Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression
Abstract
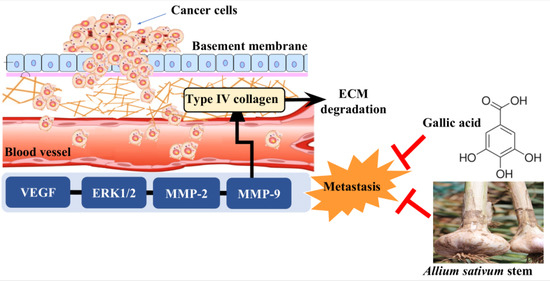
:1. Introduction
2. Results and Discussion
2.1. Effect of ASE on Melanoma Cell Growth
2.2. Effect of ASE on Melanoma Cell Migration
2.3. mRNA Expressions of VEGF, MMP-2, and MMP-9
2.4. Identification of Gallic Acid in ASE
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Extract
3.3. Cell Culture
3.4. MTT Assays
3.5. Wound Healing Assay
3.6. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
3.7. HPLC Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dang, L.; Wang, Y.; Xue, Y.; He, L.; Li, Y. Low-dose UVB irradiation prevents MMP2-induced skin hyperplasia by inhibiting inflammation and ROS. Oncol. Rep. 2015, 24, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, V.; Mitragotri, S. Nanoparticles for topical drug delivery potential for skin cancer treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines 2020, 8, 336. [Google Scholar] [CrossRef]
- Lee, W.W.; Ashly, W.; Cotliar, J.; Jung, J. Management of elderly patients with skin cancer. J. Geriatr. Oncol. 2016, 7, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, O.S.; Malyarenko, T.V.; Usoltseva, R.V.; Silchenko, A.S.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Fucoidan from brown algae Fucus evanescens potentiates the anti-proliferative efficacy of asterosaponins from starfish Asteropsis carinifera in 2D and 3D models of melanoma cells. Int. J. Biol. Macromol. 2021, 185, 31–39. [Google Scholar]
- Park, H.J. Cutaneous squamous cell carcinoma: High risk factors, staging and management. Korean Lepr. Bull. 2019, 52, 3–8. [Google Scholar] [CrossRef]
- Jo, S.J. Dermatologist’s perspective on the medical environments for skin disorders. Health Insur. Rev. Assess. Serv. Res. 2021, 1, 113–118. [Google Scholar] [CrossRef]
- Oh, B.H. Pathogenesis and prevention of skin cancer. J. Korean Med. Assoc. 2018, 61, 644–648. [Google Scholar] [CrossRef]
- Ryu, D.H.; Ryu, D.S. Anticancer and signaling mechanisms of biologically active substances from Orostachys japonicus through arrest of cell cycle in human melanoma cells. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2019, 32, 1–12. [Google Scholar]
- Kim, S.G. Primary malignant melanoma of the esophagus. Korean J. Gastroenterol. 2011, 57, 262–264. [Google Scholar] [CrossRef] [Green Version]
- Irani, S. Emerging insights into the biology of metastasis: A review article. Iran. J. Basic Med. Sci. 2019, 22, 833–847. [Google Scholar] [PubMed]
- Rice, A.; Cortes, E.; Lachowski, D.; Oertle, P.; Matellan, C.; Thorpe, S.D.; Ghose, R.; Wang, H.; Lee, D.A.; Plodinec, M.; et al. GPER activation inhibits cancer cell mechanotransduction and basement membrane invasion via RhoA. Cancers 2020, 12, 289. [Google Scholar] [CrossRef] [Green Version]
- Franchi, M.; Piperigkou, Z.; Karamanos, K.A.; Franchi, L.; Masola, V. Extracellular matrix-mediated breast cancer cells morphological alterations, invasiveness, and microvesicles/exosomes release. Cells 2020, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Stryker, Z.I.; Rajabi, M.; Davis, P.J.; Mousa, S.A. Evaluation of angiogenesis assays. Biomedicines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.J.; Song, B.; Yang, T. MMP-2, MMP-9, TIMP-1, and TIMP-2 in the peripheral blood of patients with differentiated thyroid carcinoma. Cancer Manag. Res. 2019, 11, 10675–10681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Takino, T.; Endo, Y.; Sato, H. Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis. Cancer Sci. 2017, 108, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhang, J.; Guo, J.; Jia, Y.; Han, Y.; Wang, Z. RNA interference targeting CD147 inhibits metastasis and invasion of human breast cancer MCF-7 cells by downregulating MMP-9/VEGF expression. Acta Biochim. Et Biophys. Sin. 2018, 50, 676–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Kim, S.H.; Lee, H.; Kim, B.; Kim, Y.S.; Key, J. Visualization of MMP-2 activity using dual-probe nanoparticles to detect potential metastatic cancer cells. Nanomaterials 2018, 8, 119. [Google Scholar]
- Bae, M.G.; Hwang-Bo, J.; Lee, D.Y.; Lee, Y.-H.; Chung, I.S. Effects of 6,8-diprenylgenistein on VEGF-A-induced lymphangiogenesis and lymph node metastasis in an oral cancer sentinel lymph node animal model. Int. J. Mol. Sci. 2021, 22, 770. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Javid, H.; Afshari, A.R.; Mashkani, B.; Hashemy, S.l. Substance P accelerates the progression of human esophageal squamous cell carcinoma via MMP-2, MMP-9, VEGF-A, and VEGFR1 overexpression. Mol. Biol. Rep. 2020, 47, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Boocock, C.A.; Charnock-Jones, D.S.; Sharkey, A.M.; McLaren, J.; Barker, P.J.; Wright, K.A.; Twentyman, P.R.; Smith, S.K. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J. Natl. Cancer Inst. 1995, 87, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yoneda, J.; Herrera, C.; Wood, J.; Killion, J.J.; Fidler, I.J. Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int. J. Oncol. 2000, 16, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Belotti, D.; Paganoni, P.; Manenti, L.; Garofalo, A.; Marchini, S.; Taraboletti, G.; Giavazzi, R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: Implications for ascites formation. Cancer Res. 2003, 63, 5224–5229. [Google Scholar] [PubMed]
- Gao, H.; Lan, W.; Le, S.; Xue, Y. Relationships of MMP-9, E-cadherin, and VEGF expression with clinicopathological features and response to chemosensitivity in gastric cancer. Tumor Biol. 2017, 30, 1010428317698368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, J.; Levina, N.; Van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar]
- Maron, M.F.; Camargo, A.B.; Manucha, W. Allicin pharmacology: Common molecular mechanisms against neuroinflammation and cardiovascular diseases. Life Sci. 2020, 249, 117513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, A.L.; Du, G.H. Allicin. Nat. Small Mol. Drugs Plants 2018, 10, 569–573. [Google Scholar]
- Santis, D.D.; Garzoli, S.; Vettraino, A.M. Effect of gaseous ozone treatment on the aroma and clove rot by Fusarium proliferatum during garlic postharvest storage. Heliyon 2021, 7, e06634. [Google Scholar] [CrossRef]
- Kim, R.J.; Kang, M.J.; Lee, S.J.; Shin, J.H.; Sung, N.J. Physicochemical characteristics and antioxidant activities of fermented garlic husk. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1731–1738. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lim, G.B.; Kim, S.Y.; Park, J.H.; Kim, E.H.; Sung, Y.J.; Heo, Y.J.; Kim, Y.H.; Kim, Y.H.; Lee, S.R. Application evaluation of physical and strength properties of paperboard by kraft pulp mixing made from agricultural byproducts. Korea Tech. Assoc. Pulp Pap. Ind. 2014, 46, 43–50. [Google Scholar] [CrossRef]
- Bontempo, P.; Stiuso, P.; Lama, S.; Napolitano, A.; Piacente, S.; Altucci, L.; Molinari, A.M.; De Masi, L.; Rigano, D. Metabolite profile and in vitro beneficial effects of black garlic (Allium sativum L.) polar extract. Nutrients 2021, 13, 2771. [Google Scholar] [CrossRef]
- Subramanian, M.S.; Nandagopal, M.S.G.; Amin Nordin, S.; Thilakavathy, K.; Joseph, N. Prevailing knowledge on the bioavailability and biological activities of sulphur compounds from Alliums: A potential drug candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef] [PubMed]
- An, E.J.; Kim, Y.; Lee, S.H.; Ko, H.M.; Chung, W.S.; Jang, H.J. Anti-cancer potential of oxialis obtriangulata in pancreatic cancer cell through regulation of the ERK/Src/STAT3-mediated pathway. Molecules 2020, 25, 2301. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.D.; Kang, W.S.; Kim, D.H.; Ku, J.J.; Seo, K.I. Comparison of biological activity between Stellaria aquatica seed extracts. Korean J. Food Preserv. 2019, 26, 228–237. [Google Scholar] [CrossRef]
- Shin, E.J.; Chung, S.W.; Hwang, J.T. Eff. Γ-Oryzanol Prolif. Apoptosis AGS Hum. Gastric Carcinoma Cell. Korean Soc. Biotechnol. Bioeng. J. 2017, 32, 83–89. [Google Scholar]
- Asemani, Y.; Zamani, N.; Bayat, M.; Amirghofran, Z. Allium vegetables for possible future of cancer treatment. Phytother. Res. 2019, 33, 3019–3039. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Tchounwou, P.B. In vitro assessment of oxidative stress and apoptotic mechanisms of garlic extract in the treatment of acute promyelocytic leukemia. J. Cancer Sci. Ther. 2012, 6, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Wang, C.J.; Huang, K.H.; Lee, Y.J.; Chan, W.M.; Chang, Y.C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Maghiari, A.L.; Coricovac, D.; Pinzaru, I.A.; Macașoi, I.G.; Marcovici, I.; Simu, S.; Navolan, D.; Dehelean, C. High concentrations of aspartame induce pro-angiogenic effects in ovo and cytotoxic effects in HT-29 human colorectal carcinoma cells. Nutrients 2020, 12, 3600. [Google Scholar] [CrossRef]
- Huang, T.H.; Chiu, Y.H.; Chan, Y.L.; Chiu, Y.H.; Wang, H.; Huang, K.C.; Li, T.L.; Hsu, K.H.; Wu, C.J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in lewis tumor-bearing mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef]
- Lee, Y.S. Current development and research trend of chemotherapeutic agents for head and neck squamous cell carcinoma. Korean J. Otorhinolaryngol. Head Neck Surg. 2019, 62, 487–798. [Google Scholar] [CrossRef]
- Shin, D.Y.; Yoon, M.K.; Choi, Y.W.; Gweon, O.C.; Kim, J.I.; Choi, T.H.; Choi, Y.H. Effect of aged black garlic extracts on the tight junction permeability and cell invasion in human gastric cancer cells. J. Life Sci. 2010, 20, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, A.; Quagliariello, V.; Del Vecchio, V.; Falco, M.; Luciano, A.; Amruthraj, N.J.; Nasti, G.; Ottaiano, A.; Berretta, M.; Iaffaioli, R.V.; et al. Anticancer and anti-inflammatory properties of Ganoderma lucidum extract effects on melanoma and triple-negative breast cancer treatment. Nutrients 2017, 9, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inhibition of lymphatic endothelial growth factor receptor in a murine model of oral squamous cell carcinoma. J. Korean Assoc. Maxillofac. Plast. Reconstr. Surg. 2011, 33, 1–9.
- Zhou, Y.; Xia, L.; Wang, H.; Obang, L.; Su, M.; Liu, Q.; Lin, J.; Tan, S.; Tian, Y.; Liao, Q.; et al. Cancer stem cells in progression of colorectal cancer. Oncotarget 2017, 9, 33403–33415. [Google Scholar] [CrossRef] [Green Version]
- Truffi, M.; Mazzucchelli, S.; Bonizzi, A.; Sorrentino, L.; Allevi, R.; Vanna, R.; Morasso, C.; Corsi, F. Nano-strategies to target breast cancer-associated fibroblasts: Rearranging the tumor microenvironment to achieve antitumor efficacy. Int. J. Mol. Sci. 2019, 20, 1263. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.; Kim, Y.J.; Min, J.W.; Yang, D.C. Optimization of extraction method for the quantitative analysis of gallic acid from Cornus officinallis. Korean J. Food Sci. Technol. 2009, 41, 498–502. [Google Scholar]
- Zeng, C.; Luo, S.; Feng, S.; Chen, T.; Zhou, L.; Yuan, M.; Huang, Y.; Liao, J.; Ding, C. Phenolic composition, antioxidant and anticancer potentials of extracts from Rosa banksiae ait. flowers. Molecules 2020, 25, 3068. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Zhang, S.; Xie, Y.; Zhang, Z.; Zhao, W. Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncol. Lett. 2016, 11, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [PubMed]
- Xu, M.; Gu, W.; Shen, Z.; Wang, F. Anticancer activity of phloretin against human gastric cancer cell lines involves apoptosis, cell cycle arrest, and inhibition of cell invasion and JNK signalling pathway. Med. Sci. Monit. 2018, 24, 6551–6558. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, F.; Casciaro, B.; Mangoni, M.L. A novel in vitro wound healing assay to evaluate cell migration. J. Vis. Exp. 2018, 133, 56825. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lv, Y.; Hao, J.; Shi, T.; Wang, S.; Wang, K.; Fan, X.; Guo, Y.; Zhang, J.; Li, J. N-myc downstream-regulated gene 2 promotes the protein stability of estrogen receptor beta via inhibition of ubiquitin-protein ligase E3A to suppress colorectal cancer. J. Gastrointest. Oncol. 2020, 11, 1200–1213. [Google Scholar] [CrossRef]





| Primer | Forward (5’–3’) | Reverse (5’–3’) | Size (bp) |
|---|---|---|---|
| VEGF | GCAGAATCATCACGAAGTGG | GCATGGTGATGTTGGACTCC | 169 |
| MMP-2 | CAGCCTGGGACTGCCCCCTGAT | CAGGCCCCTCCGGGTCCTTCTC | 400 |
| MMP-9 | AGTTTGGTGTCGCGGAGCAC | TACATGAGCGCTTCCGGCAC | 754 |
| β-actin | AGCACAGAGCCTCGCCTTT | CTTAATGTCACGCACGATTTCC | 697 |
| Genes | Functions | Abb. |
|---|---|---|
| Vascular endothelial growth factor | Specific growth factor for angiogenesis. A crucial factor of angiogenesis in tumor growth and metastasis. | VEGF |
| Matrix metalloproteinases-2 | Degradation of gelatin, type IV collagen, and some bioactive molecules, such as growth factor-binding proteins receptors. | MMP-2 |
| Matrix metalloproteinases-9 | Degradation of type IV collagen, proteoglycan core protein, and elastin. | MMP-9 |
| Matrix metalloproteinases | metalloproteinases capable of degrading all components of the extracellular matrix. | MMPs |
| Vascular endothelial growth factor receptor-2 | Activation of VEGF-stimulated signal transduction including endothelial cell survival, migration, proliferation, enhancing permeability. | VEGFR-2 |
| Phosphoinositide 3-kinase | Regulation of various cell functions including cell proliferation, apoptosis, tumor growth, and angiogenesis by Akt downstream. | PI3K |
| Extracellular signal-regulated protein kinases | Important messenger for extracellular and intracellular signals, which serve a vital role in processes, including proliferation, differentiation, cytoskeleton construction, and cellular senescence. Involvement in VEGF-C upregulation by inducing IGF-1. | ERK1/2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gam, D.-H.; Park, J.-H.; Kim, J.-H.; Beak, D.-H.; Kim, J.-W. Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression. Molecules 2022, 27, 21. https://doi.org/10.3390/molecules27010021
Gam D-H, Park J-H, Kim J-H, Beak D-H, Kim J-W. Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression. Molecules. 2022; 27(1):21. https://doi.org/10.3390/molecules27010021
Chicago/Turabian StyleGam, Da-Hye, Jae-Hyun Park, Jun-Hee Kim, Dong-Ho Beak, and Jin-Woo Kim. 2022. "Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression" Molecules 27, no. 1: 21. https://doi.org/10.3390/molecules27010021
APA StyleGam, D.-H., Park, J.-H., Kim, J.-H., Beak, D.-H., & Kim, J.-W. (2022). Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression. Molecules, 27(1), 21. https://doi.org/10.3390/molecules27010021