pH-Dependent Photoinduced Interconversion of Furocoumaric and Furocoumarinic Acids
Abstract
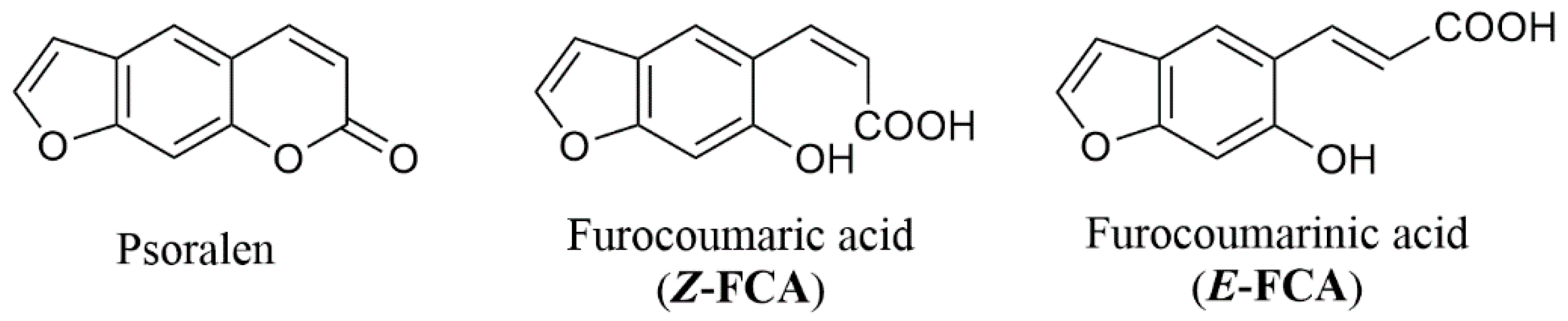
1. Introduction
2. Results and Discussion
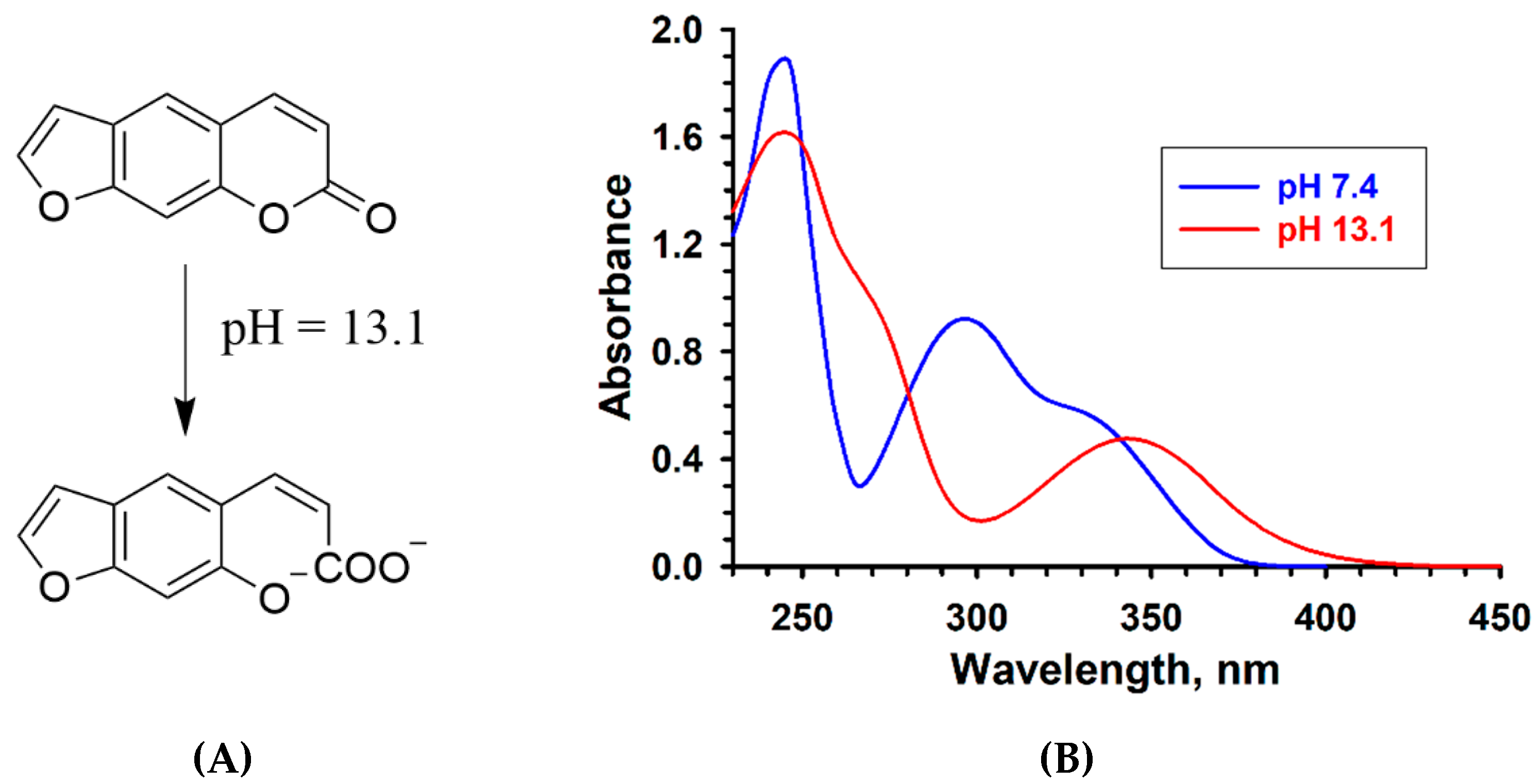
2.1. Psoralen Pyrone Ring-Opening
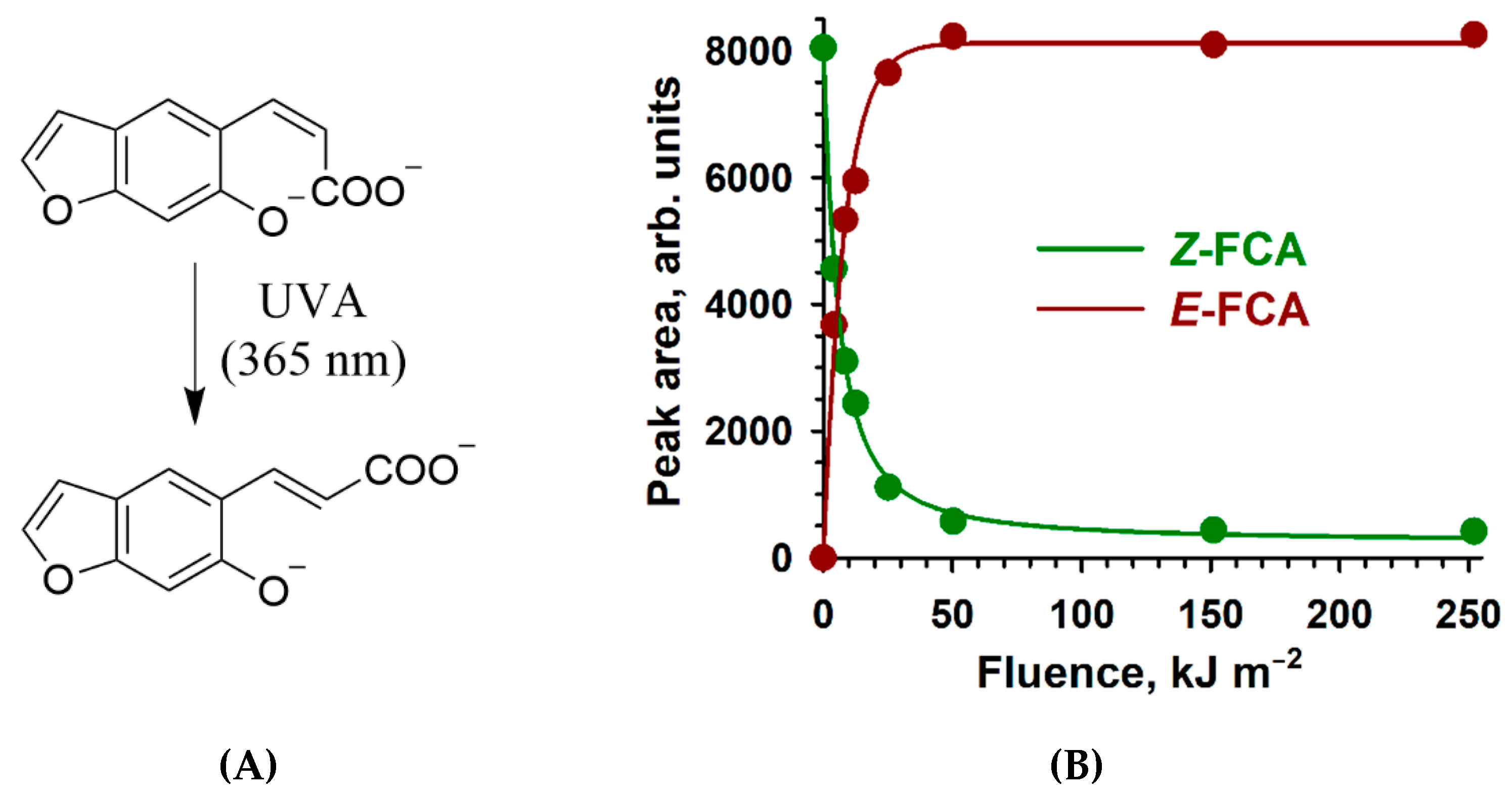
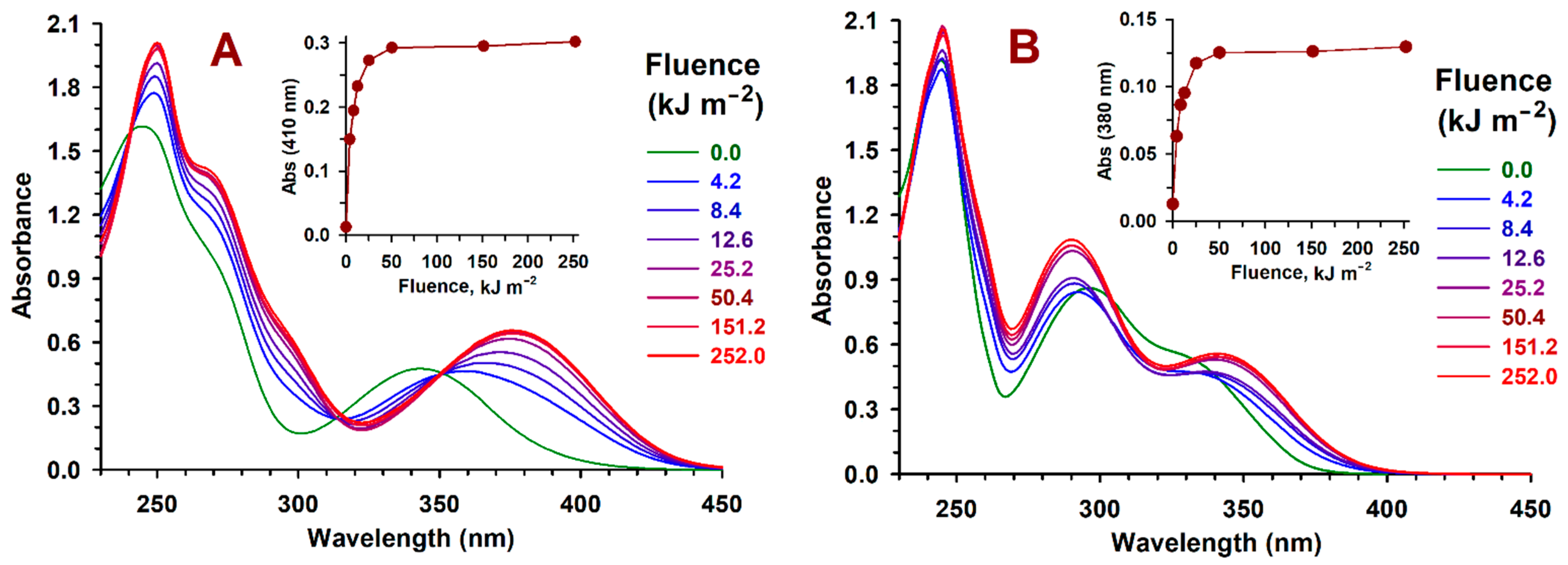
2.2. Z→ E Photoisomerization
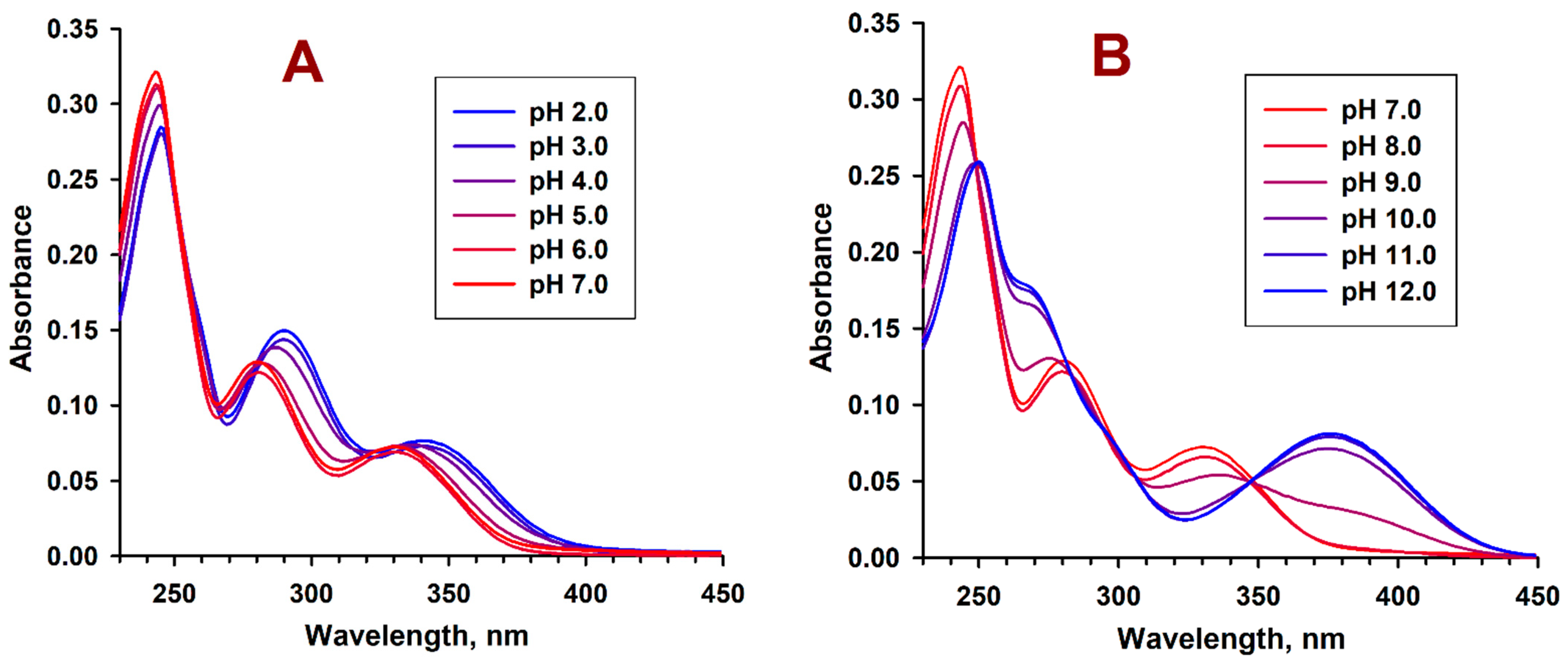
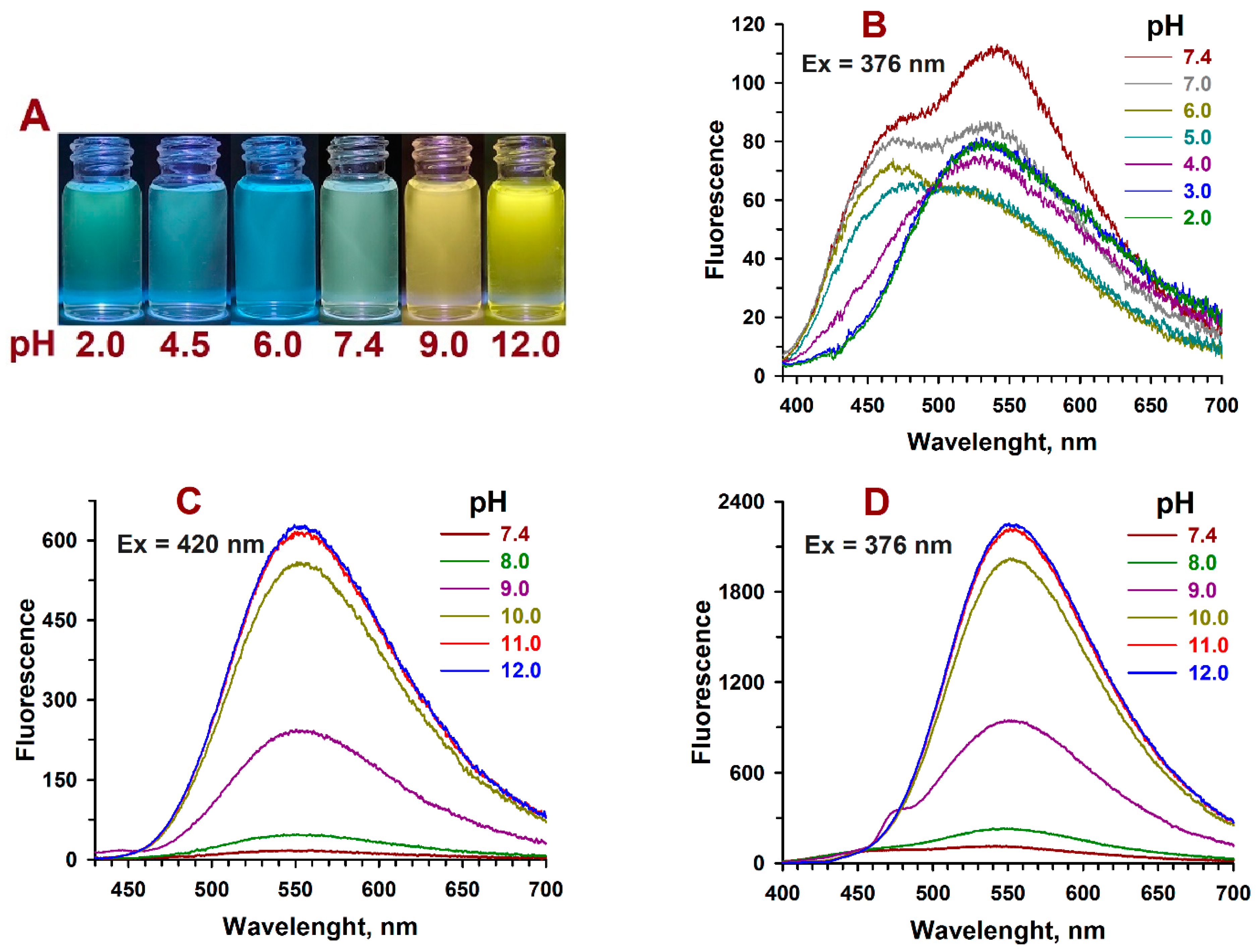
2.3. pH-Dependent Absorption and Fluorescence Characteristics of (Z/E)-FCAs
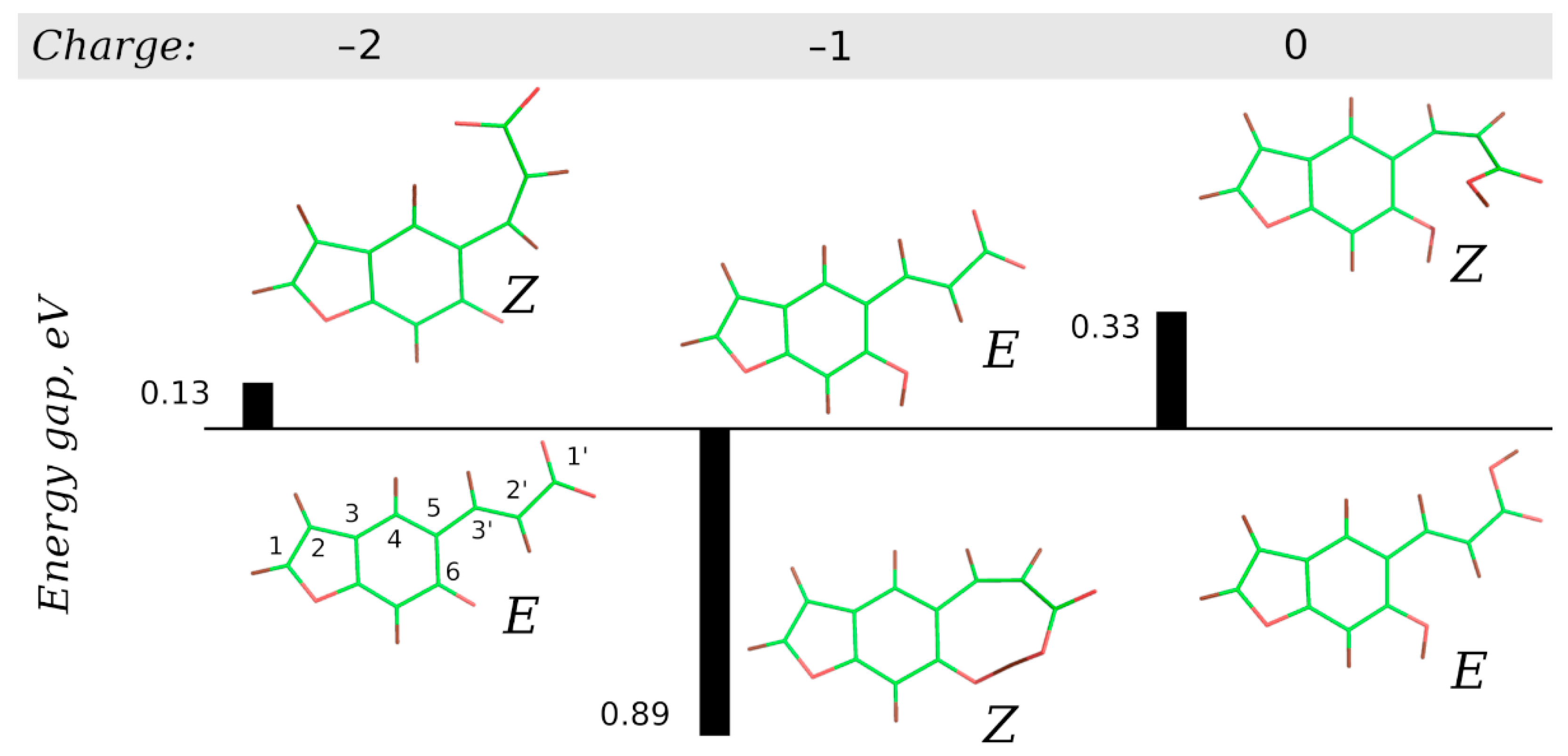
2.4. Computational Studies
2.5. E → Z Photoisomerization
3. Materials and Methods
3.1. Materials
3.2. UVA Irradiation Procedures
3.3. Analytical Procedures
3.4. Preparation and Characterization of E-FCA
3.5. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, R.; Jaswal, V.S.; Nepovimova, E.; Chaudhary, A.; Kuca, K. Psoralen: A Biological Important Coumarin with Emerging Applications. Mini-Rev. Med. Chem. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Richard, E.G.; Hönigsmann, H. Phototherapy, psoriasis, and the age of biologics. Photodermatol. Photoimmunol. Photomed. 2013, 30, 3–7. [Google Scholar] [CrossRef]
- Racz, E.; Prens, E.P. Phototherapy and Photochemotherapy for Psoriasis. Dermatol. Clin. 2015, 33, 79–89. [Google Scholar] [CrossRef]
- Torres, A.E.; Lyons, A.B.; Hamzavi, I.H.; Lim, H.W. Role of phototherapy in the era of biologics. J. Am. Acad. Dermatol. 2021, 84, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, T.; Saito, C.; Torii, K.; Nishida, E.; Yamazaki, S.; Morita, A. Photo(chemo)therapy Reduces Circulating Th17 Cells and Restores Circulating Regulatory T Cells in Psoriasis. PLoS ONE 2013, 8, e54895. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017, 49, 1–8. [Google Scholar] [CrossRef]
- Nomura, T.; Honda, T.; Kabashima, K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int. Immunol. 2018, 30, 419–428. [Google Scholar] [CrossRef]
- Ho, A.W.; Kupper, T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef]
- Sturaro, G.; Cigolini, G.; Menilli, L.; Cola, F.; Di Liddo, R.; Tasso, A.; Conconi, M.T.; Miolo, G. Antiproliferative activity of 8-methoxypsoralen on DU145 prostate cancer cells under UVA and blue light. Photochem. Photobiol. Sci. 2017, 16, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Rožman, K.; Alexander, E.M.; Ogorevc, E.; Bozovičar, K.; Sosič, I.; Aldrich, C.C.; Gobec, S. Psoralen Derivatives as Inhibitors of Mycobacterium tuberculosis Proteasome. Molecules 2020, 25, 1305. [Google Scholar] [CrossRef]
- Ren, Y.; Song, X.; Tan, L.; Guo, C.; Wang, M.; Liu, H.; Cao, Z.; Li, Y.; Peng, C. A Review of the Pharmacological Properties of Psoralen. Front. Pharmacol. 2020, 11, 571535. [Google Scholar] [CrossRef]
- Viola, G.; Salvador, A.; Vedaldi, D.; Dall’Acqua, F.; Bianchi, N.; Zuccato, C.; Borgatti, M.; Lampronti, I.; Gambari, R. Differentiation and Apoptosis in UVA-Irradiated Cells Treated with Furocoumarin Derivatives. Ann. N. Y. Acad. Sci. 2009, 1171, 334–344. [Google Scholar] [CrossRef]
- Caffieri, S. Furocoumarin photolysis: Chemical and biological aspects. Photochem. Photobiol. Sci. 2002, 1, 149–157. [Google Scholar] [CrossRef]
- Marley, K.A.; Larson, R.A.; Davenport, R. Alternative Mechanisms of Psoralen Phototoxicity. ACS Symp. Ser. 1995, 616, 179–188. [Google Scholar] [CrossRef]
- Potapenko, A.; Malakhov, M.V.; Kyagova, A.A. Photobiophysics of furocoumarins. Biophysics 2004, 49, 307–324. [Google Scholar]
- Caffieri, S.; Di Lisa, F.; Bolesani, F.; Facco, M.; Semenzato, G.C.; Dall’Acqua, F.; Canton, M. The mitochondrial effects of novel apoptogenic molecules generated by psoralen photolysis as a crucial mechanism in PUVA therapy. Blood 2007, 109, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.; Dall’Acqua, S.; Sardo, M.S.; Caffieri, S.; Vedaldi, D.; Dall’Acqua, F.; Borgatti, M.; Zuccato, C.; Bianchi, N.; Gambari, R. Erythroid Induction of Chronic Myelogenous Leukemia K562 Cells Following Treatment with a Photoproduct Derived from the UV-A Irradiation of 5-Methoxypsoralen. ChemMedChem 2010, 5, 1506–1512. [Google Scholar] [CrossRef]
- Nevezhin, E.V.; Vlasova, N.V.; Pyatnitskiy, I.A.; Lysenko, E.P.; Malakhov, M.V. On the mechanism of erythrocyte hemolysis induced by photooxidized psoralen. Biochemistry 2015, 80, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Kyagova, A.A.; Zhuravel, N.N.; Malakhov, M.V.; Lysenko, E.P.; Adam, W.; Saha-Möller, C.R.; Potapenko, A.Y. Suppression of Delayed-type Hypersensitivity and Hemolysis Induced by Previously Photooxidized Psoralen: Effect of Fluence Rate and Psoralen Concentration. Photochem. Photobiol. 1997, 65, 694–700. [Google Scholar] [CrossRef]
- Potapenko, A.Y.; Kyagova, A.A.; Bezdetnaya, L.N.; Lysenko, E.P.; Chernyakhovskaya, I.Y.; Bekhalo, V.A.; Nagurskaya, E.V.; Nesterenko, V.A.; Korotky, N.G.; Akhtyamov, S.N.; et al. Products of psoralen photooxidation possess immunomodulative and antileukemic effects. Photochem. Photobiol. 1994, 60, 171–174. [Google Scholar] [CrossRef]
- Kyagova, A.A.; Malakhov, M.V.; Potapenko, A. Immunosuppression caused by photochemo- and photodynamic therapy: Focus on photosensitizer photoproducts. In Immunosuppression: New Research; Taylor, C.B., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2009. [Google Scholar]
- Skarga, V.V.; Zadorozhny, A.D.; Shilov, B.V.; Nevezhin, E.V.; Negrebetsky, V.V.; Maslov, M.A.; Lagunin, A.A.; Malakhov, M.V. Prospective pharmacological effects of psoralen photooxidation products and their cycloadducts with aminothiols: Chemoinformatic analysis. Bull. RSMU 2020, 5, 31–39. [Google Scholar] [CrossRef]
- Décout, J.-L.; Lhomme, J. Photolysis of 8-methoxypsoralen in dilute deaerated aqueous and ethanolic solutions. Photochem. Photobiophys. 1985, 10, 113–120. [Google Scholar]
- Mohammad, T.; Morrison, H. In situ generation of psoralens by photolysis of water-soldble precursors. Photochem. Photobiol. 2008, 55, 631–638. [Google Scholar] [CrossRef]
- Mohammad, T.; Morrison, H. Light-Mediated Cyclization Of (E)-3-(5-[6-Hydroxy-7-Methoxy] Benzofuranyl)Propenoic Acid, A Water-Soluble Precursor Of 8-Methoxypsoralen. Photochem. Photobiol. 1994, 59, 248–251. [Google Scholar] [CrossRef]
- Terrian, D.L.; Mohammad, T.; Morrison, H. Photocyclization of Ortho-Substituted Cinnamic Acids. J. Org. Chem. 1995, 60, 1981–1984. [Google Scholar] [CrossRef]
- Feringa, B.L. The Art of Building Small: From Molecular Switches to Motors (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11060–11078. [Google Scholar] [CrossRef]
- García-López, V.; Liu, D.; Tour, J.M. Light-Activated Organic Molecular Motors and Their Applications. Chem. Rev. 2019, 120, 79–124. [Google Scholar] [CrossRef] [PubMed]
- Baroncini, M.; Silvi, S.; Credi, A. Photo- and Redox-Driven Artificial Molecular Motors. Chem. Rev. 2019, 120, 200–268. [Google Scholar] [CrossRef]
- Andreopoulos, F.M.; Beckman, E.J.; Russell, A.J. Photoswitchable PEG-CA Hydrogels and Factors That Affect Their Photo-sensitivity. J. Polym. Sci. A Polym. Chem. 2000, 38, 1466–1476. [Google Scholar] [CrossRef]
- Matsuhira, T.; Yamamoto, H.; Okamura, T.-A.; Ueyama, N. Manipulation of an intramolecular NHO hydrogen bond by photoswitching between stable E/Z isomers of the cinnamate framework. Org. Biomol. Chem. 2008, 6, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Taki, S.; Tsuchiya, K.; Matsumura, A.; Sakai, K.; Abe, M. Photochemical Control of Viscosity Using Sodium Cinnamate as a Photoswitchable Molecule. Chem. Lett. 2012, 41, 247–248. [Google Scholar] [CrossRef]
- Störmann, F.; Wischke, C.; Lendlein, A. Photo-Reversibility of Cinnamylidene Acetic Acid Derived Crosslinks in Poly(ε-caprolactone) Networks. MRS Proc. 2015, 1718, 49–54. [Google Scholar] [CrossRef]
- Paul, A.; Mengji, R.; Chandy, O.A.; Nandi, S.; Bera, M.; Jana, A.; Anoop, A.; Singh, N.D.P.; Singh, P.N. ESIPT-induced fluorescent o-hydroxycinnamate: A self-monitoring phototrigger for prompt image-guided uncaging of alcohols. Org. Biomol. Chem. 2017, 15, 8544–8552. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Bera, M.; Gupta, P.; Singh, N.D.P. o-Hydroxycinnamate for sequential photouncaging of two different functional groups and its application in releasing cosmeceuticals. Org. Biomol. Chem. 2019, 17, 7689–7693. [Google Scholar] [CrossRef]
- Garrett, E.R.; Lippold, B.C.; Mielck, J.B. Kinetics and Mechanisms of Lactonization of Coumarinic Acids and Hydrolysis of Coumarins, I. J. Pharm. Sci. 1971, 60, 396–405. [Google Scholar] [CrossRef]
- Lippold, B.C.; Garrett, E.R. Kinetics and Mechanisms of Lactonization of Coumarinic Acids and Hydrolysis of Coumarins II. J. Pharm. Sci. 1971, 60, 1019–1027. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Pu, S.-Z.; Sun, Q.; Fan, C.-B.; Wang, R.-J.; Liu, G. Recent advances in diarylethene-based multi-responsive molecular switches. J. Mater. Chem. C 2016, 4, 3075–3093. [Google Scholar] [CrossRef]
- Liu, D.; Yu, B.; Su, X.; Wang, X.; Zhang, Y.-M.; Li, M.; Zhang, S.X.-A.; Yu, B. Photo-/Baso-Chromisms and the Application of a Dual-Addressable Molecular Switch. Chem. Asian J. 2019, 14, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Medved’, M.; Hoorens, M.W.H.; Di Donato, M.; Laurent, A.D.; Fan, J.; Taddei, M.; Hilbers, M.; Feringa, B.L.; Buma, W.J.; Szymanski, W. Tailoring the optical and dynamic properties of iminothioindoxyl photoswitches through acidochromism. Chem. Sci. 2021, 12, 4588–4598. [Google Scholar] [CrossRef]

| Compound | Parameter 1 | pH 2.0 2 | pH 6.0 2 | pH 7.4 2 | pH 12.0 2 | Ethanol |
|---|---|---|---|---|---|---|
| Z-FCA | Absorption | 245 (15200) | ||||
| 343 (4500) | ||||||
| Emission | ‑ 3 | |||||
| E-FCA | Absorption | 245 (20700) | 243 (25200) | 243 (25100) | 250 (21200) | 245 (25600) |
| 290 (10900) | 280 (9700) | 280 (9700) | 376 (6700) | 286 (11000) | ||
| 342 (5500) | 330 (5600) | 330 (5500) | 342 (6600) | |||
| Emission | 536 (0.3) | 470 (1.6) | 543 (2.0) | 555 (2.1) | 468 (7.0) | |
| Methoxy E-FCA 4 | Absorption | 294 (16000) 4a | 365 (1000) 4b | 256 (27000) 4c | 250 (30000) | |
| 378 (7000) 4c | 289 (14500) | |||||
| 336 (5200) | ||||||
| Emission | 540 (0.4) 4d | 530 (3.0) |
| Dihedrals (°) | Angles (°) | Bond Lengths (Å) | |||||
|---|---|---|---|---|---|---|---|
| Compound (Charge) | DH 5-3′-2′-1′ | DH 6-5-3′-2′ | A 4-5-3′ | A 5-3′-2′ | A 3′-2′-1′ | B 5-3′ | B 3′-2′ |
| E-FCA (0) | 179.9 | 0.2 | 117.3 | 130.3 | 123.2 | 1.457 | 1.345 |
| E-FCA (-1) | 176.3 | 2.0 | 118.0 | 131.3 | 122.2 | 1.464 | 1.340 |
| E-FCA (-2) | 176.6 | 2.5 | 116.8 | 131.7 | 122.6 | 1.464 | 1.344 |
| Z-FCA (0) | 5.2 | 43.2 | 117.8 | 131.3 | 129.2 | 1.470 | 1.338 |
| Z-FCA (-1) | 9.4 | 26.8 | 151.1 | 138.9 | 136.0 | 1.463 | 1.352 |
| Z-FCA (-2) | 2.3 | 175.7 | 124.1 | 136.9 | 136.0 | 1.463 | 1.355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarga, V.V.; Matrosov, A.A.; Nichugovskiy, A.I.; Negrebetsky, V.V.; Maslov, M.A.; Boldyrev, I.A.; Malakhov, M.V. pH-Dependent Photoinduced Interconversion of Furocoumaric and Furocoumarinic Acids. Molecules 2021, 26, 2800. https://doi.org/10.3390/molecules26092800
Skarga VV, Matrosov AA, Nichugovskiy AI, Negrebetsky VV, Maslov MA, Boldyrev IA, Malakhov MV. pH-Dependent Photoinduced Interconversion of Furocoumaric and Furocoumarinic Acids. Molecules. 2021; 26(9):2800. https://doi.org/10.3390/molecules26092800
Chicago/Turabian StyleSkarga, Vladislav V., Anton A. Matrosov, Artemiy I. Nichugovskiy, Vadim V. Negrebetsky, Mikhail A. Maslov, Ivan A. Boldyrev, and Mikhail V. Malakhov. 2021. "pH-Dependent Photoinduced Interconversion of Furocoumaric and Furocoumarinic Acids" Molecules 26, no. 9: 2800. https://doi.org/10.3390/molecules26092800
APA StyleSkarga, V. V., Matrosov, A. A., Nichugovskiy, A. I., Negrebetsky, V. V., Maslov, M. A., Boldyrev, I. A., & Malakhov, M. V. (2021). pH-Dependent Photoinduced Interconversion of Furocoumaric and Furocoumarinic Acids. Molecules, 26(9), 2800. https://doi.org/10.3390/molecules26092800








