Coordination Chemistry of a Bis(Tetrazine) Tweezer: A Case of Host-Guest Behavior with Silver Salts
Abstract
:1. Introduction
2. Results and Discussion
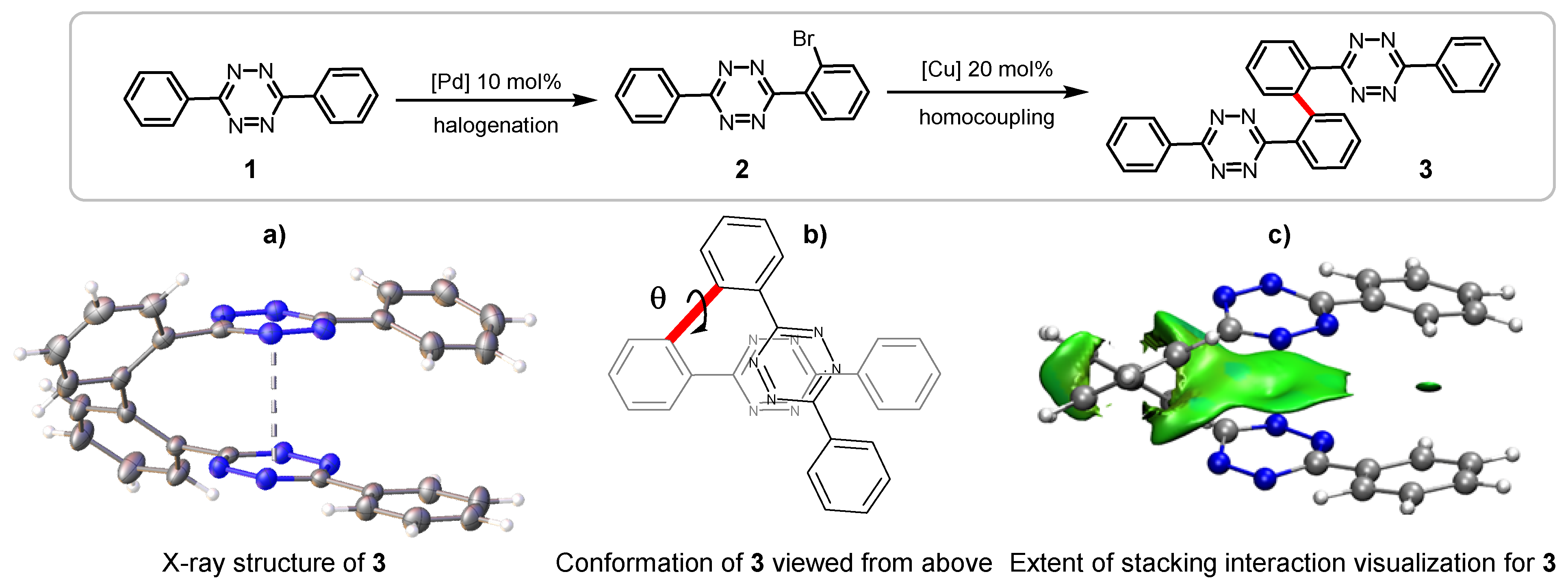
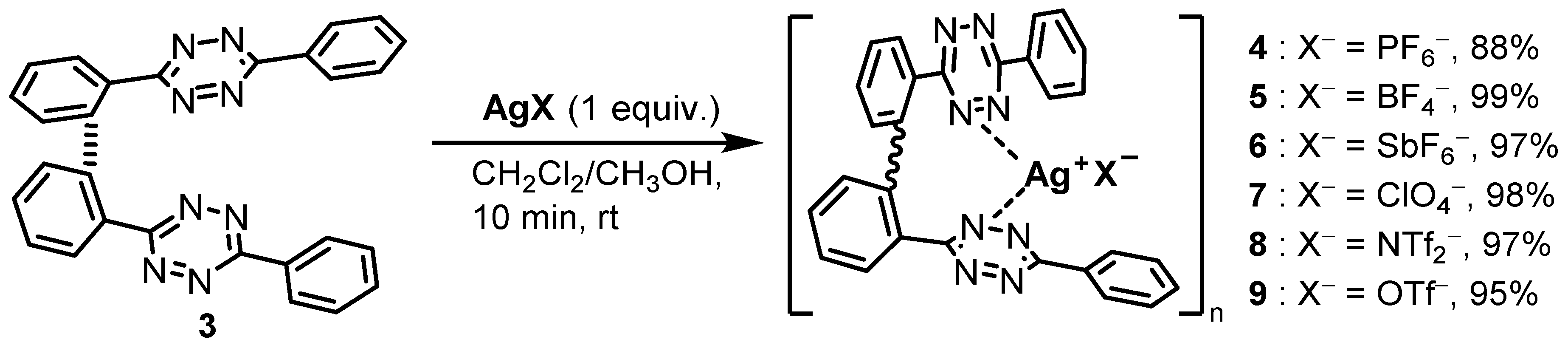
2.1. Bis(Tetrazine) 3 for Metal Complexation
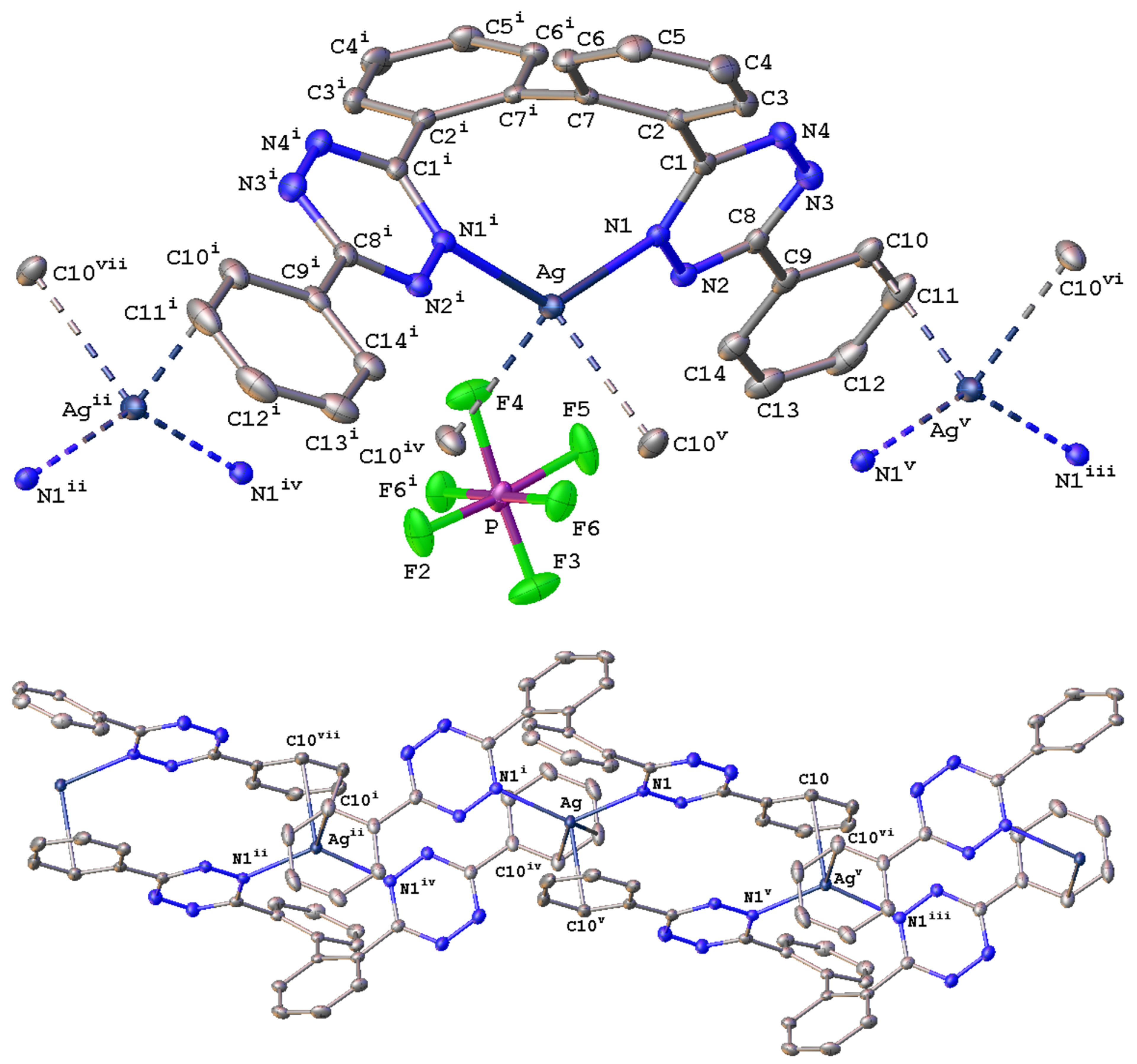
2.2. Solid-State Characterization of Silver(I) Complexes
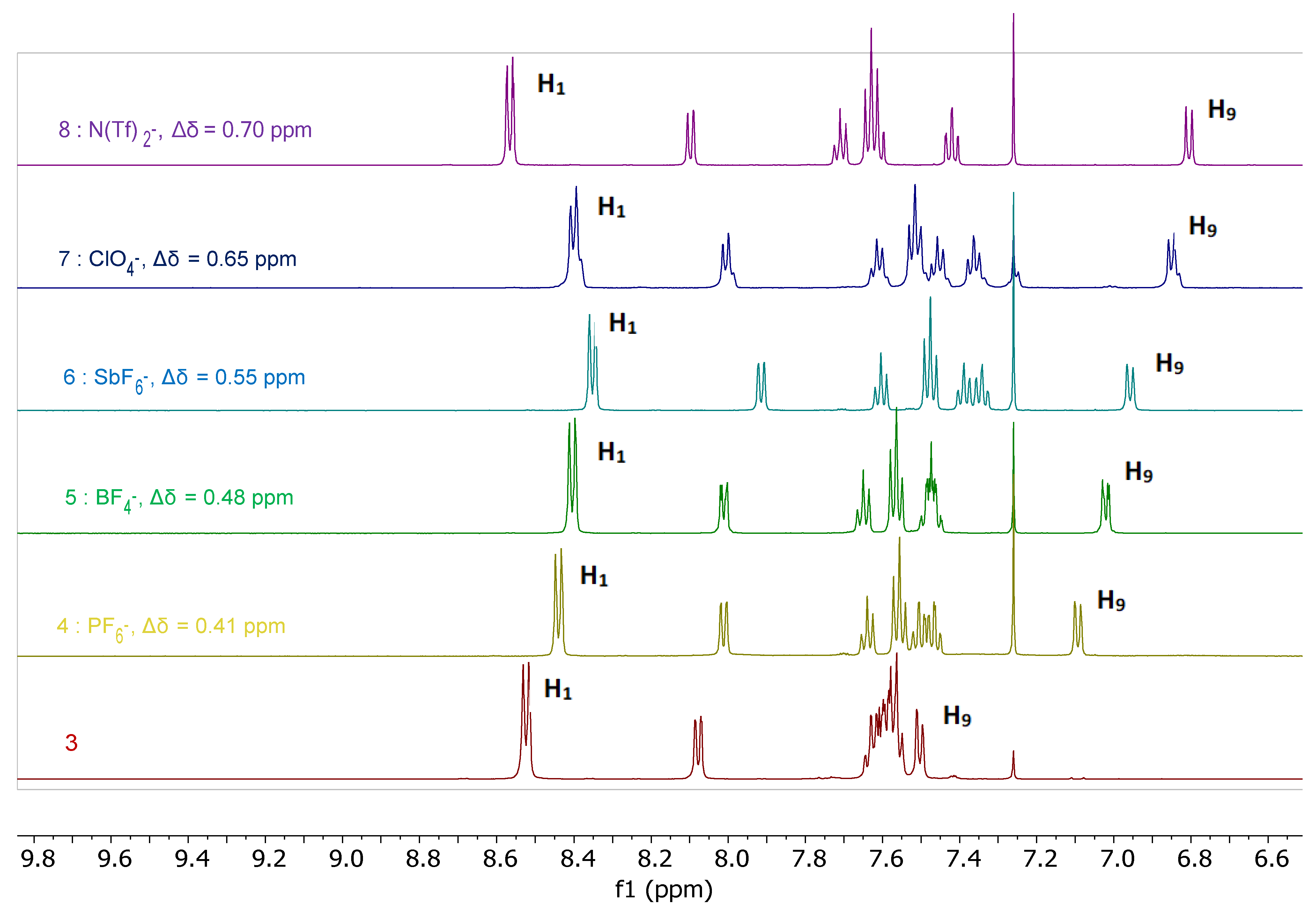
2.3. Characterization of the Bis(Tetrazine) 3 and of its Silver Complexes 4–9 in Solution
2.3.1. 1H-NMR Spectroscopy
2.3.2. Mass Spectrometry
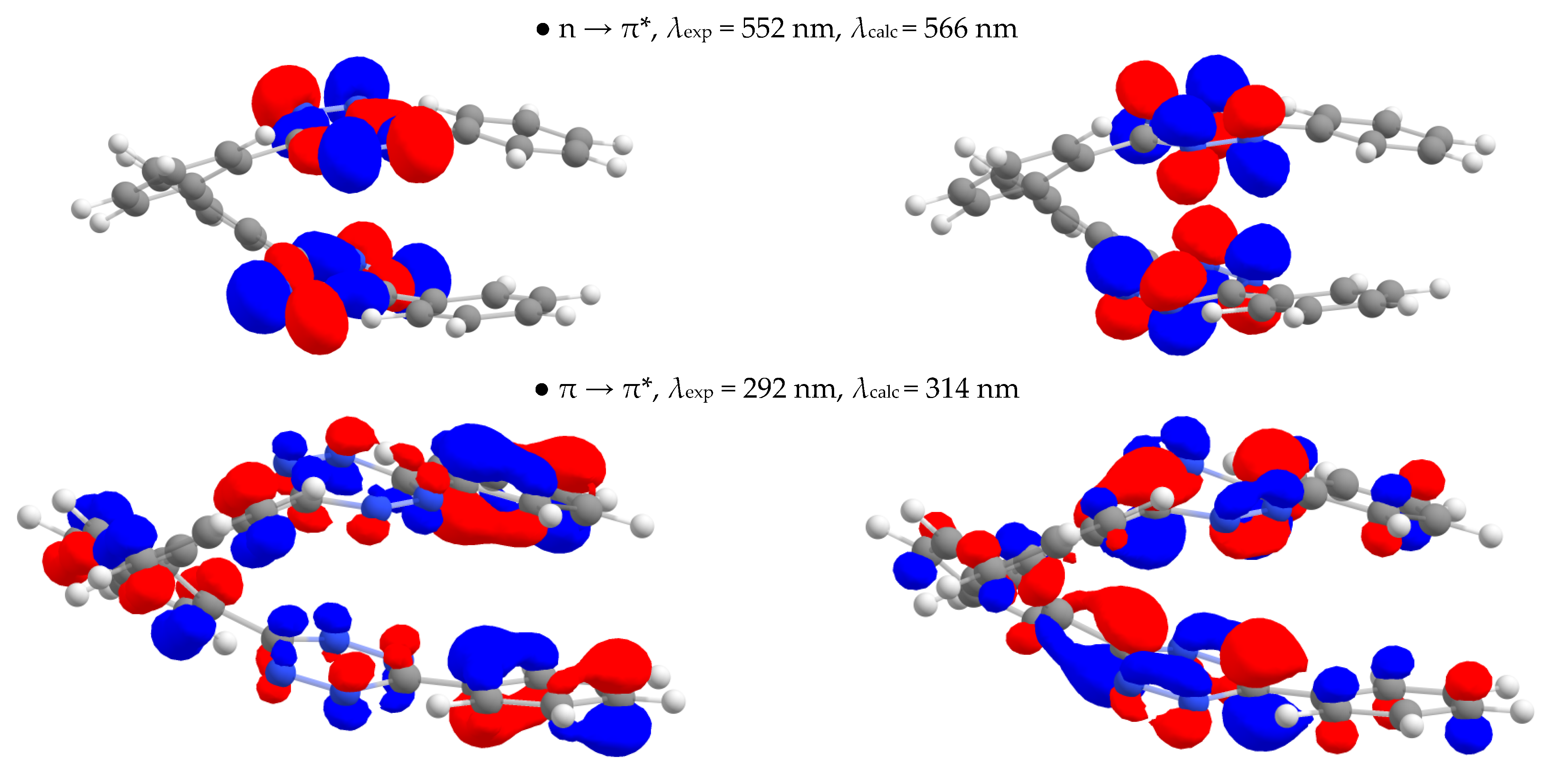
2.3.3. UV-Vis Absorption Spectroscopy
2.3.4. Electrochemical Properties
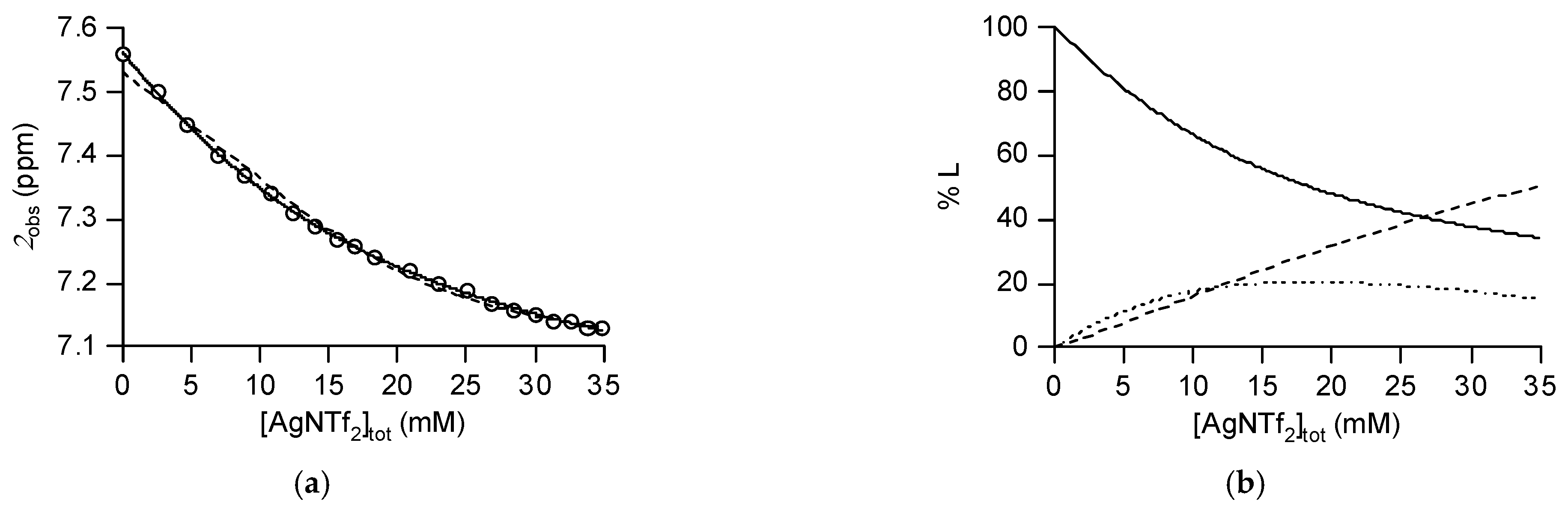
2.4. Host-Guest Behavior of Tweezer 3 with Silver Salts
3. Materials and Methods
3.1. General Synthetic Conditions
3.2. Physical Methods
3.3. Titration Procedures
3.4. Determination of the Stoichiometry of the Complexes
3.5. Determination of the Association Constants
3.6. UV-Visible Absorption and Diffuse Reflectance Spectrophotometry
3.7. Cyclic Voltammetry Measurements
3.8. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Clavier, G.; Audebert, P. s-Tetrazines as Building Blocks for New Functional Molecules and Molecular Materials. Chem. Rev. 2010, 110, 3299–3314. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mboyi, C.D.; Vivier, D.; Daher, A.; Fleurat-Lessard, P.; Cattey, H.; Devillers, H.D.; Bernhard, C.; Denat, F.; Roger, J.; Hierso, J.-C. Bridge Clamp Bis-Tetrazines Stacked by [N]8-π-Interactions and Azido-s-Aryl Tetrazines: New Classes of Doubly Clickable Tetrazines. Angew. Chem. Int. Ed. 2020, 59, 1149–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Yang, J.; Šečkutė, J.; Devaraj, N.K. In Situ Synthesis of Alkenyl Tetrazines for Highly Fluorogenic Bioorthogonal Live-Cell Imaging Probes. Angew. Chem. Int. Ed. 2014, 53, 5805–5809. [Google Scholar] [CrossRef]
- Mboyi, C.D.; Daher, A.; Khirzada, N.; Devillers, C.H.; Cattey, H.; Fleurat-Lessard, P.; Roger, J.; Hierso, J.-C. Synthesis and structural characterisation of bulky heptaaromatic (hetero)aryl o-substituted s-aryltetrazines. New J. Chem. 2020, 44, 15235–15243. [Google Scholar] [CrossRef]
- Mboyi, C.D.; Testa, C.; Reeb, S.; Genc, S.; Cattey, H.; Fleurat-Lessard, P.; Roger, J.; Hierso, J.-C. Building Diversity in ortho-Substituted s‑Aryltetrazines by Tuning N‑Directed Palladium C-H Halogenation: Unsymmetrical Polyhalogenated and Biphenyl s‑Aryltetrazines. ACS Catal. 2017, 7, 8493–8501. [Google Scholar] [CrossRef]
- Testa, C.; Gigot, E.; Genc, S.; Decréau, R.; Roger, J.; Hierso, J.-C. Ortho-Functionalized Aryltetrazines by Direct Palladium-Catalyzed C-H Halogenation: Application to Fast Electrophilic Fluorination Reactions. Angew. Chem. Int. Ed. 2016, 55, 5555–5649. [Google Scholar] [CrossRef]
- Qu, Y.; Sauvage, F.-X.; Clavier, G.; Miomandre, F.; Audebert, P. Metal-Free Synthetic Approach to 3-Monosubstituted Unsymmetrical 1,2,4,5-Tetrazines Useful for Bioorthogonal Reactions. Angew. Chem. Int. Ed. 2018, 57, 12057–12061. [Google Scholar] [CrossRef]
- Xie, Y.; Fang, Y.; Huang, Z.; Tallon, A.M.; Ende, C.W.; Fox, J.M. Divergent Synthesis of Monosubstituted and Unsymmetrical 3,6-Disubstituted Tetrazines from Carboxylic Ester Precursors. Angew. Chem. Int. Ed. 2020, 59, 16967–16973. [Google Scholar] [CrossRef]
- Kaim, W. The coordination chemistry of 1,2,4,5-tetrazines. Coord. Chem. Rev. 2002, 230, 127–139. [Google Scholar] [CrossRef]
- Myers, W.; Bjorgaard, J.A.; Brown, K.E.; Chavez, D.E.; Hanson, S.K.; Scharff, R.J.; Tretiak, S.; Veauthier, J.M. Energetic Chromophores: Low-Energy Laser Initiation in Explosive Fe(II) Tetrazine Complexes. J. Am. Chem. Soc. 2016, 138, 4685–4692. [Google Scholar] [CrossRef]
- Chavez, D.E.; Hiskey, M.A.; Gilardi, R.D. 3,3′-Azobis(6-amino-1,2,4,5-tetrazine): A Novel High-Nitrogen Energetic Material. Angew. Chem. Int. Ed. 2000, 39, 1791–1793. [Google Scholar] [CrossRef]
- Benson, C.R.; Hui, A.K.; Parimal, K.; Cook, B.J.; Chen, C.-H.; Lord, R.L.; Flood, A.H.; Caulton, K.G. Multiplying the electron storage capacity of a bis-tetrazine pincer ligand. Dalton Trans. 2014, 43, 6513–6524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kormos, A.; Koehler, C.; Fodor, E.A.; Rutkai, Z.R.; Martin, M.E.; Mezo, G.; Lemke, E.A.; Kele, P. Bistetrazine-cyanines as double-clicking fluorogenic two-point binder or cross-linker probes. Chem. Eur. J. 2018, 24, 8841–8847. [Google Scholar] [CrossRef] [PubMed]
- Audebert, P.; Miomandre, F.; Clavier, G.; Vernières, M.-C.; Badré, S.; Renault, R.M. Synthesis and Properties of New Tetrazines Substituted by Heteroatoms: Towards the World Smallest Organic Fluorophores. Chem. Eur. J. 2005, 11, 5667–5673. [Google Scholar] [CrossRef] [PubMed]
- Schottel, B.L.; Chifotides, H.T.; Shatruk, M.; Chouai, A.; Pérez, L.M.; Bacsa, J.; Dunbar, K.R. Anion-π-Interactions as Controlling Elements in Self-Assembly Reactions of Ag(I) Complexes with π-Acidic Aromatic Rings. J. Am. Chem. Soc. 2006, 128, 5895–5912. [Google Scholar] [CrossRef]
- Chifotides, H.T.; Dunbar, K.R. Anion-π Interactions in Supramolecular Architectures. Acc. Chem. Res. 2013, 46, 894–906. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Li, B.; Huang, R.-W.; Zang, S.Q.; Mak, T.C.W. Assembly of silver(I) organic frameworks from flexible supramolecular synthons with pendant ethynide arm attached to biphenyl and phenoxybenzene skeletons. Cryst. Eng. Comm. 2013, 15, 4087–4093. [Google Scholar] [CrossRef]
- Peng, P.; Li, F.F.; Bowles, F.L.; Neti, V.S.P.K.; Metta-Magana, A.J.; Olmstead, M.M.; Alan, L.; Balch, A.L.; Echegoyen, L. High yield synthesis of a new fullerene linker and its use in the formation of a linear coordination polymer by silver complexation. Chem. Commun. 2013, 49, 3209–3211. [Google Scholar] [CrossRef]
- Quinton, C.; Alain-Rizzo, V.; Dumas-Verdes, C.; Clavier, G.; Vignau, L.; Audebert, P. Triphenylamine/tetrazine based π-conjugated systems as molecular donors for organic solar cells. New J. Chem. 2015, 39, 9700–9713. [Google Scholar] [CrossRef]
- Audebert, P.; Sadki, S.; Miomandre, F.; Clavier, G.; Vernières, M.C.; Saoud, M.; Hapiot, P. Synthesis of new substituted tetrazines: Electrochemical and spectroscopic properties. New J. Chem. 2004, 28, 387–392. [Google Scholar] [CrossRef]
- Gleiter, R.; Schehlmann, V.; Spanget-Larsen, J.; Fischer, H.; Neugebauer, F.A. PE Spectra of Disubstituted 1,2,4,5-Tetrazines. J. Org. Chem. 1988, 53, 5756–5762. [Google Scholar] [CrossRef]
- Flanagan, J.B.; Margel, S.; Bard, A.J.; Anson, F.C. Electron Transfer to and from Molecules Containing Multiple, Noninteracting Redox Centers. Electrochemical Oxidation of Poly(vinylferrocene). J. Am. Chem. Soc. 1978, 100, 4248–4253. [Google Scholar] [CrossRef]
- Devillers, C.H.; Milet, A.; Moutet, J.-C.; Pécaut, J.; Royal, G.; Saint-Aman, E.; Bucher, C. Long-Range Electronic Connection in Picket-Fence like Ferrocene-Porphyrin Derivatives. Dalton Trans. 2013, 42, 1196–1209. [Google Scholar] [CrossRef]
- Ammar, F.; Savéant, J.M. Thermodynamics of Successive Electron Transferts Internal and Solvation Enthalpy and Entropy Variations in a Series of Polynitro Compounds. J. Electroanal. Chem. 1973, 47, 115–125. [Google Scholar] [CrossRef]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; Collum, D.B. Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem. Int. Ed. 2013, 52, 11998–12013. [Google Scholar] [CrossRef] [Green Version]
- Pastor, A.; Martinez-Viviente, E. NMR spectroscopy in coordination supramolecular chemistry: A unique and powerful methodology. Coord. Chem. Rev. 2008, 252, 2314–2345. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Legouin, B.; Uriac, P.; Tomasi, S.; Toupet, L.; Bondon, A.; van de Weghe, P. Novel Chiral Molecular Tweezer from (+)-Usnic Acid. Org. Lett. 2009, 11, 745–748. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, H.; Li, M.; Shao, L.; Hua, B. Host-Guest Complexation of Perethylated Pillar[6]arene toward Ferrocene Derivatives Both in Solution and Solid State: Different Binding Modes Induced by Minor Structural Changes of Guests. Org. Lett. 2020, 22, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Jaunet-Lahary, T.; Jacquemin, D.; Legouin, B.; Le Questel, J.-Y.; Cupif, J.-F.; Toupet, L.; Uriac, P.; Graton, J. Dissymmetric Molecular Tweezers in Host-Guest Complexes: Internal or External Complexation? J. Phys. Chem. C 2015, 119, 3771–3779. [Google Scholar] [CrossRef]
- Job’s method is used in analytical chemistry to determine the stoichiometry of a binding event.
- Job, P. Formation and Stability of Inorganic Complexes in Solution. Ann. Chim. 1928, 9, 113–203. [Google Scholar]
- Laverde, A.; Da Conceicao, G.; Queiroz, S.; Fujuwara, F.; Marqioli, A. An NMR tool for cyclodextrin selection in enantiomeric resolution by high-performance liquid chromatography. Magn. Reson. Chem. 2002, 40, 433–442. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Blaschke, G. Selector-select and interactions in chiral capillary electrophoresis. Electrophoresis 1999, 20, 2592–2604. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Endresz, G.; Schulte, D.; Bergenthal, D.; Blaschke, G. Capillary electrophoresis and 1H-NMR studies on chiral recognition of atropisomeric binaphthyl derivatives by cyclodextrin hosts. J. Chromatogr. A 1996, 732, 143–150. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Pintore, G.; Burjanadze, N.; Berganthal, D.; Bergander, K.; Breitkreuz, C.; Muhlenbrock, C.; Blaschke, G. Mechanistic study of opposite migration order of dimethindene enantiomers in capillary electrophoresis in the presence of native β-cyclodextrin and heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin. J. Chromatogr. A 2000, 875, 455–469. [Google Scholar] [CrossRef]
- Pitfalls of the continuous variation method and of the associated Job plot are discussed in refs. [40,41].
- Ulatowski, F.; Dąbrowa, K.; Bałakier, T.; Jurczak, J. Recognizing the Limited Applicability of Job Plots in Studying Host-Guest Interactions in Supramolecular Chemistry. J. Org. Chem. 2016, 81, 1746–1756. [Google Scholar] [CrossRef]
- Brynn-Hibbert, D.; Thordarson, P. The death of the job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef] [Green Version]
- Frassineti, C.; Alderighi, L.; Gans, P.; Sabatini, A.; Vacca, A.; Ghelli, S. Determination of protonation constants of some fluorinated polyamines by means of 13C-NMR data processed by the new computer program HypNMR2000. Protonation sequence in polyamines. Anal. Bioanal. Chem. 2003, 376, 1041–1052. [Google Scholar] [PubMed]
- Perlmutter-Hayman, B. Cooperative Binding to Macromolecules. A Formal Approach. Acc. Chem. Res. 1986, 19, 90–96. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately Extracting the Signature of Intermolecular Interactions Present in the NCI Plot of the Reduced Density Gradient versus Electron Density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Khartabil, H.; Boisson, J.-C.; Contreras-Garcia, J.; Piquemal, J.-P.; Hénon, E. Independent Gradient Model: A New Approach for Probing Strong and Weak Interactions in Molecules from Wave Function Calculations. Chem. Phys. Chem. 2018, 19, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]





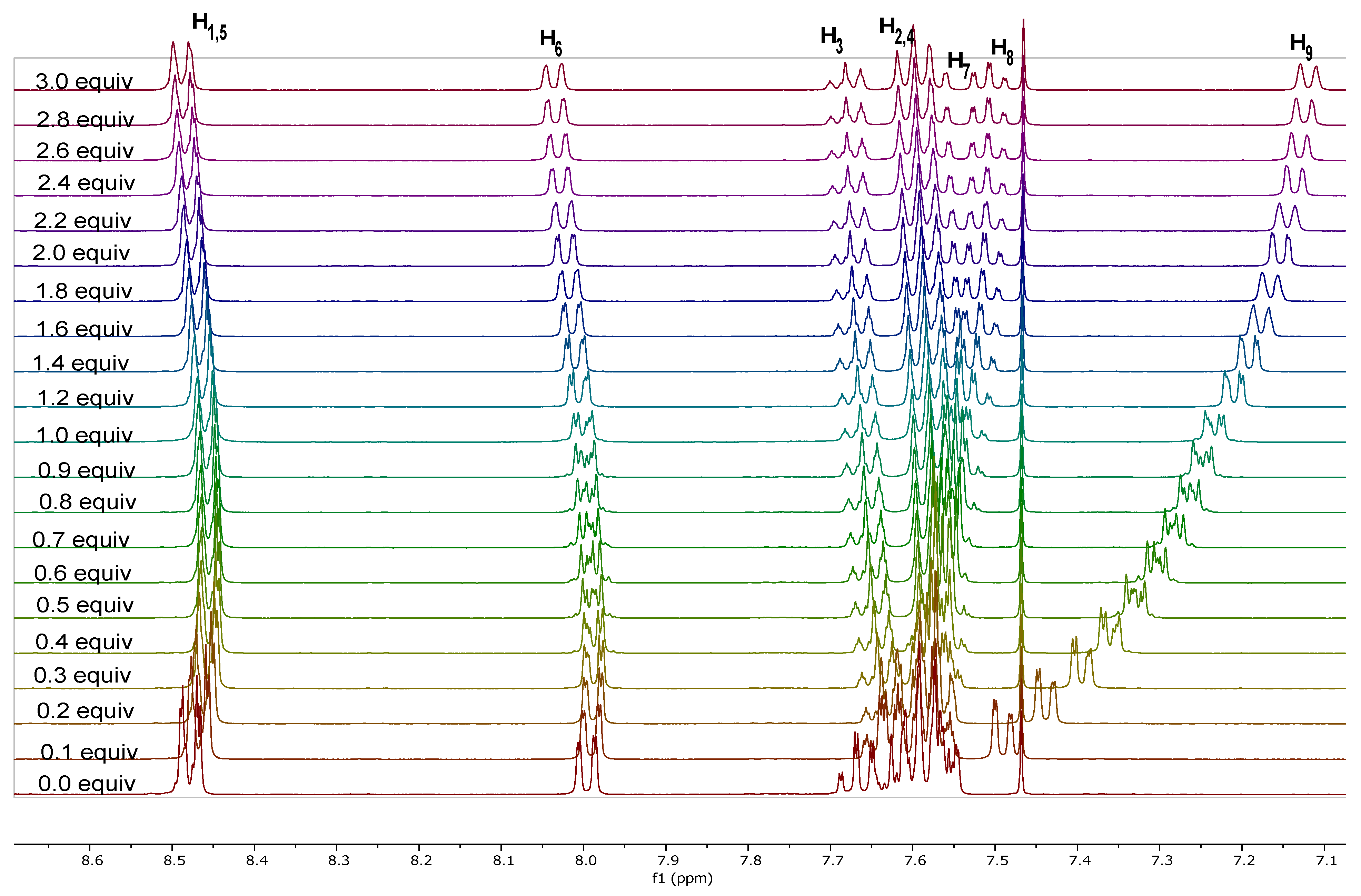

| Attribution | Chemical Shift (ppm in CD2Cl2) | SSCCs (Hz) |
|---|---|---|
| H1,5 (dd, 4H) | 8.41 | 3J1,2 = 7.4 and 4J1,3 = 1.4 |
| H6 (dd, 2H) | 7.97 | 3J6,7 = 7.6 and 4J6,8 = 1.4 |
| H3 (tt, 2H) | 7.66 | 3J3,2(4) = 7.4 and 4J3,1(5) = 1.3 |
| H2,4 (p-t, 4H) | 7.56 | 3J2,1 = 3J2,3 = 7.4 |
| H7 (ddd, 2H) | 7.50 | 3J7,6 = 3J7,8 = 7.6 and 4J7,9 = 1.3 |
| H8 (ddd, 2H) | 7.45 | 3J8,7 = 3J8,9 = 7.6 and 4J8,6 = 1.5 |
| H9 (dd, 2H) | 7.08 | 3J9,8 = 7.6 and 4J9,7 = 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mboyi, C.D.; Amamou, O.; Fleurat-Lessard, P.; Roger, J.; Cattey, H.; Devillers, C.H.; Meyer, M.; Boubaker, T.; Hierso, J.-C. Coordination Chemistry of a Bis(Tetrazine) Tweezer: A Case of Host-Guest Behavior with Silver Salts. Molecules 2021, 26, 2705. https://doi.org/10.3390/molecules26092705
Mboyi CD, Amamou O, Fleurat-Lessard P, Roger J, Cattey H, Devillers CH, Meyer M, Boubaker T, Hierso J-C. Coordination Chemistry of a Bis(Tetrazine) Tweezer: A Case of Host-Guest Behavior with Silver Salts. Molecules. 2021; 26(9):2705. https://doi.org/10.3390/molecules26092705
Chicago/Turabian StyleMboyi, Clève D., Ons Amamou, Paul Fleurat-Lessard, Julien Roger, Hélène Cattey, Charles H. Devillers, Michel Meyer, Taoufik Boubaker, and Jean-Cyrille Hierso. 2021. "Coordination Chemistry of a Bis(Tetrazine) Tweezer: A Case of Host-Guest Behavior with Silver Salts" Molecules 26, no. 9: 2705. https://doi.org/10.3390/molecules26092705








