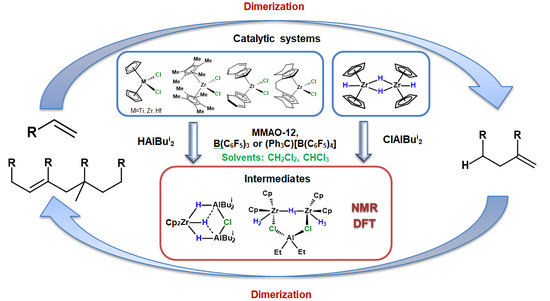
Ti Group Metallocene-Catalyzed Synthesis of 1-Hexene Dimers and Tetramers
Abstract
:1. Introduction
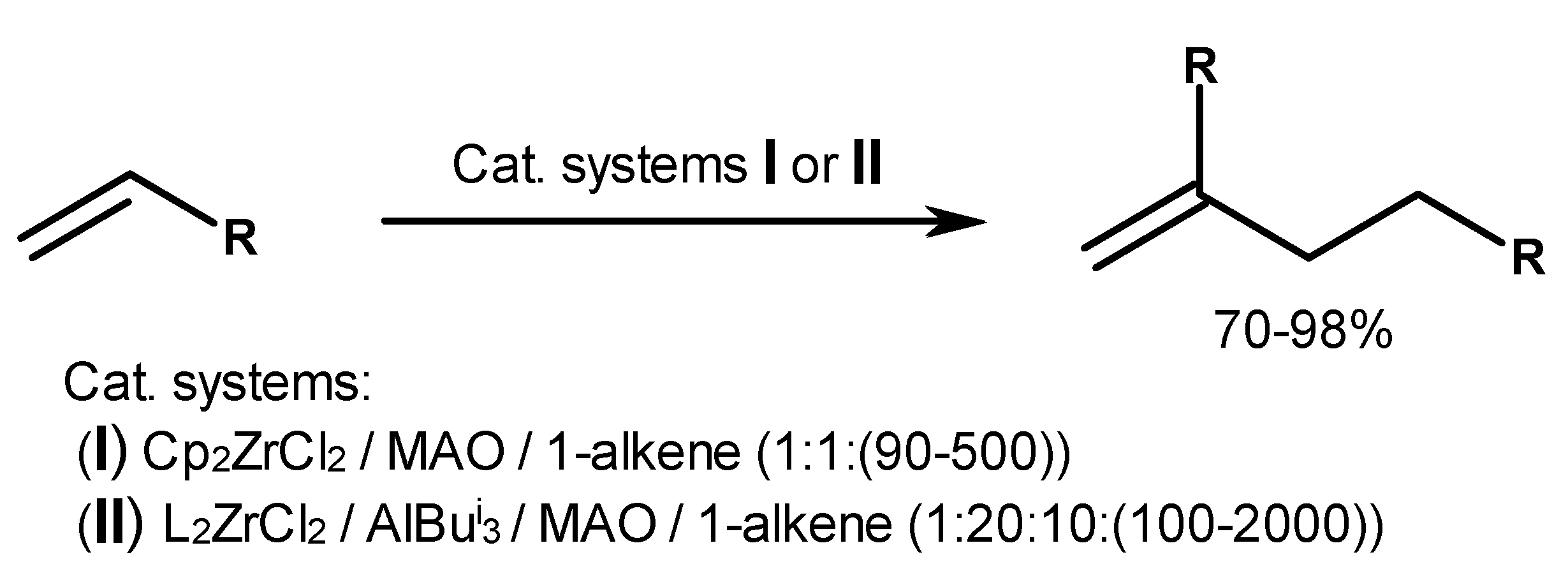
2. Results and Discussion
2.1. Transformations of 1-Hexene with Cp2MY2 (M = Ti, Zr, Hf; Y = H, Cl)-XAlBui2 (X = H, Bui) Catalytic Systems Activated by MMAO-12, B(C6F5)3, or (Ph3C)[B(C6F5)4]
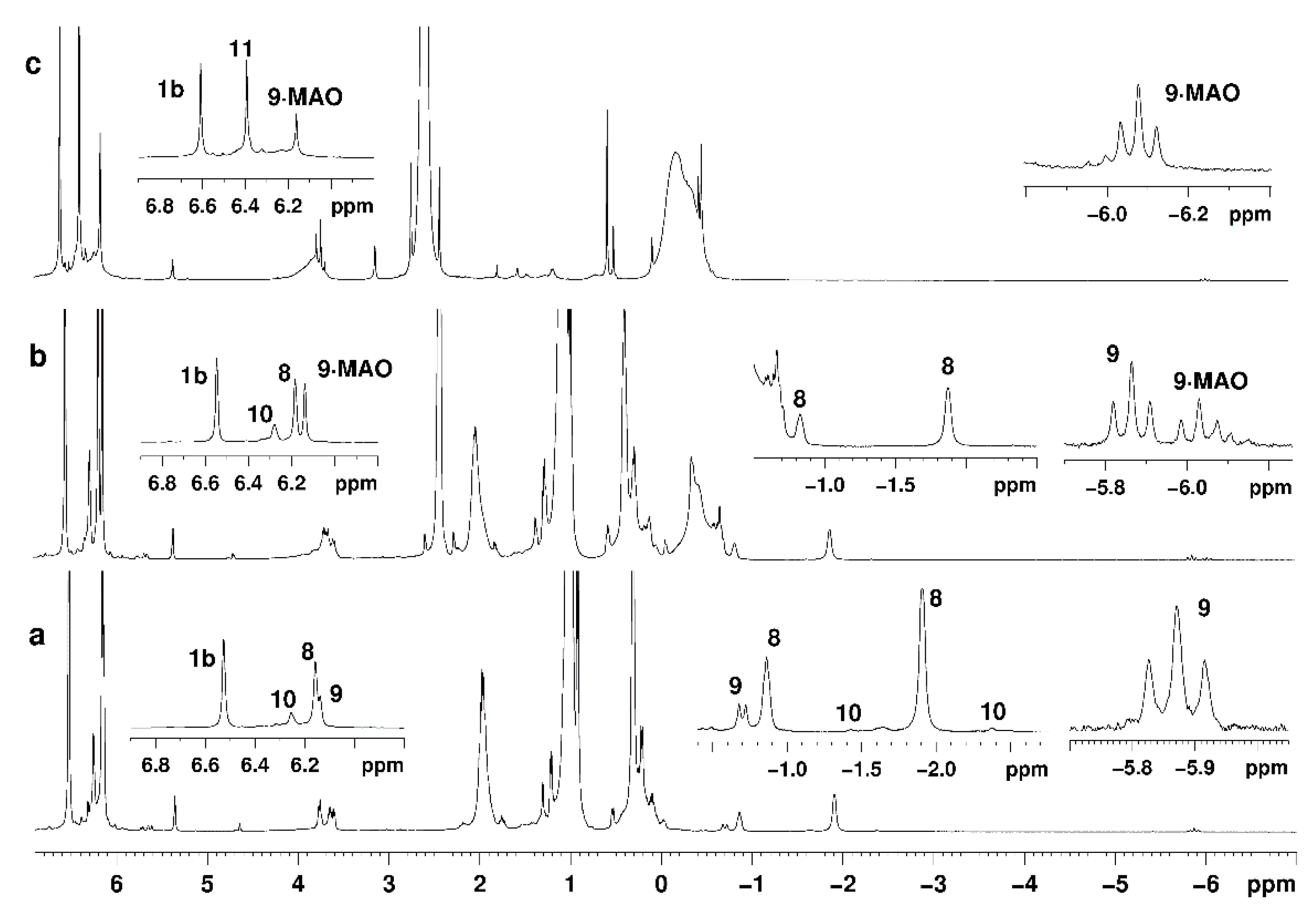
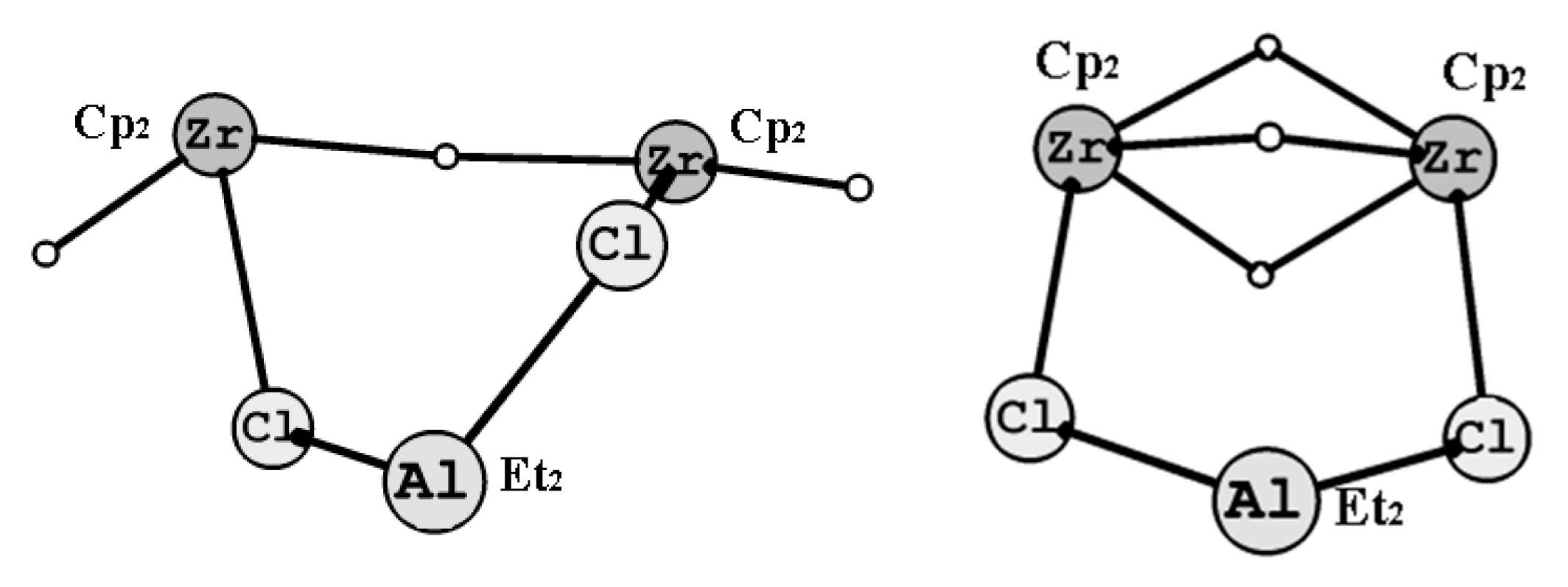
2.2. NMR Study of Intermediate Structure in MMAO-12-Activated Systems Cp2ZrY2 (Y = H, Cl)-OAC in Chlorinated Solvents
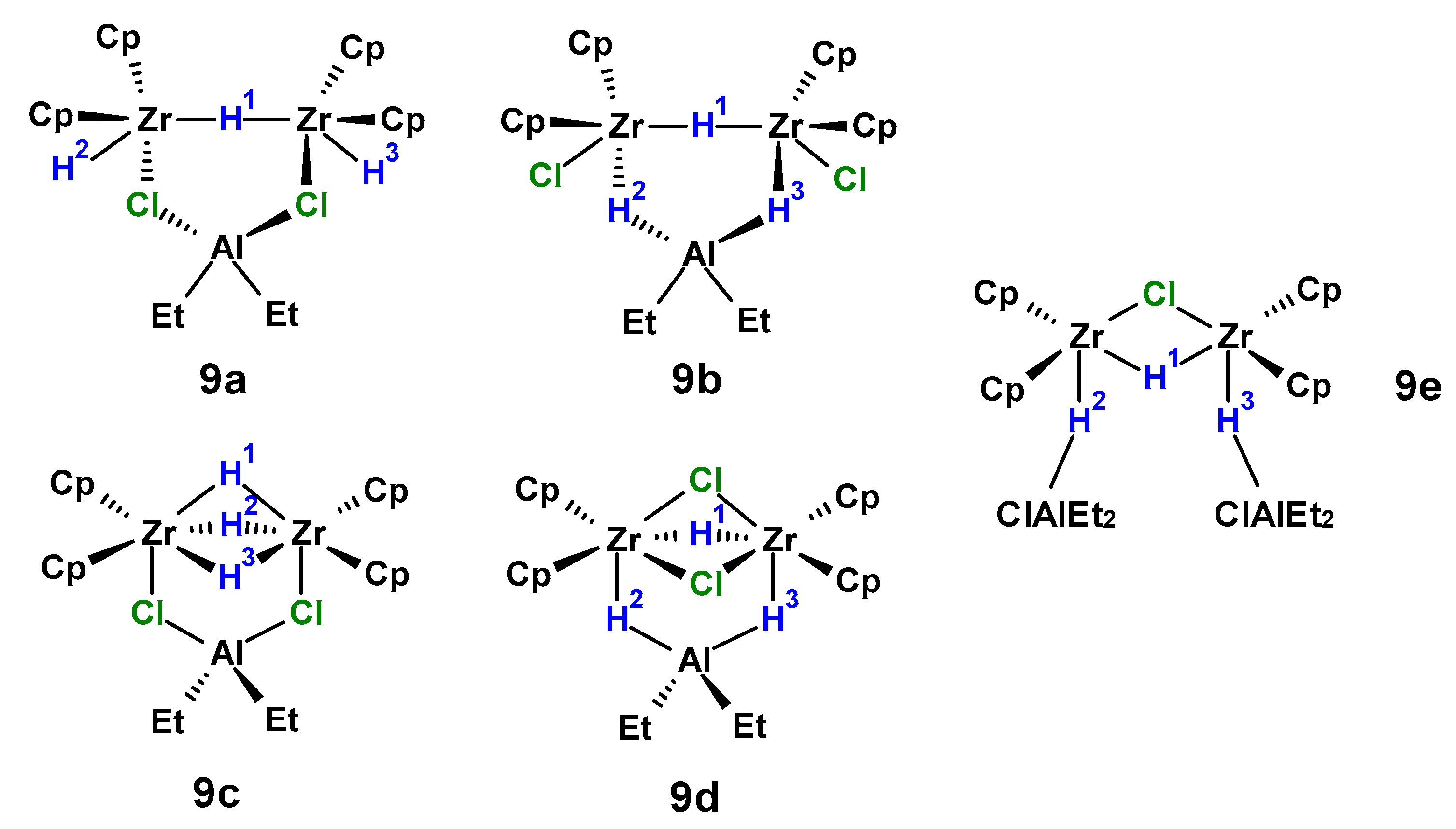
2.3. DFT Study of the Structure of Biszirconium Complex 9
3. Materials and Methods
3.1. General Procedures
3.2. Reaction of [Cp2ZrH2]2 with ClAlBui2, Activators (MMAO-12, (Ph3C)[B(C6F5)4] or B(C6F5)3) and 1-Hexene
3.3. Reaction of L2MCl2 (1a–f) with HAlB i2, MMAO-12, (Ph3C)[B(C6F5)4] or B(C6F5)3 and 1-Hexene
3.4. NMR Study of the Reaction of [Cp2ZrH2]2 with ClAlR2 and MMAO-12
3.5. NMR Study of the Reaction of Cp2ZrCl2 with HAlBui2 and MMAO-12
3.6. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Organometallic Reactions and Polymerization; Osakada, K. (Ed.) Springer-Verlag: Berlin/Heidelberg, Germany, 2014; p. 301. [Google Scholar] [CrossRef]
- Nicholas, C.P. Applications of light olefin oligomerization to the production of fuels and chemicals. Appl. Cat. A Gen. 2017, 543, 82–97. [Google Scholar] [CrossRef]
- de Klerk, A. Oligomerization. In Fischer-Tropsch Refining; de Klerk, A., Ed.; Wiley-VCH Verlag: Hoboken, NJ, USA, 2011; pp. 369–391. [Google Scholar] [CrossRef]
- McGuinness, D.S. Olefin Oligomerization via Metallacycles: Dimerization, Trimerization, Tetramerization, and Beyond. Chem. Rev. 2011, 111, 2321–2341. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta Catalysis in a-Olefin Transformations: Reaction Mechanisms and Product Design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and Related Catalysts for Olefin, Alkyne and Silane Dimerization and Oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Comyns, A.E. Encyclopedic Dictionary of Named Processes in Chemical Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 416. [Google Scholar] [CrossRef]
- Harvey, B.G.; Meylemans, H.A. 1-Hexene: A Renewable C6 Platform for Full-performance Jet and Diesel fuels. Green Chem. 2014, 16, 770–776. [Google Scholar] [CrossRef]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure−Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W. The Discovery of Metallocene Catalysts and Their Present State of the Art. J. Polym. Sci. A Polym. Chem. 2004, 42, 3911–3921. [Google Scholar] [CrossRef]
- Collins, R.A.; Russell, A.F.; Mountford, P. Group 4 Metal Complexes for Homogeneous Olefin Polymerisation: A Short Tutorial Review. App. Petroch. Res. 2015, 5, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Christoffers, J.; Bergman, R.G. Catalytic Dimerization Reactions of α-Olefins and α,ω-Dienes with Cp2ZrCl2/Poly(methylalumoxane): Formation of Dimers, Carbocycles, and Oligomers. J. Am. Chem. Soc. 1996, 118, 4715–4716. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Zirconocene-alumoxane (1:1)—A Catalyst for the Selective Dimerization of α-Olefins. Inorg. Chim. Acta 1998, 270, 20–27. [Google Scholar] [CrossRef]
- Nifant'ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-Catalyzed Dimerization of 1-Hexene: Two-stage Activation and Structure–Catalytic Performance Relationship. Cat. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally Uniform 1-Hexene, 1-Octene, and 1-Decene Oligomers: Zirconocene/MAO-Catalyzed Preparation, Characterization, and Prospects of Their Use as low-viscosity Low-temperature Oil Base Stocks. Appl. Cat. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Nifant'ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Bagrov, V.V.; Kashulin, I.A.; Roznyatovsky, V.A.; Grishin, Y.K.; Ivchenko, P.V. The Catalytic Behavior of Heterocenes Activated by TIBA and MMAO under a Low Al/Zr Ratios in 1-Octene Polymerization. Appl. Cat. A Gen. 2019, 571, 12–24. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers 2020, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K. Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization. Molecules 2020, 25, 2216. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R. Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization. Catalysts 2021, 11, 39. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules 2019, 24, 3565. [Google Scholar] [CrossRef] [Green Version]
- Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of bimetallic Zr,Al- hydride complexes with methylaluminoxane: NMR and DFT study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Dong, S.Q.; Mi, P.K.; Xu, S.; Zhang, J.; Zhao, R.D. Preparation and Characterization of Single-Component Poly-α-olefin Oil Base Stocks. Energy Fuels 2019, 33, 9796–9804. [Google Scholar] [CrossRef]
- Zhao, R.; Mi, P.; Xu, S.; Dong, S. Structure and Properties of Poly-α-olefins Containing Quaternary Carbon Centers. ACS Omega 2020, 5, 9142–9150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, H.; Gu, X.; Wang, R.; Wang, X.; Jiang, T.; Guo, X. Preparation of Lubricant Base Stocks with High Viscosity Index through 1-Decene Oligomerization Catalyzed by Alkylaluminum Chloride Promoted by Metal Chloride. Energy Fuels 2020, 34, 2214–2220. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Gabdrakhmanov, V.Z.; Istomina, G.P.; Ivchenko, P.V.; Nifant'Ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Ligand Exchange Processes in Zirconocene Dichloride-Trimethylaluminum Bimetallic Systems and Their Catalytic Properties in Reaction with Alkenes. Dalton Trans. 2018, 47, 16918–16937. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, L.V.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanism of Cp2ZrCl2-Catalyzed Olefin Hydroalumination by Alkylalanes. Russ. Chem. Bull. 2005, 54, 316–327. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Nifant’ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Role of Zr,Al Hydride Intermediate Structure and Dynamics in Alkene Hydroalumination with XAlBui2 (X = H, Cl, Bui), Catalyzed by Zr η5-Complexes. Organometallics 2015, 34, 3559–3570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Laikov, D.N. Razvitiye ekonomnogo podkhoda k raschetu molekul metodom funktsionala plotnosti i yego primeneniye k resheniyu slozhnykh khimicheskikh zadach. Ph.D Thesis, Moscow State University, Moscow, Russia, 2000. (In Russian). [Google Scholar]
- Laikov, D.N. Fast Evaluation of Density Functional Exchange-Correlation Terms Using the Expansion of the Electron Density in Auxiliary Basis Sets. Chem. Phys. Lett. 1997, 281, 151–156. [Google Scholar] [CrossRef]
- Freidlina, R.K.; Brainina, E.M.; Nesmeyanov, A.N. The Synthesis of Mixed Pincerlike Cyclopentadienyl Compounds of Zirconium. Dokl. Acad. Nauk SSSR 1961, 138, 1369–1372. [Google Scholar]
- Shoer, L.I.; Gell, K.I.; Schwartz, J. Mixed-Metal Hydride Complexes Containing Zr-H-Al Bridges. Synthesis and Relation to Transition-Metal-Catalyzed Reactions of Aluminum Hydrides. J. Organomet. Chem. 1977, 136, c19–c22. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent Perturbation Theory of Diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient Implementation of the Gauge-independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Rev. D.01; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Rocchigiani, L.; Fernandez-Cestau, J.; Chambrier, I.; Hrobárik, P.; Bochmann, M. Unlocking Structural Diversity in Gold(III) Hydrides: Unexpected Interplay of cis/trans-Influence on Stability, Insertion Chemistry, and NMR Chemical Shifts. J. Am. Chem. Soc. 2018, 140, 8287–8302. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Zhurko, G.A.; Zhurko, D.A. ChemCraft 1.6; Informer Technologies, Inc.: Roseau, Dominica, 2009. [Google Scholar]



| Entry | Catalytic Systems | [Zr]: [Al]: [Activator]:[1-Hexene] | Solvent | T, °C | Time, min | Alkene Conversion, % | Product Composition, % h | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr Complex | OAC a | Activator | 4 | 5 | 6 | 7 | ||||||||
| n = 1 | n = 2 | n = 3 | ||||||||||||
| 1 [19] | [Cp2ZrH2]2 | ClAlBui2 | MMAO-12 | 1:3:30:100 | C6H5CH3 | 40 | 15 | >99 b | 86 | |||||
| 2 | ClAlBui2 | MMAO-12 | 1:3:30:400 | CH2Cl2 | 40 | 15 | >99 | 98 | 1.3 | |||||
| 3 | 180 | 98.2 | 1.8 | |||||||||||
| 4 | ClAlBui2 | MMAO-12 | 1:3:30:400 | CHCl3 | 40 | 15 | 92 | 92 | ||||||
| 5 | 60 | >99 | 98 | |||||||||||
| 6 | 105 | 97 | 1.3 | 1.6 | ||||||||||
| 7 | 180 | 9 | 12.6 | 1.4 | 2 | 75 | ||||||||
| 8 | 960 | 17 | - | 1.4 | 2.6 | 79 | ||||||||
| 9 | - | MMAO-12 | 1:30:400 | CHCl3 | 40 | 30 | 96 | 1 | 91 | 1 | ||||
| 10 | CH2Cl2 | >99 | <1 | 89 | 8 | |||||||||
| 11 | o-Cl2C6H4 | 60 | 86 | 1 | 73 | 10 | <1 | |||||||
| 12 | 960 | 93 | <1 | 74 | 13 | 3 | 1 | |||||||
| 13 | (CH2Cl)2 | 60 | 90 | 83 | 5 | |||||||||
| 14 | 960 | 99 | 91 | 4 | 1 | |||||||||
| 15 [20] | ClAlBui2 | B(C6F5)3 | 4:8:1:400 | C6H6 | 40 | 90 | 81 | 81 | ||||||
| 16 | - | B(C6F5)3 | 4:1:400 | CHCl3 | 40 | 960 | 0 | |||||||
| 17 | CH2Cl2 | 960 | 0 | |||||||||||
| 18 | ClAlBui2 | 4:16:1:400 | CHCl3 | 180 | 42 | 40 | 2 | |||||||
| 19 | 960 | 75 | 71 | 4 | ||||||||||
| 20 | CH2Cl2 | 960 | 0 | |||||||||||
| 21 [20] | ClAlEt2 | (Ph3C)[B(C6F5)4] | 4:8:1:400 | C6H6 | 40 | 90 | 91 c | 86 | ||||||
| 22 | - | (Ph3C)[B(C6F5)4] | 4:1:400 | CHCl3 | 40 | 960 | 0 | |||||||
| 23 | CH2Cl2 | 960 | 0 | |||||||||||
| 24 | ClAlBui2 | 4:16:1:400 | CHCl3 | 180 | 44 | 9 | 20 | - | 15 | |||||
| 25 | 960 | 81 | 17 | 36 | 13 | 15 | ||||||||
| 26 | CH2Cl2 | 180 | >99 | 13 | 18 | 69 | ||||||||
| 27 [19] | Cp2ZrCl2 | HAlBui2 | MMAO-12 | 1:3:30:100 | C6H5CH3 | 40 | 15 | >99 d | 91 | |||||
| 28 | HAlBui2 | MMAO-12 | 1:3:30:400 | CH2Cl2 | 40 | 30 | 98 | 97 | 1 | |||||
| 29 | 60 | 99 | 98 | 1 | ||||||||||
| 30 | - | 1:30:400 | CH2Cl2 | 40 | 180 | 98 | 96 | - | - | |||||
| 31 | 960 | 99 | 92 | 4 | <1 | |||||||||
| 32 | HAlBui2 | MMAO-12 | 1:3:30:1000 | CH2Cl2 | 40 | 30 | 82 | 80 | 2 | |||||
| 33 | 60 | 88 | 77 | 2 | 9 | |||||||||
| 34 | HAlBui2 | MMAO-12 | 1:3:30:400 | CHCl3 | 40 | 30 | >99 | 98 | 2 | |||||
| 35 | HAlBui2 | MMAO-12 | 1:3:30:1000 | CHCl3 | 40 | 30 | >99 | 90 | 7 | 3 | ||||
| 36 | HAlBui2 | MMAO-12 | 1:3:30:400 | CHCl3 | 20 | 180 | >99 | 97 | 2 | |||||
| 37 | - | MMAO-12 | 1:30:400 | CHCl3 | 40 | 180 | >99 | 5 | 3 | 2 | 89 | |||
| 38 | - | MMAO-12 | 1:10:400 | CHCl3 | 40 | 960 | 91 | 91 | <1 | |||||
| 39 | - | MMAO-12 | 1:30:400 | o-Cl2C6H4 | 40 | 30 | 99 | 93 | 4 | - | ||||
| 40 | 960 | 99 | 91 | 5 | 1 | |||||||||
| 41 | - | MMAO-12 | 1:30:400 | (CH2Cl)2 | 40 | 30 | 99 | 95 | 2 | |||||
| 42 | 960 | >99 | 96 | 2 | ||||||||||
| 43 [20] | HAlBui2 | B(C6F5)3 | 4:16:1:1000 | C6H6 | 40 | 60 | >99 e | 93 | ||||||
| 44 | HAlBui2 | B(C6F5)3 | 4:16:1:1000 | CH2Cl2 | 40 | 60 | >99 | 99 | 1 | |||||
| 45 | CHCl3 | 60 | 83 | 82 | 1 | |||||||||
| 46 | - | B(C6F5)3 | 4:1:1000 | CH2Cl2 | 40 | 960 | 0 | |||||||
| 47 | CHCl3 | 40 | 960 | 0 | ||||||||||
| 48 [20] | HAlBui2 | (Ph3C)[B(C6F5)4] | 4:16:1:1000 | C6H6 | 60 | 90 | 97 f | 67 | ||||||
| 49 | HAlBui2 | (Ph3C)[B(C6F5)4] | 4:16:1:1000 | CH2Cl2 | 20 | 180 | >99 | 92 | 6 | 2 | ||||
| 50 | 960 | 1 | 55 | 8 | 3 | 33 | ||||||||
| 51 | HAlBui2 | (Ph3C)[B(C6F5)4] | 4:16:1:1000 | CH2Cl2 | 40 | 30 | >99 g | 3 | 60 | 6 | 1 | 27 | ||
| 52 | 960 | 1 | 2 | 1 | 24 | 43 | ||||||||
| 53 | HAlBui2 | (Ph3C)[B(C6F5)4] | 4:16:1:1000 | CHCl3 | 20 | 180 | >99 | 76 | 8 | 2 | 13 | |||
| 54 | 40 | 180 | 15 | 8 | 11 | 65 | ||||||||
| 55 | 960 | 8 | 8 | 12 | 72 | |||||||||
| 56 | - | (Ph3C)[B(C6F5)4] | 4:1:1000 | CH2Cl2 | 40 | 960 | 0 | |||||||
| 57 | CHCl3 | 960 | 0 | |||||||||||
| Entry | Catalytic Systems | [Zr]: [Al]: [Activator]:[1-Alkene] | Solvent | T, °C | Time, min | Alkene Conversion, % | Product Composition, % d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr Complex | OAC | Activator | 4 | 5 | 6 | 7 | ||||||||
| n = 1 | n = 2 | n = 3 | ||||||||||||
| 1 | Cp2TiCl2 a | HAlBui2 | MMAO-12 | 1:3:30:400 | CH2Cl2 | 40 | 60 | 80 | 20 | 34 | 4 | 15 | ||
| 2 | 180 | 91 | 17 | 44 | 5 | 20 | ||||||||
| 3 | CHCl3 | 60 | 93 | 16 | 36 | 6 | 5 | 28 | ||||||
| 4 | 960 | >99 | 5 | 4 | 41 | 11 | 7 | 30 | ||||||
| 5 | Cp2HfCl2 | HAlBui2 | MMAO-12 | 1:3:30:400 | CH2Cl2 | 40 | 120 | 84 | 2 | 53 | 20 | 6 | 2 | |
| 6 | 180 | 94 | 2 | 54 | 21 | 8 | 2 | |||||||
| 7 | 960 | 96 | - | 59 | 22 | 8 | 3 | 4 | ||||||
| 8 | MMAO-12 | 1:3:30:400 | CHCl3 | 40 | 60 | 60 | - | 42 | 15 | 3 | 1 | |||
| 9 | 120 | 74 b | - | 30 | 16 | 4 | 5 | |||||||
| 10 | 960 | >99 c | 6 | 17 | 17 | 15 | 9 | |||||||
| 11 | Cp2TiCl2 | HAlBui2 | (Ph3C)[B(C6F5)4] | 4:16:1:400 | CH2Cl2 | 40 | 960 | 0 | ||||||
| 12 | CHCl3 | 960 | 0 | |||||||||||
| 13 | Cp2HfCl2 | HAlBui2 | (Ph3C)[B(C6F5)4] | CH2Cl2 | 40 | 960 | 0 | |||||||
| 14 | CHCl3 | 960 | 0 | |||||||||||
| Entry | Catalytic Systems | [Zr]: [Al]: [activator]:[1-alkene] | Solvent | T, °C | Time, min | Alkene Conversion, % | Product Composition, % e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr Complex | OAC | Activator | 4 | 5 | 6 | ||||||||
| n = 1 | n = 2 | n = 3 | |||||||||||
| 1 | (C5Me5)2ZrCl2 | HAlBui2 | MMAO-12 | 1:3:30:400 | CHCl3 | 40 | 30 | >99 a | |||||
| 2 | CH2Cl2 | 30 | |||||||||||
| 3 | Ind2ZrCl2 | HAlBui2 | MMAO-12 | 1:3:30:400 | CH2Cl2 | 40 | 30 | 19 | 11 | 5 | 2 | 1 | |
| 4 | 180 | 48 | 20 | 19 | 6 | 3 | |||||||
| 5 | 960 | 85 b | 9 | 18 | 18 | 16 | |||||||
| 6 | CHCl3 | 40 | 180 | >99 | 13 | 51 | 24 | 12 | |||||
| 7 | 960 | >99 c | 16 | 21 | 19 | 18 | |||||||
| 8 | rac-H4C2[THInd]2ZrCl2 | HAlBui2 | MMAO-12 | 1:3:30:400 | CHCl3 | 40 | 30 | >99 d | 39 | 38 | 16 | 4 | |
| 9 | CH2Cl2 | 960 | 0 | ||||||||||
| Complex | ∆E, Hartree | ∆EZPVE, Hartree | ∆H, kcal/mol | ∆G, kcal/mol | T∆S, cal/mol |
|---|---|---|---|---|---|
| 9a | 0.000000 | 0.000000 | 0.0 | 0.0 | 2475.2 |
| 9b | 0.013103 | 0.012948 | 8.2 | 9.6 | 1139.2 |
| 9c | 0.003022 | 0.003398 | 2.4 | 3.3 | 1517.2 |
| 9d | 0.022146 | 0.023111 | 14.4 | 16.9 | 0.0 |
| Complex | δ(H 1), ppm | δ(H 2), ppm | δ(H 3), ppm | δ(Cp), ppm |
|---|---|---|---|---|
| 9a | −4.2 | 2.9 | 2.9 | 5.9 |
| 9b | −2.3 | 1.8 | 1.8 | 6.2 |
| 9c | −3.6 | 3.7 | 3.7 | 6.2 |
| 9d | 0.7 | 0.8 | 0.8 | 6.0 |
| 9e | −1.6 | −0.4 | −0.4 | 6.1 |
| 9 (experimental) | −5.9 | −0.7 | −0.7 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovyazin, P.V.; Bikmeeva, A.K.; Islamov, D.N.; Yanybin, V.M.; Tyumkina, T.V.; Parfenova, L.V. Ti Group Metallocene-Catalyzed Synthesis of 1-Hexene Dimers and Tetramers. Molecules 2021, 26, 2775. https://doi.org/10.3390/molecules26092775
Kovyazin PV, Bikmeeva AK, Islamov DN, Yanybin VM, Tyumkina TV, Parfenova LV. Ti Group Metallocene-Catalyzed Synthesis of 1-Hexene Dimers and Tetramers. Molecules. 2021; 26(9):2775. https://doi.org/10.3390/molecules26092775
Chicago/Turabian StyleKovyazin, Pavel V., Almira Kh. Bikmeeva, Denis N. Islamov, Vasiliy M. Yanybin, Tatyana V. Tyumkina, and Lyudmila V. Parfenova. 2021. "Ti Group Metallocene-Catalyzed Synthesis of 1-Hexene Dimers and Tetramers" Molecules 26, no. 9: 2775. https://doi.org/10.3390/molecules26092775
APA StyleKovyazin, P. V., Bikmeeva, A. K., Islamov, D. N., Yanybin, V. M., Tyumkina, T. V., & Parfenova, L. V. (2021). Ti Group Metallocene-Catalyzed Synthesis of 1-Hexene Dimers and Tetramers. Molecules, 26(9), 2775. https://doi.org/10.3390/molecules26092775