direct influence on biota. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; NATO Science Series IV: Earth and Environmental Sciences; Perminova, I.V., Hatfield, K., Hertkorn, N., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 52, pp. 285–310. [Google Scholar]
- Ouyang, K.; Walker, S.L.; Yu, X.-Y.; Gao, C.-H.; Huang, Q.; Cai, P. Metabolism, survival, and gene expression of Pseudomonas putida to hematite nanoparticles mediated by surface-bound humic acid. Environ. Sci. Nano 2018, 5, 682–695. [Google Scholar] [CrossRef]
- Nikolaev, Y.A.; Loiko, N.G.; Demkina, E.V.; Atroshchik, E.A.; Konstantinov, A.I.; Perminova, I.V.; El’-Registan, G.I. Functional activity of humic substances in survival prolongation of populations of hydrocarbon-oxidizing bacteria Acinetobacter junii. Microbiology 2020, 89, 74–85. [Google Scholar] [CrossRef]
- Cervantes, F.J.; Dijksma, W.; Duong-Dac, T.; Ivanova, A.; Lettinga, G.; Field, J.A. Anaerobic mineralization of toluene by enriched sediments with quinones and humus as terminal electron acceptors. Appl. Environ. Microbiol. 2001, 67, 4471–4478. [Google Scholar] [CrossRef]
- Cervantes, F.J.; Martinez, C.M.; Gonzalez-Estrella, J.; Marquez, A.; Arriaga, S. Kinetics during the redox biotransformation of pollutants mediated by immobilized and soluble humic acids. Appl. Microbiol. Biotechnol. 2013, 97, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Xu, Y.; Fan, M.J.; Chen, Y.W.; Shen, S.B. The stimulatory effect of humic acid on the co-metabolic biodegradation of tetrabromobisphenol A in bioelectrochemical system. J. Environ. Manag. 2019, 235, 350–356. [Google Scholar] [CrossRef]
- Hong, Y.G.; Wu, P.; Li, W.R.; Gu, J.G.; Duan, S.S. Humic analog AQDS and AQS as an electron mediator can enhance chromate reduction by Bacillus sp. strain 3C(3). Appl. Microbiol. Biotechnol. 2012, 93, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Jin, R.F.; Liu, G.F.; Tian, T.; Gu, C.; Zhou, J.T.; Xing, D. Effects of sludge lysate for Cr(VI) bioreduction and analysis of bioaugmentation mechanism of sludge humic acid. Environ. Sci. Poll. Res. 2019, 26, 5065–5075. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Yu, L.; Fang, Y.; Ashry, N.; Riahi, Y.; Uddin, I.; Dai, K.; Huang, Q. Iron mineral-humic acid complex enhanced Cr(VI) reduction by Shewanella oneidensis MR-1. Chemosphere 2020, 247, 125902. [Google Scholar] [CrossRef]
- Olk, D.C.; Dinnes, D.L.; Scoresby, J.R.; Callaway, C.R.; Darlington, J.W. Humic products in agriculture: Potential benefits and research challenges—A review. J. Soil Sediments 2018, 18, 2881–2891. [Google Scholar] [CrossRef]
- Lehtonen, K.; Hanninen, K.; Ketola, M. Structurally bound lipids in peat humic acids. Org. Geochem. 2001, 32, 33–43. [Google Scholar] [CrossRef]
- Van Trump, J.I.; Vega Fransheska, J.R.; Coates, J.D. Natural organic matter as global antennae for primary production. Astrobiology 2013, 3, 476–482. [Google Scholar] [CrossRef]
- Hong, Y.G.; Gu, J.; Xu, Z.C.; Xu, M.Y.; Sun, G.P. Humic substances act as electron acceptor and redox mediator for microbial dissimilatory azoreduction by Shewanella decolorationis S12. J. Microbiol. Biotechnol. 2007, 17, 428–437. [Google Scholar] [PubMed]
- Bu, X.; Han, F.; Ruan, H.; Zhu, L. Changes in chemical composition and spectral characteristics of dissolved organic matter from soils induced by biodegradation. Soil Sci. 2014, 179, 197–204. [Google Scholar] [CrossRef]
- Nagao, S.; Kodama, H.; Aramaki, T.; Fujitake, N.; Uchida, M.; Shibata, Y. Carbon isotope composition of dissolved humic and fulvic acids in the Tokachi river system. Radiat. Prot. Dosim. 2011, 146, 322–325. [Google Scholar] [CrossRef]
- Jouraiphy, A.; Amir, S.; Winterton, P.; El Gharous, M.; Revel, J.-C.; Hafidi, M. Structural study of the fulvic fraction during composting of activated sludge-plant matter: Elemental analysis, FTIR and 13C NMR. Bioresour. Technol. 2008, 99, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Hertkorn, N.; Frommberger, M.; Witt, M.; Koch, B.P.; Schmitt-Kopplin, P.; Perdue, E.M. Natural organic matter and the event horizon of mass spectrometry. Anal. Chem. 2008, 80, 8908–8919. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Brune, A.; Ji, R. Selective digestion of the proteinaceous component of humic substances by the geophagous earthworms Metaphire guillelmi and Amynthas corrugatus. Soil Biol. Biochem. 2010, 42, 1455–1462. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Simpson, A.J. Humic substances in soils: Are they really chemically distinct? Environ. Sci. Technol. 2006, 40, 4605–4611. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Gerke, J. Concepts and misconceptions of humic substances as the stable part of soil organic matter: A review. Agronomy 2018, 8, 76. [Google Scholar] [CrossRef]
- Baveye, P.C.; Wander, M. The (bio)chemistry of soil humus and humic substances: Why is the “new view” still considered novel after more than 80 years? Front. Environ. Sci. 2019, 7, 27. [Google Scholar] [CrossRef]
- Filip, Z.K.; Bielek, P.; Demnerova, K. Prerequisites and susceptibility of humic acids to microbial utilization and transformation—A review. Arch. Agron. Soil Sci. 2011, 57, 445–454. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Badun, G.A.; Kulikova, N.A.; Perminova, I.V. Behavior of humic substances in the liquid-liquid system directly measured using tritium label. Chemosphere 2020, 238, 124646. [Google Scholar] [CrossRef]
- Perminova, I.V.; Kulikova, N.A.; Zhilin, D.M.; Grechischeva, N.Y.; Kovalevskii, D.V.; Lebedeva, G.F.; Matorin, D.N.; Venediktov, P.S.; Konstantinov, A.I.; Kholodov, V.A.; et al. Mediating effects of humic substances in the contaminated environments. In Soil and Water Pollution Monitoring, Protection and Remediation; NATO Science Series; Twardowska, I., Allen, H.E., Häggblom, M.M., Stefaniak, S., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 69, pp. 249–273. [Google Scholar] [CrossRef]
- Tchaikovskaya, O.N.; Yudina, N.V.; Maltseva, E.V.; Nechaev, L.V.; Svetlichnyi, V.A. Interaction of humic acids with organic toxicants. Russ. Phys. J. 2016, 59, 597–603. [Google Scholar] [CrossRef]
- Perminova, I.V.; Hatfield, K. Remediation chemistry of humic substances: Theory and implications for technology. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; NATO Science Series IV: Earth and Environmental Sciences; Perminova, I.V., Hatfield, K., Hertkorn, N., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 52, pp. 3–36. [Google Scholar]
- Liang, Y.N.; Britt, D.W.; McLean, J.E.; Sorensen, D.L.; Sims, R.C. Humic acid effect on pyrene degradation: Finding an optimal range for pyrene solubility and mineralization enhancement. Appl. Microbiol. Biotechnol. 2007, 74, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Qiu, S.; Liu, B.; Pu, Y.; Gao, Z.; Wang, J.; Jin, R.; Zhou, J. Microbial reduction of Fe (III)-bearing clay minerals in the presence of humic acids. Sci. Rep. 2017, 7, 45354. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.; Edling, H.; Bechemin, C. Interactions between a marine dinoflagellate (Alexandrium catenella) and a bacterial community utilizing riverine humic substances. Aquat. Microb. Ecol. 1998, 16, 65–80. [Google Scholar] [CrossRef]
- Dong, L.H.; Yuan, H.L. Nitrogen incorporation into lignite humic acids during microbial degradation. Geomicrobiol. J. 2009, 26, 484–490. [Google Scholar] [CrossRef]
- Makarov, M.I.; Malysheva, T.I.; Haumaier, L.; Alt, H.G.; Zech, W. The forms of phosphorus in humic and fulvic acids of a toposequence of alpine soils in the northern Caucasus. Geoderma 1997, 80, 61–73. [Google Scholar] [CrossRef]
- Kuhn, K.M.; Maurice, P.A. Accessibility of humic-associated Fe to a microbial siderophore: Implications for bioavailability. Environ. Sci. Technol. 2014, 48, 1015–1022. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Perminova, I.V. A comparative study of elemental composition of water-soluble humic substances, humic acids, and fulvic acids extracted from sod—Podzolic soils. Moscow Univ. Soil. Sci. Bull. 2010, 65, 151–154. [Google Scholar] [CrossRef]
- Rosenstock, B.; Simon, M. Consumption of dissolved amino acids and carbohydrates by limnetic bacterioplankton according to molecular weight fractions and proportions bound to humic matter. Microb. Ecol. 2003, 45, 433–443. [Google Scholar] [CrossRef]
- Rosenstock, B.; Zwisler, W.; Simon, M. Bacterial consumption of humic and non-humic low and high molecular weight DOM and the effect of solar irradiation on the turnover of labile DOM in the Southern Ocean. Microb. Ecol. 2005, 50, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Thorn, K.A.; Mikita, M.A. Ammonia fixation by humic substances: A nitrogen-15 and carbon-13 NMR study. Sci. Total Environ. 1992, 113, 67–87. [Google Scholar] [CrossRef]
- Trubetskaya, O.E.; Reznikova, O.I.; Afanas’eva, G.V.; Markova, L.F.; Muranova, T.A.; Trubetskoj, O.A. Amino acid distribution in soil humic acids fractionated by tandem size exclusion chromatography polyacrylamide gel electrophoresis. Environ. Int. 1998, 24, 573–581. [Google Scholar] [CrossRef]
- Bracewell, J.M.; Robertson, G.W. Quantitative comparison of the nitrogen-containing pyrolysis products and amino acid composition of soil humic acids. J. Anal. Appl. Pyrolysis 1984, 6, 19–29. [Google Scholar] [CrossRef]
- Vialykh, E.A.; Ilarionov, S.A.; Abdelrahman, H.M.; Vialykh, I.A. Changes in amino acids content of humic acids sequentially extracted from peat and sod-Podzolic soil. Can. J. Soil Sci. 2014, 94, 575–583. [Google Scholar] [CrossRef]
- Itoh, K.; Watanabe, A.; Tsutsuki, K.; Kuwatsuka, S. Monosaccharide composition of four humus fractions in an Andosol and a Cambisol. Soil Sci. Plant. Nutr. 2007, 53, 7–11. [Google Scholar] [CrossRef]
- Watanabe, A.; Kuwatsuka, S. Ethanol-soluble and insoluble fractions of humic substances in soil fulvic acids. Soil Sci. Plant. Nutr. 1992, 38, 391–399. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Hermosin, B.; Yruela, I.; Saiz-Jimenez, C. Lipids of soil humic acids. II. Residual components after hymatomelanic acid extraction. Sci. Total Environ. 1989, 81–82, 421–428. [Google Scholar] [CrossRef]
- Nielsen, P.; Petersen, S.O. Ester-linked polar lipid fatty acid profiles of soil microbial communities: A comparison of extraction methods and evaluation of interference from humic acids. Soil Biol. Biochem. 2000, 32, 1241–1249. [Google Scholar] [CrossRef]
- Schnitzer, M.; Neyroud, J.A. Alkanes and fatty acids in humic substances. Fuel 1975, 54, 17–19. [Google Scholar] [CrossRef]
- Hajje, N.; Jaffe, R. Molecular characterization of Cladium peat from the Florida Everglades: Biomarker associations with humic fractions. Hydrobiologia 2006, 569, 99–112. [Google Scholar] [CrossRef]
- De Sanfilippo, E.C.; Argoello, J.A.; Abdala, G.; Orioli, G.A. Content of auxin-, inhibitor- and gibbereilin-like substances in humic acids. Biologia Plantarum 1990, 32, 346–351. [Google Scholar] [CrossRef]
- Trevisan, S.; Pizzeghello, D.; Ruperti, B.; Francioso, O.; Sassi, A.; Palme, K.; Quaggiotti, S.; Nardi, S. Humic substances induce lateral root formation and expression of the early auxin-responsive IAA19 gene and DR5 synthetic element in Arabidopsis. Plant. Biol. 2009, 12, 604–614. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Reniero, F.; Rascio, N. Chemical and biochemical properties of humic substances isolated from forest soils and plant growth. Soil Sci. Soc. Am. J. 2000, 64, 639–645. [Google Scholar] [CrossRef]
- Hertkorn, N.; Claus, H.; Schmitt-Kopplin, P.; Perdue, E.M.; Filip, Z. Utilization and transformation of aquatic humic substances by autochthonous microorganisms. Environ. Sci. Technol. 2002, 36, 4334–4345. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H.G.; Kim, S.J.; Gocke, K. Microbial decomposition in aquatic environments: Combined process of extracellular enzyme activity and substrate uptake. Appl. Environ. Microbiol. 1988, 54, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Munster, U.; Einio, P.; Nurminen, J.; Overbeck, J. Extracellular enzymes in a polyhumic lake: Important regulators in detritus processing. Hydrobiologia 1992, 229, 225–238. [Google Scholar] [CrossRef]
- Lu, X.Q.; Hanna, J.V.; Johnson, W.D. Source indicators of humic substances: An elemental composition, solid state 13C CP/MAS NMR and Py-GC/MS study. Appl. Geochem. 2000, 15, 1019–1033. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Wei, Z.M.; Zhang, F.; Li, X.; Tan, W.B.; Xi, B.D. Role of humic acid chemical structure derived from different biomass feedstocks on Fe(III) bioreduction activity: Implication for sustainable use of bioresources. Catalysts 2019, 9, 450. [Google Scholar] [CrossRef]
- Rimmer, D.L.; Abbott, G.D. Phenolic compounds in NaOH extracts of UK soils and their contribution to antioxidant capacity. Eur. J. Soil Sci. 2011, 62, 285–294. [Google Scholar] [CrossRef]
- Rimmer, D.L.; Smith, A.M. Antioxidants in soil organic matter and in associated plant materials. Eur. J. Soil Sci. 2009, 60, 170–175. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Antioxidant activity of humic substances via bioluminescent monitoring in vitro. Environ. Monit. Assess. 2015, 187, 89. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.I.; Kulikova, N.A.; Filimonov, I.S.; Koroleva, O.V.; Konstantinov, A.I. Long-term kinetics study and quantitative characterization of the antioxidant capacities of humic and humic-like substances. J. Soils Sediments 2018, 18, 1355–1364. [Google Scholar] [CrossRef]
- Feificova, D.; Snajdr, J.; Siglova, M.; Cejkova, A.; Masak, J.; Jirku, V. Influence of humic acids on the growth of the microorganisms utilizing toxic compounds (comparison between yeast and bacteria). Chimia 2005, 59, 749–752. [Google Scholar] [CrossRef]
- Klein, O.I.; Isakova, E.P.; Deryabina, Y.I.; Kulikova, N.A.; Badun, G.A.; Chernysheva, M.G.; Stepanova, E.V.; Koroleva, O.V. Humic substances enhance growth and respiration in the basidiomycetes Trametes maxima under carbon limited conditions. J. Chem. Ecol. 2014, 40, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shi, H.T.; Jiao, Y.Q.; Han, S.Y.; Akindolie, M.S.; Yang, Y.; Chen, Z.; Zhang, Y. Effects of humic acid on the biodegradation of di-n-butyl phthalate in mollisol. J. Clean Prod. 2020, 249, 119404. [Google Scholar] [CrossRef]
- Scott, D.T.; McKnight, D.M.; Blunt-Harris, E.L.; Kolesar, S.E.; Lovley, D.R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 1998, 32, 2984–2989. [Google Scholar] [CrossRef]
- Struyk, Z.; Sposito, G. Redox properties of standard humic acids. Geoderma 2001, 102, 329–346. [Google Scholar] [CrossRef]
- Hernández-Montoya, V.; Alvarez, L.H.; Montes-Morán, M.A.; Cervantes, F.J. Reduction of quinone and non-quinone redox functional groups in different humic acid samples by Geobacter sulfurreducens. Geoderma 2012, 183–184, 25–31. [Google Scholar] [CrossRef]
- Cervantes, F.J.; van der Velde, S.; Lettinga, G.; Field, J.A. Quinones as terminal electron acceptors for anaerobic microbial oxidation of phenolic compounds. Biodegradation 2000, 11, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.J.; Vu-Thi-Thu, L.; Lettinga, G.; Field, J.A. Quinone-respiration improves dechlorination of carbon tetrachloride by anaerobic sludge. Appl. Microbiol. Biotechnol. 2004, 64, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Ratasuk, N.; Nanny, M.A. Characterization and quantification of reversible redox sites in humic substances. Environ. Sci. Technol. 2007, 41, 7844–7850. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Vergari, D.; Schwarzenbach, R.P.; Sander, M. Electrochemical analysis of proton and electron transfer equilibria of the reducible moieties in humic acids. Environ. Sci. Technol. 2011, 45, 8385–8394. [Google Scholar] [CrossRef] [PubMed]
- Varshovi, A.; Sartain, J.B. Chemical characteristics and microbial-degradation of humate. Comm. Soil Sci. Plant. Anal. 1993, 24, 2493–2505. [Google Scholar] [CrossRef]
- Saadi, I.; Borisover, M.; Armon, R.; Laor, Y. Monitoring of effluent DOM biodegradation using fluorescence, UV and DOC measurements. Chemosphere 2006, 63, 530–539. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Hofle, M.G. Bacterial growth in mixed cultures on dissolved organic carbon from humic and clear waters. Appl. Environ. Microbiol. 1987, 53, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lim, K.H. Spectroscopic and elemental investigation of microbial decomposition of aquatic fulvic acid in biological process of drinking water treatment. Biodegradation 1996, 7, 287–295. [Google Scholar] [CrossRef]
- Tóth, N.; Vörös, L.; Mózes, A.; Balogh, K.V. Biological availability and humic properties of dissolved organic carbon in Lake Balaton (Hungary). Hydrobiologia 2007, 592, 281–290. [Google Scholar] [CrossRef]
- Ylla, I.; Romani, A.M.; Sabater, S. Labile and recalcitrant organic matter utilization by river biofilm under increasing water temperature. Microb. Ecol. 2012, 64, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, I. Bacterial utilization of humic substances from the Arctic Ocean. Aquat. Microb. Ecol. 1999, 19, 37–45. [Google Scholar] [CrossRef][Green Version]
- Esham, E.C.; Ye, W.; Moran, M.A. Identification and characterization of humic substances-degrading bacterial isolates from an estuarine environment. FEMS Microbiol. Ecol. 2000, 34, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Marthur, S.P.; Paul, E.A. Microbial utilization of soil humic acids. Can. J. Microbiol. 1967, 13, 573–580. [Google Scholar] [CrossRef]
- Bhardwaj, K.K.R.; Gaur, A.C. Isolation and characterization of some humic acid decomposing bacteria and fungi from soil. Zbl. Bakt. II. 1971, 126, 307–312. [Google Scholar]
- Konchou, C.Y.; Blondeau, R. Effect of heterotrophic bacteria on different humic substances in mixed batch cultures. Can. J. Soil Sci. 1990, 70, 51–59. [Google Scholar] [CrossRef]
- De Haan, H. Effect of a fulvic acid fraction on the growth of a Pseudomonas from Tjeukemeer (the Netherlands). Fresh Biol. 1974, 4, 301–310. [Google Scholar] [CrossRef]
- Ueno, A.; Shimizu, S.; Tamamura, S.; Okuyama, H.; Naganuma, T.; Kaneko, K. Anaerobic decomposition of humic substances by Clostridium from the deep subsurface. Sci. Rep. 2016, 6, 18990. [Google Scholar] [CrossRef] [PubMed]
- Hutalle-Schmelzer, K.M.L.; Grossart, H.-P. Changes in the bacterioplankton community of oligotrophic Lake Stechlin (northeastern Germany) after humic matter addition. Aquat Microb. Ecol. 2009, 55, 155–168. [Google Scholar] [CrossRef]
- Byzov, B.A.; Tikhonov, V.V.; Nechitailo, T.Y.; Demin, V.V.; Zvyagintsev, D.G. Taxonomic composition and physiological and biochemical properties of bacteria in the digestive tracts of earthworms. Euras. Soil Sci. 2015, 48, 268–275. [Google Scholar] [CrossRef]
- Filip, Z.; Bielek, P. Susceptibility of humic acids from soils with various contents of metals to microbial utilization and transformation. Biol. Fertil. Soils 2002, 36, 426–433. [Google Scholar] [CrossRef]
- Crawford, D.L.; Gupta, R.K. Characterization of extracellular bacterial enzymes which depolymerize a soluble lignite coal polymer. Fuel 1991, 70, 577–580. [Google Scholar] [CrossRef]
- Ghosh, S.; Leff, L.G. Impacts of labile organic carbon concentration on organic and inorganic nitrogen utilization by a stream biofilm bacterial community. Appl. Environ. Microbiol. 2013, 79, 7130–7141. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.; Shimizu, S.; Tamamura, S.; Naganuma, T.; Ohmi, Y.; Kaneko, K. Structural alteration of humic acids by Pseudomonas spp. from deep terrestrial subsurface diatomite formations in northernmost Japan. Geomicrobiol. J. 2014, 31, 654–663. [Google Scholar] [CrossRef]
- Fu, D.L.; Liu, M.Y.; Chang, Q.R.; Liu, L.W.; Lv, A.H. Degradation capacity of humic acids-degrading bacteria on humic acids extracted from arable soil. Zemdirb. Agric. 2017, 104, 9–14. [Google Scholar] [CrossRef][Green Version]
- Hayakawa, M.; Nonomura, H. Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J. Ferment. Technol. 1987, 65, 501–509. [Google Scholar] [CrossRef]
- Khandelwal, K.C.; Gaur, A.C. Degradation of humic acids, extracted from manure and soil by some streptomycetes and fungi. Zentralbl. Bakteriol. Naturwiss. 1980, 135, 119–122. [Google Scholar] [CrossRef]
- Dari, K.; Béchet, M.; Blondeau, R. Isolation of soil Streptomyces strains capable of degrading humic acids and analysis of their peroxidase activity. FEMS Microbiol. Ecol. 1995, 16, 115–122. [Google Scholar] [CrossRef]
- Dehorter, B.; Blondeau, R. Extracellular enzyme activities during humic acid degradation by the white rot fungi Phanerochaete chrysosporium and Trametes versicolor. FEMS Microbiol. Lett. 1992, 94, 209–216. [Google Scholar] [CrossRef]
- Badis, A.; Ferradji, F.Z.; Boucherit, A.; Fodil, D.; Boutoumi, H. Removal of natural humic acids by decolorizing actinomycetes isolated from different soils (Algeria) for application in water purification. Desalination 2010, 259, 216–222. [Google Scholar] [CrossRef]
- Alphaproteobacteria. Available online: https://hyperleap.com/topic/Alphaproteobacteria (accessed on 20 March 2021).
- See, J.H.; Bronk, D.A.; Lewitus, A.J. Uptake of Spartina derived humic nitrogen by estuarine phytoplankton in axenic and non-axenic culture. Limnol. Oceanogr. 2006, 51, 2290–2299. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Perminova, I.V.; Badun, G.A.; Chernysheva, M.G.; Koroleva, O.V.; Tsvetkova, E.A. Estimation of uptake of humic substances from different sources by Escherichia coli cells under optimum and salt stress conditions by use of tritium-labeled humic materials. Appl. Environ. Microbiol. 2010, 76, 6223–6230. [Google Scholar] [CrossRef] [PubMed]
- Farjalla, V.F.; Amado, A.M.; Suhett, A.L.; Meirelles-Pereira, F. DOC removal paradigms in highly humic aquatic ecosystems. Environ. Sci. Pollut. Res. Int. 2009, 16, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bronk, D.A.; See, J.H.; Bradley, P.; Killberg, L. DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences 2007, 4, 283–296. [Google Scholar] [CrossRef]
- Schnitzer, M. Nature of nitrogen in humic substances. In Humic Substances in Soil, Sediment, and Water; Aiken, G.R., McKnight, D.M., Wershaw, R.L., Eds.; John Wiley and Sons: New York, NY, USA, 1985; pp. 303–328. [Google Scholar]
- Stern, N.; Mejia, J.; He, S.M.; Yang, Y.; Ginder-Vogel, M.; Roden, E.E. Dual role of humic substances as electron donor and shuttle for dissimilatory iron reduction. Environ. Sci. Technol. 2018, 52, 5619–5691. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Fraga, J.L.; Coates, J.D.; Blunt-Harris, E.L. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1999, 1, 89–98. [Google Scholar] [CrossRef]
- Brune, A.; Ohkuma, M. Role of the termite gut microbiota in symbiotic digestion. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 439–475. [Google Scholar]
- Donovan, S.E.; Eggleton, P.; Bignell, D.E. Gut content analysis and a new feeding group classification of termites. Ecol. Entomol. 2001, 26, 356–366. [Google Scholar] [CrossRef]
- Eggleton, P.; Tayasu, I. Feeding groups, life types and the global ecology of termites. Ecol. Res. 2001, 16, 941–960. [Google Scholar] [CrossRef]
- Eggleton, P.; Bignell, D.E.; Sands, W.A.; Waite, B.; Wood, T.G.; Lawton, J.H. The species richness of termites (Isoptera) under differing levels of forest disturbance in the Mbalmayo Forest Reserve, southern Cameroon. J. Trop. Ecol. 1995, 11, 85–98. [Google Scholar] [CrossRef]
- Ji, R.; Brune, A. Transformation and mineralization of 14C-labeled bacterial cells, protein, peptidoglycan, and cellulose by soil-feeding termites. Biol. Fertil. Soils 2001, 33, 166–174. [Google Scholar] [CrossRef]
- Bignell, D.E. Soil feeding and gut morphology in higher termites. In Nourishment and Evolution in Insects Societies; Hunt, J.H.N., Nalepa, C.A., Eds.; Westview Press: Boulder, CO, USA, 1994; pp. 131–158. [Google Scholar]
- Brune, A.; Kühl, M. pH profiles of the extremely alkaline hindguts of soil-feeding termites (Isoptera: Termitidae) determined with microelectrodes. J. Insect Physiol. 1996, 42, 1121–1127. [Google Scholar] [CrossRef]
- Ebert, A.; Brune, A. Hydrogen concentration profiles at the oxic-anoxic interface: A microsensor study of the hindgut of the wood-feeding lower termite Reticulitermes flavipes (Kollar). Appl. Environ. Microbiol. 1997, 63, 4039–4046. [Google Scholar] [CrossRef]
- Schmitt-Wagner, D.; Brune, A. Hydrogens profiles and localization of methanogenic activities in the highly compartmentalized hindgut of soil feeding higher termites. Appl. Environ. Microbiol. 1999, 65, 4490–4496. [Google Scholar] [CrossRef] [PubMed]
- Brauman, A. Effect of gut transit and mound deposit on soil organic matter transformations in the soil feeding termite: A review. Eur. J. Soil. Biol. 2000, 36, 117–125. [Google Scholar] [CrossRef]
- Bignell, D.E.; Oskarsson, H.; Anderson, J.M. Distribution and abundance of bacteria in the gut of a soil-feeding termite Procubitermes aburiensis (Termitidae, Termitinae). J. Gen. Microbiol. 1980, 117, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Kappler, A.; Brune, A. Transformation and mineralization of synthetic 14C-labeled humic model compounds by soil-feeding termites. Soil Biol. Biochem. 2000, 32, 1281–1291. [Google Scholar] [CrossRef]
- Kappler, A.; Ji, R.; Brune, A. Synthesis and characterization of specifically 14C-labeled humic model compounds for feeding trials with soil-feeding termites. Soil Biol. Biochem. 2000, 32, 1271–1280. [Google Scholar] [CrossRef]
- Kappler, A.; Brune, A. Influence of gut alkalinity and oxygen status on mobilization and size-class distribution of humic acids in the hindgut of soil-feeding termites. Appl. Soil Ecol. 1999, 13, 219–229. [Google Scholar] [CrossRef]
- Kappler, A.; Brune, A. Dynamics of redox potential and changes in redox state of iron and humic acids during gut passage in soil-feeding termites (Cubitermes spp.). Soil Biol. Biochem. 2002, 34, 221–227. [Google Scholar] [CrossRef]
- Andert, J.; Marten, A.; Brandl, R.; Brune, A. Inter- and intraspecific comparison of the bacterial assemblages in the hindgut of humivorous scarab beetle larvae (Pachnoda spp.). FEMS Microbiol. Ecol. 2010, 74, 439–449. [Google Scholar] [CrossRef]
- Li, X.; Brune, A. Transformation and mineralization of soil organic nitrogen by the humivorous larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Plant Soil 2007, 301, 233–244. [Google Scholar] [CrossRef]
- Hobbie, S.N.; Li, X.; Basen, M.; Stingl, U.; Brune, A. Humic substance-mediated Fe(III) reduction by a fermenting Bacillus strain from the alkaline gut of a humus-feeding scarab beetle larva. Syst. Appl. Microbiol. 2012, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ji, R.; Schäffer, A.; Brune, A. Mobilization of soil phosphorus during passage through the gut of larvae of Pachnoda ephippiata (Coleoptera: Searabaeidae). Plant. Soil 2006, 288, 263–270. [Google Scholar] [CrossRef]
- Lavelle, P.; Bignell, D.; Lepage, M.; Wolters, V.; Roger, P.; Ineson, P.; Heal, O.W.; Dhillion, S. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 1997, 33, 159–193. [Google Scholar]
- Gramss, G. Activity of oxidative enzymes in fungal mycelia from grassland and forest soils. J. Basic Microbiol. 1997, 37, 407–423. [Google Scholar] [CrossRef]
- Řezáčová, V.; Hršelová, H.; Gryndlerová, H.; Mikšík, I.; Gryndler, M. Modifications of degradation-resistant soil organic matter by soil saprobic microfungi. Soil Biol. Biochem. 2006, 38, 2292–2299. [Google Scholar] [CrossRef]
- Khundzhua, D.A.; Patsaeva, S.V.; Terekhova, V.A.; Yuzhakov, V.I. Spectral characterization of fungal metabolites in aqueous medium with humus substances. J. Spectrosc. 2013, 2013, 538608. [Google Scholar] [CrossRef]
- Claus, H.; Filip, Z. Degradation and transformation of aquatic humic substances by laccase-producing fungi Cladosporium cladosporioides and Polyporus versicolor. Acta Hydrochim. Hydrobiol. 1998, 26, 180–185. [Google Scholar] [CrossRef]
- Rojas-Jimenez, K.; Fonvielle, J.A.; Ma, H.; Grossart, H.P. Transformation of humic substances by the freshwater Ascomycete Cladosporium sp. Limnol. Oceanogr. 2017, 62, 1955–1962. [Google Scholar] [CrossRef]
- Mishra, B.; Srivastava, L.L. Degradation of humic acids of a forest soil by some fungal isolates. Plant. Soil 1986, 96, 413–416. [Google Scholar] [CrossRef]
- Fakoussa, R.M.; Frost, P.J. In vivo-decolourization of coal-derived humic acids by laccase excreting fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1999, 52, 60–65. [Google Scholar] [CrossRef]
- Kluczek-Turpeinen, B.; Steffen, K.T.; Tuomela, M.; Hatakka, A.; Hofrichter, M. Modification of humic acids by the compost-dwelling deuteromycete Paecilomyces inflatus. Appl. Microbiol. Biotechnol. 2005, 66, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Monistrol, I.F.; Luna, N.; Fernández, M. Processes of liquefaction/solubilization of Spanish coals by microorganisms. Appl. Microbiol. Biotechnol. 1999, 52, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Koukol, O.; Gryndler, M.; Novak, F.; Vosátka, M. Effect of Chalara longipes on decomposition of humic acids from Picea abies needle litter. Folia Microbiol. 2004, 49, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Ziegenhagen, D.; Sorge, S. Degradation of soil humic extract by wood- and soil-associated fungi, bacteria, and commercial enzymes. Microbial. Ecol. 1999, 37, 140–151. [Google Scholar] [CrossRef]
- Belcarz, R.; Ginalska, G.; Korniłłowicz-Kowalska, T. Extracellular enzyme activities of Bjerkandera adusta R59 soil strain, capable for daunomycin and humic acids degradation. Appl. Microbiol. Biotechnol. 2005, 68, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M.; Fritsche, W. Depolymerization of low-rank coal by extracellular fungal enzyme systems. II. The ligninolytic enzymes of the coal-humic-acid-depolymerizing fungus Nematoloma frowardii b19. Appl. Microbiol. Biotechnol. 1997, 47, 419–424. [Google Scholar] [CrossRef]
- Wunderwald, U.; Kreisel, G.; Braun, M.; Schulz, M.; Jäger, C.; Hofrichter, M. Formation and degradation of a synthetic humic acid derived from 3-fluorocatechol. Appl. Microbiol. Biotechnol. 2000, 53, 441–446. [Google Scholar] [CrossRef]
- Yanagi, Y.; Tamaki, H.; Otsuka, H.; Fujitake, N. Comparison of decolorization by microorganisms of humic acids with different 13C NMR properties. Soil Biol. Biochem. 2002, 34, 729–731. [Google Scholar] [CrossRef]
- Haider, K.; Martin, J.P. Mineralization of 14C-labelled humic acids and humic-acid bound 14C-xenobiotics by Phanerochaete chrysosporium. Soil Biol. Biochem. 1988, 20, 425–429. [Google Scholar] [CrossRef]
- Ralph, J.P.; Catcheside, D.E.A. Decolorization and depolymerization of solubilized low-rank coal by the white-rot basidiomycete Phanerochaete-Chrysosporium. Appl. Microbiol. Biotechnol. 1994, 42, 536–542. [Google Scholar] [CrossRef]
- Elbeyli, I.Y.; Palantoken, A.; Piskin, S.; Peksel, A.; Kuzu, H. Bio-liquefaction/solubilization of lignitic humic acids by white-rot fungus (Phanerochaete chrysosporium). Energy Source Part A 2006, 28, 1051–1061. [Google Scholar] [CrossRef]
- Granit, T.; Chen, Y.; Hadar, Y. Humic acids bleaching by white rot fungi isolated from biosolids compost. Soil Biol. Biochem. 2007, 39, 1040–1046. [Google Scholar] [CrossRef]
- Temp, U.; Meyrahn, H.; Eggert, C. Extracellular phenol oxidase patterns during depolymerization of low-rank coal by three basidiomycetes. Biotechnol. Lett. 1999, 21, 281–287. [Google Scholar] [CrossRef]
- Steffen, K.T.; Hatakka, A.; Hofrichter, M. Degradation of humic acids by the litter-decomposing basidiomycete Collybia dryophila. Appl. Environ. Microbiol. 2002, 68, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, T.M.; Merritt, C.S.; Reddy, C.A. Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Appl. Environ. Microbiol. 1999, 65, 5307–5313. [Google Scholar] [CrossRef]
- Baldrian, P.; Kolařík, M.; Štursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Žifčáková, L.; Šnajdr, J.; Rídl, J.; Vlček, Č.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef]
- Weber, C.F.; Vilgalys, R.; Kuske, C.R. Changes in fungal community composition in response to elevated atmospheric CO2 and nitrogen fertilization varies with soil horizon. Front. Microbiol. 2013, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Voříšková, J.; Brabcováa, V.; Cajthaml, T.; Baldrian, P. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2014, 201, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Cozzolino, A.; Conte, P.; Spaccini, R. Polymerization of humic substances by an enzyme-catalyzed oxidative coupling. Naturwissenschaften 2000, 87, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Jansa, J.; Bukovská, P.; Gryndler, M. Mycorrhizal hyphae as ecological niche for highly specialized hypersymbionts—Or just soil free-riders? Front. Plant. Sci. 2013, 4, 134. [Google Scholar] [CrossRef]
- Collado, S.; Oulego, P.; Suárez-Iglesias, O.; Díaz, M. Leachates and natural organic matter. A review of their biotreatment using fungi. Waste Manag. 2019, 96, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, V.; Zavgorodnyaya, J.; Demin, V.; Byzov, B. Transformation of soil humic acids by Aporrectodea caliginosa earthworm: Effect of gut fluid and gut associated bacteria. Eur. J. Soil Biol. 2016, 75, 47–53. [Google Scholar] [CrossRef]
- Filip, Z.; Berthelin, J. Analytical determination of the microbial utilization and transformation of humic acids extracted from municipal refuse. Fresenius J. Anal. Chem. 2001, 371, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Filip, Z.; Pecher, W.; Berthelin, J. Microbial utilization and transformation of humic acid-like substances extracted from a mixture of municipal refuse and sewage sludge disposed of in a landfill. Environ. Pollut. 2000, 109, 83–89. [Google Scholar] [CrossRef]
- Filip, Z.; Kubat, J. Aerobic short-term microbial utilization and degradation of humic acids extracted from soils of long-term field experiments. Eur. J. Soil Biol. 2003, 39, 175–182. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Schmerwitz, J.; Kaiser, K.; Haumaier, L.; Glaser, B.; Ellerbrock, R.; Leinweber, P. Changes in properties of soil-derived dissolved organic matter induced by biodegradation. Soil Biol. Biochem. 2003, 35, 1129–1142. [Google Scholar] [CrossRef]
- Wei, Z.-M.; Xi, B.-D.; Li, M.-X.; Wang, S.-P.; Zhao, Y.; Jiang, Y.-H.; Su, J. Study on three-dimensional fluorescence spectroscopy characteristics of humic acid during composting with microbes inoculation. Spectrosc. Spect. Anal. 2008, 28, 2895–2899. [Google Scholar] [CrossRef]
- Li, X.Z.; Brune, A. Selective digestion of the peptide and polysaccharide components of synthetic humic acids by the hurnivorous larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Soil Biol. Biochem. 2005, 37, 1476–1483. [Google Scholar] [CrossRef]
- Wang, S.; Dou, S.; Liu, Y.-L.; Li, H.-M.; Cui, J.-T.; Zhang, W.; Wang, C.-Y. Characterization of soil humus by FTIR spectroscopic analyses after being inoculated with different microorganisms plus wheat straw. Spectrosc. Spect. Anal. 2012, 32, 2409–2413. [Google Scholar] [CrossRef]
- Dec, J.; Bollag, J.M. Phenoloxidase-mediated interactions of phenols and anilines with humic materials. J. Environ. Qual. 2000, 29, 665–676. [Google Scholar] [CrossRef]
- Huang, Z.S.; Wei, Z.S.; Xiao, X.L.; Tang, M.R.; Li, B.L.; Ming, S.; Cheng, X. Bio-oxidation of elemental mercury into mercury sulfide and humic acid-bound mercury by sulfate reduction for Hg0 removal in flue gas. Environ. Sci. Technol. 2019, 53, 12923–12934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Tan, W.B.; Peng, J.J.; Dang, Q.L.; Zhang, H.; Xi, B.D. Biowaste-source-dependent synthetic pathways of redox functional groups within humic acids favoring pentachlorophenol dechlorination in composting process. Environ. Int. 2020, 135, 105380. [Google Scholar] [CrossRef]
- Voordeckers, J.W.; Kim, B.C.; Izallalen, M.; Lovley, D.R. Role of Geobacter sulfurreducens outer surface c-type cytochromes in reduction of soil humic acid and anthraquinone-2,6-disulfonate. Appl. Environ. Microbiol. 2010, 76, 2371–2375. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Zhuang, L.; Zhou, S.-G.; Yuan, Y.; Yuan, T.; Li, F.-B. Humic substance-mediated reduction of iron(III) oxides and degradation of 2,4-D by an alkaliphilic bacterium, Corynebacterium humireducens MFC-5. Microb. Biotechnol. 2013, 6, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.N.; Wang, X.N.; Wu, J.; Lu, Y.Z.; Fu, L.; Zhang, F.; Lau, T.-C.; Zeng, R.J. Humic substances as electron acceptors for anaerobic oxidation of methane driven by ANME-2d. Water Res. 2019, 164, 114935. [Google Scholar] [CrossRef]
- Piepenbrock, A.; Schröder, C.; Kappler, A. Electron transfer from humic substances to biogenic and abiogenic Fe(III) oxyhydroxide minerals. Environ. Sci. Technol. 2014, 48, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Klüpfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nature Geosci. 2014, 7, 195–200. [Google Scholar] [CrossRef]
- Coates, J.D.; Cole, K.A.; Chakraborty, R.; O’Connor, S.M.; Achenbach, L.A. Diversity and ubiquity of bacteria capable of utilizing humic substances as electron donors for anaerobic respiration. Appl. Environ. Microbiol. 2002, 68, 2445–2452. [Google Scholar] [CrossRef]
- Yu, Z.-G.; Orsetti, S.; Haderlein, S.B.; Knorr, K.-H. Electron transfer between sulfide and humic acid: Electrochemical evaluation of the reactivity of Sigma-Aldrich humic acid toward sulfide. Aquat. Geochem. 2016, 22, 117–130. [Google Scholar] [CrossRef]
- Finneran, K.; Forbush, H.M.; VanPraagh, C.V.G.; Lovley, D.R. Desulfitobacterium metallireducens sp. nov., an anaerobic bacterium that couples growth to the reduction of metals, humics, and chlorinated compounds. Int. J. Syst. Evol. Microbiol. 2002, 52, 1929–1935. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef] [PubMed]
- Roden, E.E.; Kappler, A.; Bauer, I.; Jiang, J.; Paul, A.; Stoesser, R.; Konishi, H.; Xu, H. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat. Geosci. 2010, 3, 417–421. [Google Scholar] [CrossRef]
- Rios-del Toro, E.E.; Valenzuela, E.I.; Ramírez, J.E.; Loopez-Lozano, N.E.; Cervantes, F.J. Anaerobic ammonium oxidation linked to microbial reduction of natural organic matter in marine sediments. Environ. Sci. Technol. Lett. 2018, 5, 571–577. [Google Scholar] [CrossRef]
- Xi, B.D.; Zhao, X.Y.; He, X.S.; Huang, C.H.; Tan, W.B.; Gao, R.T.; Zhang, H.; Li, D. Successions and diversity of humic-reducing microorganisms and their association with physical-chemical parameters during composting. Bioresour. Technol. 2016, 219, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Wu, C.Y.; Wang, X.J.; Zhou, S.G. The role of humic substances in the anaerobic reductive dechlorination of 2,4-dichlorophenoxyacetic acid by Comamonas koreensis strain CY01. J. Hazard. Mater. 2009, 164, 941–947. [Google Scholar] [CrossRef]
- Jiang, J.; Kappler, A. Kinetics of microbial and chemical reduction of humic substances: Implications for electron shuttling. Environ. Sci. Technol. 2008, 42, 3563–3569. [Google Scholar] [CrossRef]
- Kwon, M.; Finneran, K. Biotransformation products and mineralization potential for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in abiotic versus biological degradation pathways with anthraquinone-2,6-disulfonate (AQDS) and Geobacter metallireducens. Biodegradation 2008, 19, 705–715. [Google Scholar] [CrossRef]
- Chen, M.J.; Tong, H.; Liu, C.S.; Chen, D.D.; Li, F.B.; Qiao, J.T. A humic substance analogue AQDS stimulates Geobacter sp abundance and enhances pentachlorophenol transformation in a paddy soil. Chemosphere 2016, 160, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Liu, T.X.; Liu, L.; Li, F.B. Dependence of the electron transfer capacity on the kinetics of quinone-mediated Fe(III) reduction by two iron/humic reducing bacteria. RSC Adv. 2014, 4, 2284–2290. [Google Scholar] [CrossRef]
- Collins, R.; Picardal, F. Enhanced anaerobic transformations of carbon tetrachloride by soil organic matter. Environ. Toxicol. Chem. 1999, 18, 2703–2710. [Google Scholar] [CrossRef]
- Amstaetter, K.; Borch, T.; Kappler, A. Influence of humic acid imposed changes of ferrihydrite aggregation on microbial Fe(III) reduction. Geochim. Cosmochim. Acta 2012, 85, 326–341. [Google Scholar] [CrossRef]
- Cooper, R.E.; Eusterhues, K.; Wegner, C.-E.; Totsche, K.U.; Küsel, K. Ferrihydrite-associated organic matter (OM) stimulates reduction by Shewanella oneidensis MR-1 and a complex microbial consortia. Biogeosciences 2017, 14, 5171–5188. [Google Scholar] [CrossRef]
- Hädrich, A.; Taillefert, M.; Akob, D.M.; Cooper, R.E.; Litzba, U.; Wagner, F.E.; Nietzsche, S.; Ciobota, V.; Räsch, P.; Popp, J.; et al. Microbial Fe(II) oxidation by Sideroxydans lithotrophicus ES-1 in the presence of Schlöppnerbrunnen fen-derived humic acids. FEMS Microbiol. Ecol. 2019, 95, fiz034. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Halasz, A.; Hawari, J. Effect of iron(III), humic acids and anthraquinone-2,6-disulfonate on biodegradation of cyclic nitramines by Clostridium sp. EDB2. J. Appl. Microbiol. 2006, 100, 555–563. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Chen, N.; Li, H.; Li, Q.-F. Kocuria rosea HN01, a newly alkaliphilic humus-reducing bacterium isolated from cassava dreg compost. J. Soils Sediments 2014, 14, 423–431. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Zhuang, L.; Zhou, S.G.; Li, F.B.; He, J. Corynebacterium humireducens sp. nov., an alkaliphilic, humic acid-reducing bacterium isolated from a microbial fuel cell. Int. J. Syst. Evol. Microbiol. 2011, 61 Pt 4, 882–887. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Kostandarithes, H.M.; Li, S.W.; Plymale, A.E.; Daly, M.J. Reduction of Fe(III), Cr (VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl. Environ. Microbiol. 2000, 66, 2006–2011. [Google Scholar] [CrossRef]
- Piepenbrock, A.; Behrens, S.; Kappler, A. Comparison of humic substance- and Fe(III)-reducing microbial communities in anoxic aquifers. Geomicrobiol. J. 2014, 31, 917–928. [Google Scholar] [CrossRef]
- Cervantes, F.J.; de Bok, F.A.M.; Tuan, D.D.; Stams, A.J.M.; Lettinga, G.; Field, J.A. Reduction of humic substances by halorespiring, sulphate-reducing and methanogenic microorganisms. Environ. Microbiol. 2002, 4, 51–57. [Google Scholar] [CrossRef]
- Benz, M.; Schink, B.; Brune, A. Humic acid reduction by Propionibacterium freudenreichii and other fermenting bacteria. Appl. Environ. Microbiol. 1998, 64, 4507–4512. [Google Scholar] [CrossRef]
- Emmerich, M.; Kappler, A. Absence of microbial humic substance reduction at acidic pH: Implications for stimulation of natural organohalogen formation and for the mechanism of acidophilic Fe (III) reduction. Biogeochemistry 2012, 109, 219–231. [Google Scholar] [CrossRef]
- Skogerboe, R.K.; Wilson, S.A. Reduction of ionic species by fulvic acid. Anal. Chem. 1981, 53, 228–232. [Google Scholar] [CrossRef]
- Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [CrossRef]
- Smith, J.A.; Nevin, K.P.; Lovley, D.R. Syntrophic growth via quinone-mediated interspecies electron transfer. Front. Microbiol. 2015, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Lei, Y.Q.; Liu, Z.; Xue, Y.T.; Sun, D.Z.; Wang, L.Y.; Holmes, D.E. Impact of fulvic acids on bio-methanogenic treatment of municipal solid waste incineration leachate. Water Res. 2016, 106, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Van Trump, J.I.; Wrighton, K.C.; Thrash, J.C.; Weber, K.A.; Andersen, G.L.; Coates, J.D. Humic acid-oxidizing, nitrate-reducing bacteria in agricultural soils. mBio 2011, 2, e00044-11. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.; Kretzschmar, R.; Kappler, A.; van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Perminova, I.V. From green chemistry and nature-like technologies towards ecoadaptive chemistry and technology. Pure Appl. Chem. 2019, 9, 851–864. [Google Scholar] [CrossRef]
- Dong, S.; Li, M.; Chen, Y. Inherent humic substance promotes microbial denitrification of landfill leachate via shifting bacterial community, improving enzyme activity and up-regulating gene. Sci. Rep. 2017, 7, 12215. [Google Scholar] [CrossRef]
- Cervantes, F.J.; Mancilla, A.R.; Rios-del Toro, E.E.; Alpuche-Solis, A.G.; Montoya-Lorenzana, L. Anaerobic degradation of benzene by enriched consortia with humic acids as terminal electron acceptors. J. Hazard. Mater. 2011, 195, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Di Maio, V.; Ferri, T.; Majone, M. The humic acid analogue antraquinone-2,6-disulfonate (AQDS) serves as an electron shuttle in the electricity-driven microbial dechlorination of trichloroethene to cis-dichloroethene. Bioresour. Technol. 2010, 101, 9728–9733. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, Y.G.; Su, Y.L.; Wan, R.; Zheng, X. Effect of fulvic acids with different characteristics on biological denitrification. RSC Adv. 2016, 6, 14993–15001. [Google Scholar] [CrossRef]
- Gertsen, L.M.; Dmitrieva, E.D. Utilization of hexadecane by biocomposition based on humic acids of peats and oil-degrading microorganisms of the genus Rhodococcus in aqueous media. Moroc. J. Chem. 2020, 8, 392–399. [Google Scholar] [CrossRef]
- Yuan, Y.; Xi, B.D.; He, X.S.; Tan, W.B.; Zhang, H.; Li, D.; Yang, C.; Zhao, X. Polarity and molecular weight of compost-derived humic acids impact bio-dechlorination of pentachlorophenol. J. Agric. Food Chem. 2019, 67, 4726–4733. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Fernandes, E.; Bollag, J.M. Enzymatic transformation and binding of labeled 2,4,6-trinitrotoluene to humic substances during an anaerobic/aerobic incubation. J. Environ. Qual. 2002, 31, 437–444. [Google Scholar] [CrossRef]
- Tong, H.; Chen, M.J.; Li, F.B.; Liu, C.S.; Li, B.; Qiao, J.T. Effects of humic acid on pentachlorophenol biodegrading microorganisms elucidated by stable isotope probing and high-throughput sequencing approaches. Eur. J. Soil Sci. 2018, 69, 380–391. [Google Scholar] [CrossRef]
- Filatov, D.A.; Ivanov, A.A.; Svarovskaya, L.I.; Yudina, N.V. Activation of the biochemical processes in an oil-contaminated soil using a light-correcting film and humic acids. Euras. Soil Sci. 2011, 44, 204–210. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, A.J.; Qiu, L.N.; Li, J.R.; Li, F.K. Effect of copper ion and soil humic acid on biodegradation of decabromodiphenyl ether (BDE-209) by Pseudomonas aeruginosa. MicrobiologyOpen 2017, 6, e00439. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singla, S.; Sharma, M.; Singh, D.P.; Prasad, R.; Thakur, V.K.; Singh, J. Kinetic study of the biodegradation of acephate by indigenous soil bacterial isolates in the presence of humic acid and metal ions. Biomolecules 2020, 10, 433. [Google Scholar] [CrossRef]
- Tan, W.B.; Jia, Y.F.; Huang, C.H.; Zhang, H.; Li, D.; Zhao, X.Y.; Wang, G.; Jiang, J.; Xi, B. Increased suppression of methane production by humic substances in response to warming in anoxic environments. J. Environ. Manag. 2018, 206, 602–606. [Google Scholar] [CrossRef]
- Ho, L.; Ho, G. Mitigating ammonia inhibition of thermophilic anaerobic treatment of digested piggery wastewater: Use of pH reduction, zeolite, biomass and humic acid. Water Res. 2012, 46, 4339–4350. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Z.; Lv, Z.; Gao, T.; Yang, J.; Qin, Z.; Yuan, H. The biosolubilization of lignite by Bacillus sp. Y7 and characterization of the soluble products. Fuel 2013, 103, 639–645. [Google Scholar] [CrossRef]
- Yuan, H.L.; Yang, J.S.; Wang, F.Q.; Chen, W.X. Degradation and solubilization of Chinese lignite by Penicillium sp P6. Appl. Biochem. Microbiol. 2006, 42, 52–55. [Google Scholar] [CrossRef]
- Gokcay, C.F.; Kolankaya, N.; Dilek, F.B. Microbial solubilisation of lignite. Fuel 2001, 80, 1421–1433. [Google Scholar] [CrossRef]
- Füchtenbusch, B.; Steinbüchel, A. Biosynthesis of polyhydroxyalkanoates from low-rank coal liquefaction products by Pseudomonas oleovorans and Rhodococcus ruber. Appl. Microbiol. Biotechnol. 1999, 52, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Sabar, M.A.; Ali, M.I.; Fatima, N.; Malik, A.Y.; Jamal, A.; Liaquat, R.; He, H.; Liu, F.J.; Guo, H.; Urynowicz, M.; et al. Evaluation of humic acids produced from Pakistani subbituminous coal by chemical and fungal treatments. Fuel 2020, 278, 118301. [Google Scholar] [CrossRef]
- Kabe, Y.; Osawa, T.; Ishihara, A.; Kabe, T. Decolorization of coal humic acid by extracellular enzymes produced by white-rot fungi. Int. J. Coal Prep. Util. 2005, 25, 211–220. [Google Scholar] [CrossRef]
- Wolf, M.; Kappler, A.; Jiang, J.; Meckenstock, R.U. Effects of humic substances and quinones at low concentrations on ferrihydrite reduction by Geobacter metallireducens. Environ. Sci. Technol. 2009, 43, 5679–5685. [Google Scholar] [CrossRef]
- Castro, L.; Garcia-Balboa, C.; Gonzalez, F.; Ballester, A.; Blazquez, M.L.; Munoz, J.A. Effectiveness of anaerobic iron bio-reduction of jarosite and the influence of humic substances. Hydrometallurgy 2013, 131, 29–33. [Google Scholar] [CrossRef]
- Huang, L.; Angelidaki, I. Effect of humic acids on electricity generation integrated with xylose degradation in microbial fuel cells. Biotechnol. Bioeng. 2008, 100, 413–422. [Google Scholar] [CrossRef]
- Van der Zee, F.P.; Cervantes, F.J. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: A review. Biotechnol. Adv. 2009, 27, 256–277. [Google Scholar] [CrossRef]
- Field, J.A.; Cervantes, F.J. Microbial redox reactions mediated by humus and structurally related quinones. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; Perminova, I.V., Hatfield, K., Hertkorn, N., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 52, pp. 343–352. [Google Scholar]
- Valenzuela, E.I.; Avendano, K.A.; Balagurusamy, N.; Arriaga, S.; Nieto-Delgado, C.; Thalasso, F.; Cervantes, F.J. Electron shuttling mediated by humic substances fuels anaerobic methane oxidation and carbon burial in wetland sediments. Sci. Total Environ. 2019, 650, 2674–2684. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Prieto-Davo, A.; Lopez-Lozano, N.E.; Hernandez-Eligio, A.; Vega-Alvarado, L.; Juarez, K.; Garcia-Gonzalez, A.S.; Lopez, M.G.; Cervantes, F.J. Anaerobic methane oxidation driven by microbial reduction of natural organic matter in a tropical wetland. Appl. Environ. Microbiol. 2017, 83, e00645-17. [Google Scholar] [CrossRef]
- Scheutz, C.; Pedersen, R.B.; Petersen, P.H.; Jorgensen, J.H.B.; Ucendo, I.M.B.; Monster, J.G.; Samuelsson, J.; Kjeldsen, P. Mitigation of methane emission from an old unlined landfill in Klintholm, Denmark using a passive biocover system. Waste Manag. 2014, 34, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.D.; Astals, S.; Lu, Y.; Peces, M.; Jensen, P.D.; Batstone, D.J.; Tait, S. Humic acid inhibition of hydrolysis and methanogenesis with different anaerobic inocula. Waste Manag. 2018, 80, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef]
- Smith, S.R.; Lang, N.L.; Cheung, K.H.M.; Spanoudaki, K. Factors controlling pathogen destruction during anaerobic digestion of biowastes. Waste Manag. 2005, 25, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Yang, Y.; Liu, F.; Ding, J. Heterogeneous reaction mechanism of elemental mercury oxidation by oxygen species over MnO2 catalyst. Proc. Combust. Inst. 2019, 37, 2967–2975. [Google Scholar] [CrossRef]
- Gu, B.; Bian, Y.; Miller, C.L.; Dong, W.; Jiang, X.; Liang, L. Mercury reduction and complexation by natural organic matter in anoxic environments. Proc. Natl. Acad. Sci. USA 2011, 108, 1479–1483. [Google Scholar] [CrossRef]
- Yin, S.D.; Tao, X.X.; Shi, K.Y.; Tan, Z.C. Biosolubilisation of Chinese lignite. Energy 2009, 34, 775–781. [Google Scholar] [CrossRef]
- Fakoussa, R.M.; Hofrichter, M. Biotechnology and microbiology of coal degradation. Appl. Microbiol. Biotechnol. 1999, 52, 25–40. [Google Scholar] [CrossRef]
- Cohen, M.S.; Gabriele, P.D. Degradation of coal by the fungi Polyporus versicolor and Poria monticolar. Appl. Environ. Microbiol. 1982, 44, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.H.; Yuan, Q.; Yuan, H.L. Changes of chemical properties of humic acids from crude and fungal transformed lignite. Fuel 2006, 85, 2402–2407. [Google Scholar] [CrossRef]
- Strandberg, G.W.; Lewis, S.N. Solubilization of coal by an extracellular product from Streptomyces setonii 75Vi2. J. Industrial Microbiol. 1987, 1, 371–375. [Google Scholar] [CrossRef]
- Valero, N.; Gómez, L.; Pantoja, M.; Ramírez, R. Production of humic substances through coal-solubilizing bacteria. Braz. J. Microbiol. 2014, 45, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Pyne, J.; Stewart, D.L.; Fredrickson, J.K.; Cohen, M.S. Method for Solubilization of Low-Rank Coal Using a Cell-Free Enzymatic System. U.S. Patent 4882274, 21 November 1989. [Google Scholar]
- Stewart, D.L.; Fredrickson, J.K.; Campbell, J.A.; Pyne, J.W., Jr.; Bean, R.M.; Wilson, B.W. Method for Solubilization of Low-Rank Coal Using Low Molecular Weight Cell-Free Filtrates Derived from Cultures of Coriolus versicolor. U.S. Patent 5084160, 28 January 1992. [Google Scholar]
- Urynowicz, M.A.; Huang, Z. Enzymatic Depolymerization and Solubilization of Chemically Pretreated Coal and Coal-Derived Constituents. U.S. Patent 2014346090, 27 November 2014. [Google Scholar]
- Strandberg, G.W.; Lewis, S.N. Microbial Solzubilization of Coal. U.S. Patent US4914024, 3 April 1990. [Google Scholar]
- Sekhohola, L.M.; Igbinigie, E.E.; Cowan, A.K. Biological degradation and solubilisation of coal. Biodegradation 2013, 24, 305–318. [Google Scholar] [CrossRef]
- Qi, B.C.; Aldrich, C.; Lorenzen, L.; WolfaardtInd, G.M. Degradation of humic acids in a microbial film consortium from landfill compost. Ind. Eng. Chem. Res. 2004, 43, 6309–6316. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef]
- Li, S.S.; Wu, S.Y.; Ma, B.R.; Gao, M.C.; Wu, Y.Y.; She, Z.L. Insights into the effects of single and combined divalent copper and humic acid on the performance, microbial community and enzymatic activity of activated sludge from sequencing batch reactor. Chemosphere 2020, 249, 126165. [Google Scholar] [CrossRef] [PubMed]
- Perminova, I.V.; Kovalenko, A.N.; Schmitt-Kopplin, P.; Hatfield, K.; Hertkorn, N.; Belyaeva, E.Y.; Petrosyan, V.S. Design of quinonoid-enriched humic materials with enhanced redox properties. Environ. Sci. Technol. 2005, 39, 8518–8524. [Google Scholar] [CrossRef] [PubMed]
| Biologically Active Compounds | HSs | Content, % | Ref. |
|---|---|---|---|
| Amino acids 1 | |||
| A sum of Ala, Arg, Asp, Cys, Glu, Gly, His, Ile, Leu, Lys, Met, Phe, Pro, Ser, Thr, Tyr, Val | Soil HAs | 6–17 | [61] |
| Soil HAs | 9–16 | [62] | |
| Soil HAs | 6–8 | [63] | |
| Peat HAs | 3–7 | ||
| Soil HAs | 9 | [5] | |
| Soil FAs | 7 | ||
| Riverine FAs | 3 | ||
| Riverine HAs | 6 | ||
| Marine FAs | 4 | ||
| Carbohydrates | |||
| A sum of fructose, galactose, glucose, mannose, rhamnose, and xylose | Soil FAs | 4 | [5] |
| Soil HAs | 10 | ||
| Riverine FAs | 0.1 | ||
| Riverine HAs | 0.1 | ||
| Marine FAs | 1 | ||
| A sum of glucose, galactose, mannose, xylose, arabinose, fucose, and rhamnose | Soil HAs | 3–9 | [64] |
| Soil FAs | 3 | ||
| A sum of hexose, pentose, and uronic acid | Soil FAs | 4–8 | [65] |
| Lipids | |||
| Fatty acids | Soil HMA | 5–10 | [66] |
| Soil HAs | 41–375 nmol/g | [67] | |
| Soil HAs | 0.1–10 | [68] | |
| Soil FAs | 0.1–9 | ||
| Aromatic acid saponification byproducts | Peat HAs | 2 × 10−3 | [69] |
| Peat FAs | 9 × 10−4 | ||
| Plant hormones | |||
| Gibberellin-like substances | Soil HAs | ≥1 × 10−5 | [70] |
| Indole-3-acetic acid | Vermicompost HAs | 0.33 | [71] |
| Soil HAs | 0.01–0.05 | [72] |
| Genus | HSs | Ref. |
|---|---|---|
| Phylum Proteobacteria (Gram-Negative) | ||
| Class Alphaproteobacteria | ||
| Agrobacterium | Soil HAs | [101] |
| Aquaspirillum, Erythrobacter | Aquatic HAs from estuarine water | [100] |
| Ahrensia, Erythrobacter, Oceanibulbus, Roseovarius, Sphingobium, Sphingopyxis, Sulfitobacter, Thalassospira | Aquatic HAs from freshwater stream in a peat bog | [6,107] |
| Aminobacter, Ochrobactrum, Sphingopyxis | Coal HAs | [107] |
| Class Betaproteobacteria | ||
| Acidovorax, Herbaspirillum, Methylophilus, Polynucleobacter | Aquatic HSs from a humic lake | [106] |
| Delftia | Coal HAs | [107] |
| Variovorax | Soil HAs | [108] |
| Class Gammaproteobacteria | ||
| Acinetobacter, Aeromonas, Buttiauxella | Coal HAs | [107] |
| Alteromonas | Aquatic HAs from freshwater stream in a peat bog | [6] |
| Pseudomonas | Soil HAs | [12] |
| Soil HAs | [101] | |
| Aquatic HSs from a humic lake | [104] | |
| Soil HAs and FAs | [103] | |
| Lignite HAs | [109] | |
| Soil HAs | [108] | |
| HSs from Quercus rubra, Hamamelis virginiana, and Zea mays leaves | [110] | |
| Coal HAs | [111] | |
| Coal HAs | [107] | |
| Phylum Bacteroidetes (Gram-Negative) | ||
| Class Bacteroidetes | ||
| Bacteroides | HSs from landfill leachate | [2] |
| Class Flavobacteriia | ||
| Chryseobacterium | Coal HAs | [107] |
| Phylum Firmicutes (Gram-Positive) | ||
| Class Bacilli | ||
| Bacillus | Soil HAs | [101] |
| Soil HAs and FAs | [103] | |
| HSs from landfill leachate | [2] | |
| Aquatic HAs from estuarine water | [100] | |
| Leonardite HAs | [20] | |
| Coal HAs | [107] | |
| Soil HAs | [112] | |
| Paenibacillus | Aquatic HAs from estuarine water | [100] |
| HSs from landfill leachate | [2] | |
| Coal HAs | [107] | |
| Staphylococcus | HSs from landfill leachate | [2] |
| Class Clostridia | ||
| Clostridium | Coal HAs and HAs from diatomite layer | [105] |
| Phylum Actinobacteria (Gram-Positive) | ||
| Class Actinobacteria | ||
| Arthrobacter | Soil HAs | [101,107] |
| Agromyces, Kocuria, Nocardioides | Coal HAs | [107] |
| Dactylosporangium, Micromonospora, Microtetraspora, Nocardia, Streptosporangium, Thermomonospora | Soil HAs | [113] |
| Microbacterium | Soil HAs | [112] |
| Streptomyces | Manure and soil HAs | [114] |
| Soil HAs | [113] | |
| Soil HAs and FAs | [103] | |
| Soil HAs | [115] | |
| Soil HAs | [116] | |
| Soil HAs | [117] | |
| Coal HAs | [107] | |
| Genus | HSs | Ref. |
|---|---|---|
| Phylum Ascomycota | ||
| Class Dothideomycetes | ||
| Alternaria | Soil HAs and FAs | [147] |
| Leonardite HAs | [148] | |
| Cladosporium | Aquatic HAs from a bog lake | [149] |
| Leonardite HAs | [148] | |
| Riverine HAs | [150] | |
| Phoma | Soil HAs and FAs | [147] |
| Leonardite HAs | [148] | |
| Class Eurotiomycetes | ||
| Aspergillus | Manure and soil HAs | [114] |
| Soil HAs | [151] | |
| Paecilomyces | Coal HAs | [152] |
| Soil HAs and FAs | [153] | |
| Soil HAs and FAs | [147] | |
| Penicillium | Soil HAs | [101] |
| Manure and soil HAs | [114] | |
| Soil HAs | [151] | |
| Coal HAs | [154] | |
| Class Leotiomycetes | ||
| Geomyces | Leonardite HAs | [148] |
| Class Sordariomycetes | ||
| Chalara | HAs from Picea abies | [155] |
| Clonostachys | Soil HAs and FAs | [147] |
| Fusarium | Manure and soil HAs | [114] |
| Leonardite HAs | [148] | |
| Trichoderma | Coal HAs | [154] |
| Phylum Basidiomycota | ||
| Class Agaricomycetes | ||
| Bjerkandera | Soil HAs | [156] |
| Coal HAs | [157] | |
| Clitocybula | Soil HAs | [156] |
| Gymnopilus | Soil HAs | [156] |
| Hypholoma (Naematoloma) | Coal HAs | [158] |
| Soil HAs | [156] | |
| Synthetic HAs | [159] | |
| Kuehneromyces | Soil HAs | [156] |
| Lenzites | Soil HAs | [160] |
| Phanerochaete | Soil HAs | [161] |
| Soil HAs and FAs | [116] | |
| Coal HAs | [162] | |
| Coal HAs | [152] | |
| Lignite HAs | [163] | |
| HAs from biosolids compost | [164] | |
| Pleurotus | Coal HAs | [152] |
| Soil HAs | [156] | |
| Polyporus | Aquatic HAs from a bog lake | [149] |
| Coal HAs | [165] | |
| Pycnoporus | Coal HAs | [165] |
| Trametes (Coriolus) | Soil HAs and FAs | [116] |
| Coal HAs | [152] | |
| Soil HAs | [160] | |
| Leonardite HAs, peat HAs, HAs from biosolids compost | [164] | |
| Coal HAs | [84] | |
| Stropharia | Soil HAs | [156] |
| Class Basidiomycetes | ||
| Collybia | Soil-litter and litter HAs | [166] |
| Genus | HSs | Ref. |
|---|---|---|
| Phylum Proteobacteria (Gram-Negative) | ||
| Class Alphaproteobacteria | ||
| Brevundimonas, Devosia, Phyllobacterium, Rhodobacter | Compost HAs | [196] |
| Class Betaproteobacteria | ||
| Comamonas | Model HA (AQDS) | [197] |
| Compost HAs | [196] | |
| Pusillimonas, Rubrivivax, Janthinobacterium | Compost HAs | [196] |
| Class Deltaproteobacteria | ||
| Desulfobacca | Compost HAs | [196] |
| Geobacter | Soil HAs | [16] |
| Soil HAs and Model HA (AQDS) | [90] | |
| Riverine, soil, peat, and coal HAs | [198] | |
| Model HA (AQDS) | [199] | |
| Soil, leonardite, and compost HAs | [88] | |
| Model HA (AQDS) | [200] | |
| Class Gammaproteobacteria | ||
| Acinetobacter, Psychrobacter, Pseudomonas, Pseudoxanthomonas, Pantoea | Compost HAs | [196] |
| Aeromonas | Model HAs (AQC, AQS, AQDS, 2-HNQ, 5-HNQ) | [201] |
| Shewanella | Model HAs (AQDS, AQS) | [35] |
| Riverine, soil, peat, and coal HAs | [198] | |
| Soil HAs and FAs and humin | [202] | |
| Peat, riverine, soil, and leonardite HAs | [189] | |
| Model HAs (AQC, AQS, AQDS, 2-HNQ, 5-HNQ) | [201] | |
| Peat HAs | [203] | |
| Soil DOM | [204] | |
| Compost HAs | [78] | |
| Sideroxydans | Peat HAs | [205] |
| Phylum Bacteroidetes (Gram-Negative) | ||
| Class Sphingobacteriia | ||
| Sphingobacterium | Compost HAs | [196] |
| Phylum Firmicutes (Gram-Positive) | ||
| Class Bacilli | ||
| Bacillus | Soil HAs, HAs from midgut, hindgut, and feces of Pachnoda ephippiata | [143] |
| Model HAs (AQDS, AQS) | [29] | |
| Compost HAs | [196] | |
| Paenibacillus, Lysinibacillus, Sprorosarcina, Ureibacillus, Facklamia | Compost HAs | [196] |
| Class Clostridia | ||
| Clostridium | Coal HAs | [206] |
| Desulfitobacterium | Coal HAs, model HA (AQDS) | [192] |
| Sedimentibacter, Tissierella, Proteiniborus, Coprococcus | Compost HAs | [196] |
| Phylum Actinobacteria (Gram-Positive) | ||
| Class Actinobacteria | ||
| Kocuria | Model HA (AQDS) | [207] |
| Corynebacterium | Model HA (AQDS) | [208] |
| Coal FAs and HAs, model HAs (AQDS, AQS, AQC), | [186] | |
| Arthrobacter, Corynebacterium, Dietzia, Leucobacter | Compost HAs | [196] |
| Phylum Deinococcus-Thermus (Gram-Positive) | ||
| Class Deinococci | ||
| Deinococcus radiodurans | Model HA (AQDS) | [209] |
| Biological Agent | HSs | Effect | Ref. |
|---|---|---|---|
| Water purification/treatment | |||
| Consortium of microorganisms from activated sludge | Coal HAs | The dominance of Thauera after long-term exposure to HSs resulted in increased denitrification | [221] |
| Consortium of microorganisms from biofilm | Coal HAs | Enhanced TBBPA biodegradation in the bioelectrochemical system | [28] |
| Consortium of microorganisms from sludge | Sludge HAs | Increased anaerobic bioreduction of Cr(VI) | [30] |
| Consortium of microorganisms from sediment | Soil HAs, model HA (AQDS) | Increased toluene biodegradation | [26] |
| Consortium of microorganisms from soil and sediment | Soil HAs | Increased reductive benzene degradation | [222] |
| Consortium of microorganisms from soil, sediment, and anaerobic granular sludge | Sulfonated leonardite HAs, soluble or immobilized onto anion exchange resin | Increased reductive decolorization of azo dye Reactive Red 2 and reductive dechlorination of CCl4 | [27] |
| Bacillus sp. 3C3 | Model HAs (AQS, AQDS) | Enhanced Cr(VI) reduction | [29] |
| Clostridium sp. EDB2 | Coal HAs, model HA (AQDS) | Enhanced degradation of RDX and HMX | [206] |
| Comamonas koreensis CY01 | Model HA (AQDS) | Enhanced reductive dechlorination of 2,4-D | [197] |
| Corynebacterium humireducens MFC-5 | Coal HAs and FAs | Biodegradation of 2,4-D | [186] |
| Dehalococcoides spp. | Model HA (AQDS) | Increased reductive dechlorination of C2HCl3 | [223] |
| Deinococcus radiodurans R1 | Model HA (AQDS) | Increased reduction of Tc(VII) and U(VI) | [209] |
| Paracoccus denitrificans | Coal FAs | Enhanced denitrification | [224] |
| Rhodococcus erythropolis S67 and X5 | Peat HA | Increased utilization of C16H34 | [225] |
| Shewanella decolorationis S12 | Model HAs (AQS, AQDS) | Acceleration or inhibition of azoreduction depending HA concentration | [35] |
| Sh. oneidensis MR-1 | Compost HAs | Facilitated bio-dechlorination of PCP under Fe(III) reduction conditions | [226] |
| Sh. oneidensis MR-1 | Complex goethite-reduced HAs | Enhanced reduction of Cr(VI) to Cr(III) | [31] |
| Sh. oneidensis MR-1 | Compost HAs | Enhanced anaerobic transformation of PCP | [184] |
| Streptomyces sp. | Soil HAs | Increased decolorization of water | [117] |
| Soil/slurry/sediment remediation/biosolid treatment | |||
| Consortium of anaerobic microorganisms from cow manure | Soil HAs | Increased transformation and covalent binding of 2,4,6-TNT in the presence of laccase | [227] |
| Consortium of microorganisms from paddy soil | Soil HAs | Enhanced PCP biodegradation attributed to the quinine groups in HAs that functioned as redox mediators | [228] |
| Consortium of microorganisms from soil | Lignite HAs | Increased decomposition of PAHs due to increased bioavailability | [19] |
| Consortium of microorganisms from soil | HAs from mechanically activated peat | Increased biochemical oxidation of oil hydrocarbons | [229] |
| Consortium of microorganisms from soil | Soil HAs | Increased phenanthrene biodegradation due to increased bioavailability | [21] |
| Consortium of microorganisms from soil | Soil HAs | Increased or decreased pyrene biomineralization depending on concentration due to increased bioavailability | [51] |
| Consortium of microorganisms from soil | Coal HAs | Enhanced biodegradation of dibutyl phthalate due to mitigating activity of HSs | [85] |
| Phenoloxidases | HSs present in soil | Covalent binding of phenols and anilines | [182] |
| Pseudomonas aeruginosa | Soil HAs | Increased biodegradation of DBDE due to mitigating effect of HSs on copper | [230] |
| P. azotoformanss ACP1, P. aeruginosa ACP2, P. putida ACP3 from soil | Coal HAs | Enhanced decompositions of acephate due to mitigating activity of HSs | [231] |
| Methane consumption/production/suppression | |||
| Consortium of microorganisms from anaerobic granular sludge | FAs from MSW leachate | Decreased CH4 production | [217] |
| Consortium of microorganisms from paddy and wetland soils | Soil, peat, riverine HAs | Suppression of CH4 production under anoxic environments | [232] |
| Consortium of microorganisms from piggery wastewater | Coal HAs | Reduction or increase in CH4 production depending on HA concentration and pH | [233] |
| Nitrate-reducing AOM microorganisms | Coal HAs | Mitigation of CH4 emission | [187] |
| Value-added product production | |||
| Bacillus sp. Y7 | Lignite | HAs with high N/O and C/O ratios | [234] |
| Penicillium sp. P6 | Lignite | HSs with high content of FAs | [235] |
| Penicillium sp. P6 | Lignite | HAs with high N content | [54] |
| Phanerochaete chrysosporium | Lignite | Solubilized lignite for CH4 production | [236] |
| Phanerochaete chrysosporium | Lignite HAs | Raw material for production of valuable chemicals and extending the commercial utilization of coal | [163] |
| Pseudomonas oleovorans and Rhodococcus ruber | Lignite | PHAs accumulated in the microbial cells | [237] |
| Rhizopus oryzae AD-1 | Subbituminous coal | HAs with high N content | [238] |
| White-rot fungal strains extracted from decaying woods | Coal HAs | Decolorization and depolymerization of HAs | [239] |
| Bacterial communities | Leonardite | HAs with plant-hormone-like activity | [20] |
| Fungal isolate MW1 | Lignite | A variety of aromatic and aliphatic compounds, which could serve as chemical feedstock for subsequent processes such as methanogenesis | [22] |
| Mining processes | |||
| Corynebacterium humireducens MFC-5 | Coal HAs and FAs | Bioreduction of goethite | [186] |
| Geobacter metallireducens | Aquatic HAs and FAs from groundwater | Bioreduction of ferrihydrite | [240] |
| Shewanella putrefaciens and a natural consortium | Model HA (AQDS) | Bioreduction of jarosite/bioleaching/metal recovery | [241] |
| Flue gas cleaning/demercuration | |||
| Mercury-oxidizing/sulfate-reducing bacteria | HSs extracted from biofilm | HgS and HA-Hg are two dominant products of Hg0 bio-oxidation | [183] |
| Electricity generation | |||
| Consortium of microorganisms from domestic wastewater | Coal HAs | Increase in power density and Coulombic efficiency | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, N.A.; Perminova, I.V. Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies. Molecules 2021, 26, 2706. https://doi.org/10.3390/molecules26092706
Kulikova NA, Perminova IV. Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies. Molecules. 2021; 26(9):2706. https://doi.org/10.3390/molecules26092706
Chicago/Turabian StyleKulikova, Natalia A., and Irina V. Perminova. 2021. "Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies" Molecules 26, no. 9: 2706. https://doi.org/10.3390/molecules26092706
APA StyleKulikova, N. A., & Perminova, I. V. (2021). Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies. Molecules, 26(9), 2706. https://doi.org/10.3390/molecules26092706






