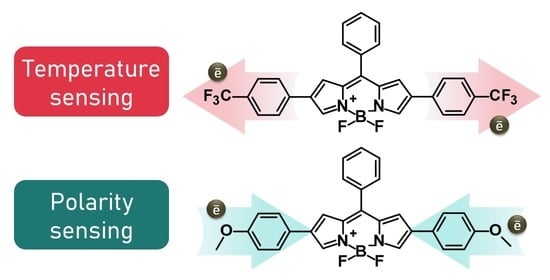
Give or Take: Effects of Electron-Accepting/-Withdrawing Groups in Red-Fluorescent BODIPY Molecular Rotors
Abstract
:1. Introduction
2. Results and Discussion
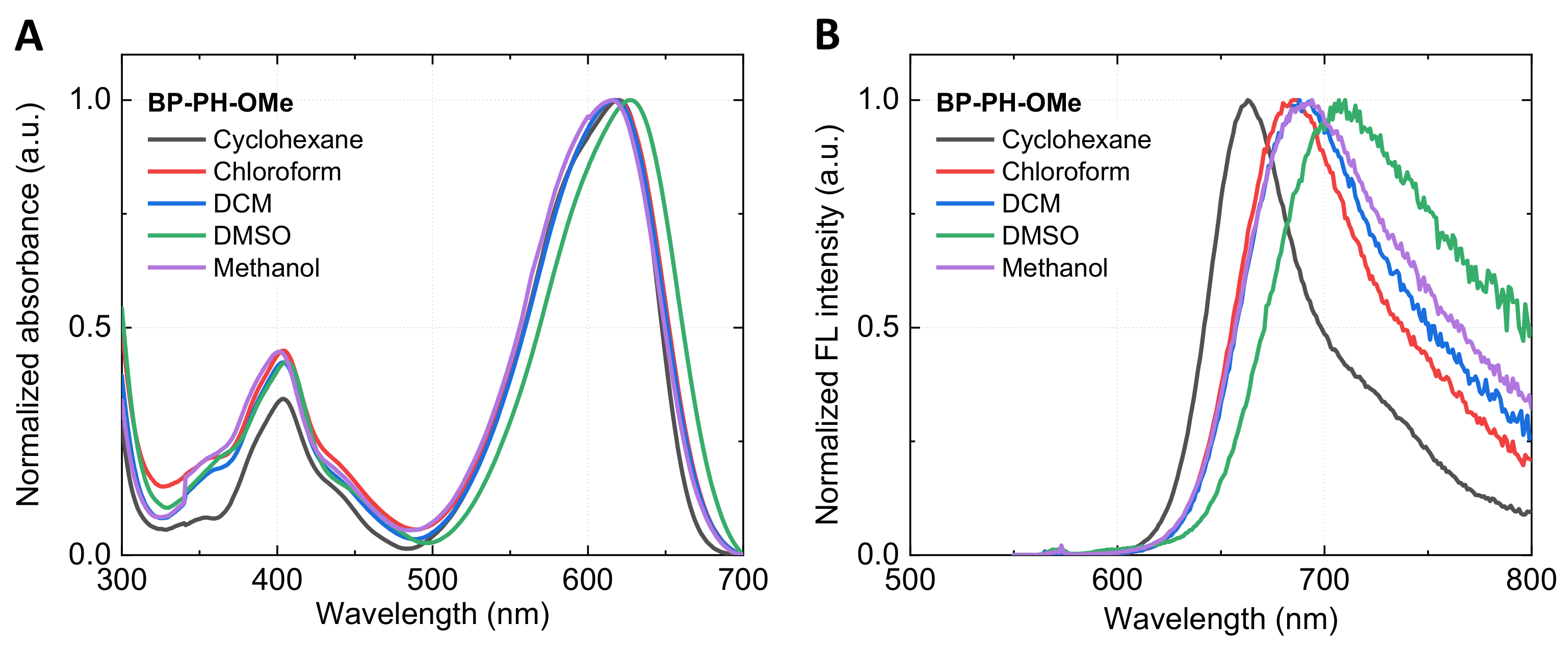
2.1. Absorbance and Fluorescence Spectra
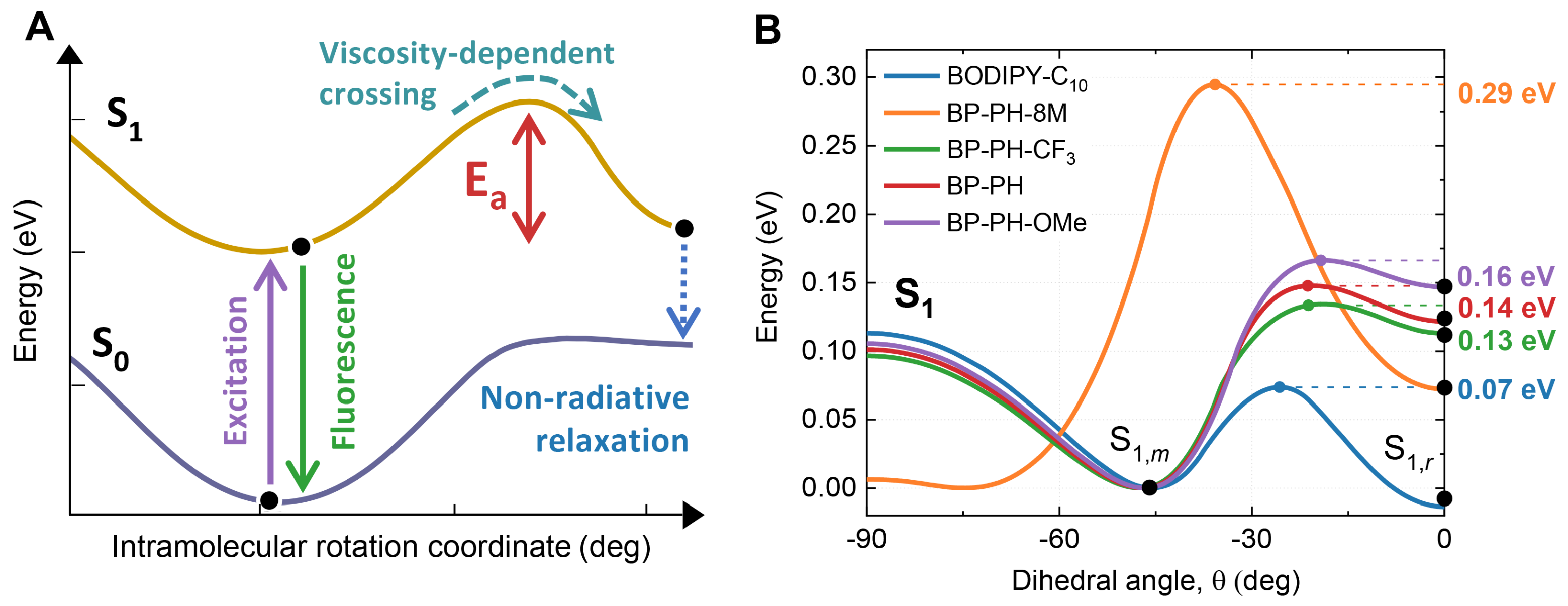
2.2. Theoretical Calculations
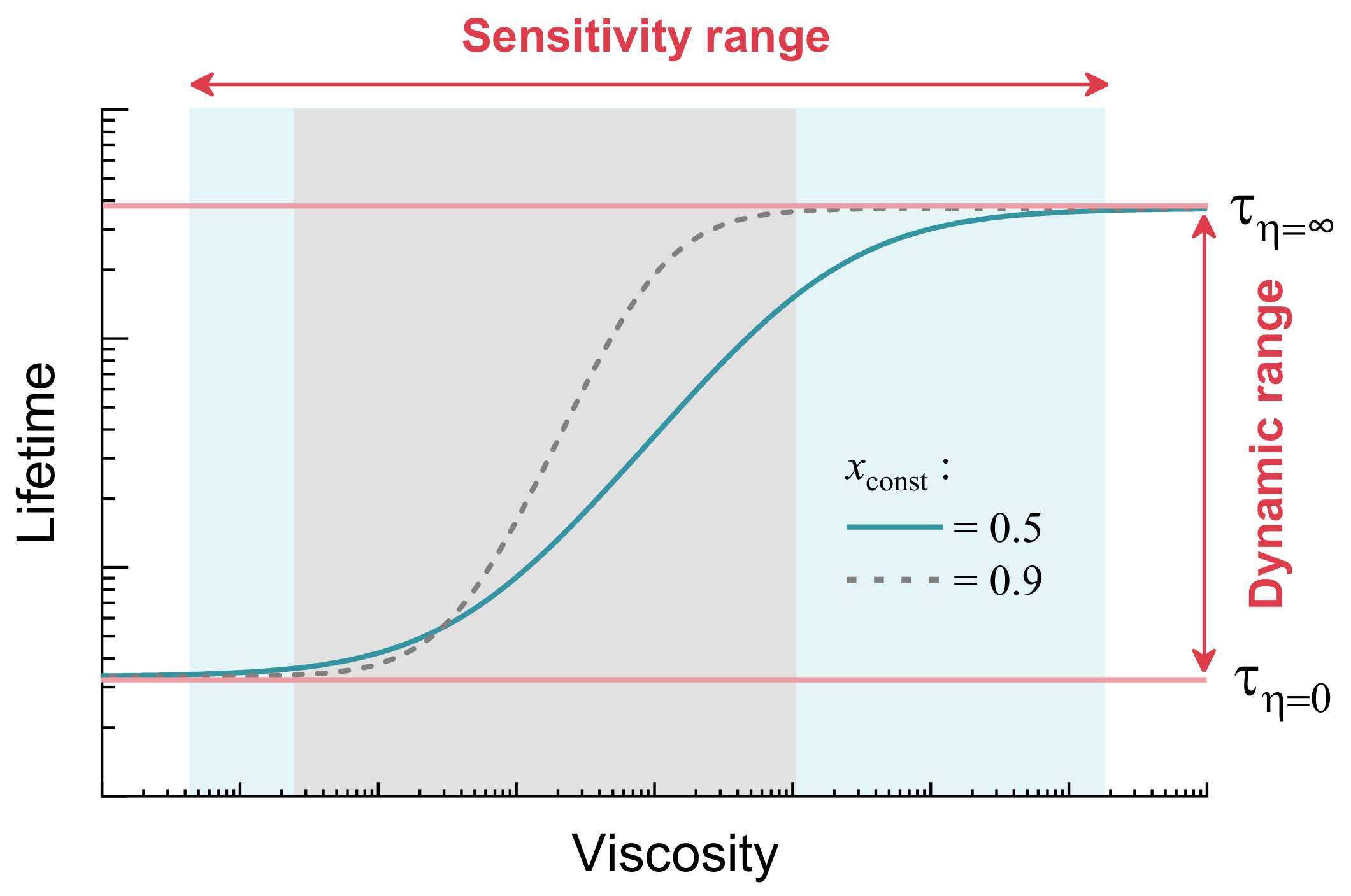
2.3. Time-Resolved Fluorescence and Its Sensitivity to Viscosity, Temperature and Polarity
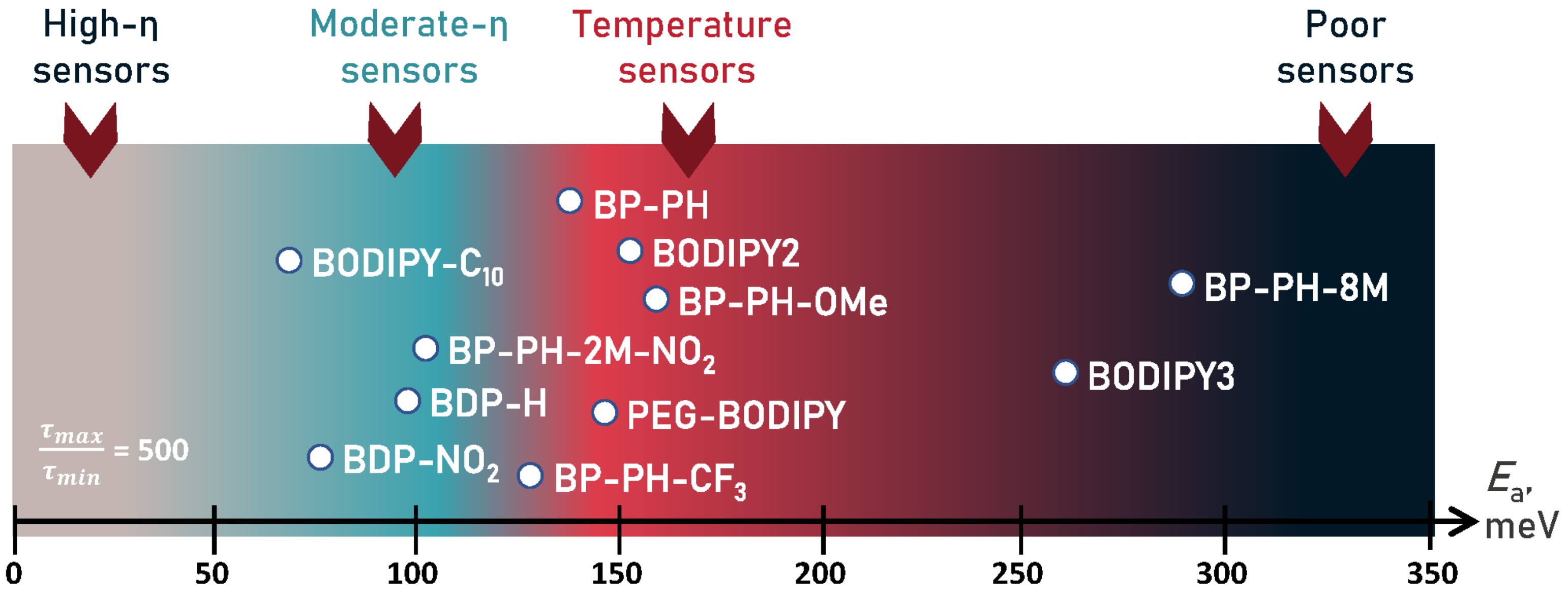
2.4. The Role of Energy Barrier for Determining Sensitivity to Viscosity and Temperature
3. Materials and Methods
3.1. Dyes, Reagents, and Solvents
3.2. Absorption, Steady-State, Time-Resolved Fluorescence, and Quantum Yields
3.3. Theoretical Calculations
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T. Design and synthesis of a library of BODIPY-based environmental polarity sensors utilizing photoinduced electron-transfer-controlled fluorescence ON/OFF switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, P.; Tang, B. Recent progresses in fluorescent probes for detection of polarity. Coord. Chem. Rev. 2021, 427, 213582. [Google Scholar] [CrossRef]
- Homma, M.; Takei, Y.; Murata, A.; Inoue, T.; Takeoka, S. A ratiometric fluorescent molecular probe for visualization of mitochondrial temperature in living cells. Chem. Commun. 2015, 51, 6194–6197. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.M.; Smith McWilliams, A.D.; Jiang, B.; Martí, A.A. Latest Trends in Temperature Sensing by Molecular Probes. ChemPhotoChem 2020, 4, 255–270. [Google Scholar] [CrossRef]
- Kubánková, M.; López-Duarte, I.; Kiryushko, D.; Kuimova, M.K. Molecular rotors report on changes in live cell plasma membrane microviscosity upon interaction with beta-amyloid aggregates. Soft Matter 2018, 14, 9466–9474. [Google Scholar] [CrossRef]
- Chambers, J.E.; Kubánková, M.; Huber, R.G.; López-Duarte, I.; Avezov, E.; Bond, P.J.; Marciniak, S.J.; Kuimova, M.K. An Optical Technique for Mapping Microviscosity Dynamics in Cellular Organelles. ACS Nano 2018, 12, 4398–4407. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Li, P.; Tang, B. Small Molecular Fluorescent Probes for Imaging of Viscosity in Living Biosystems. Chem. A Eur. J. 2021, 27, 6880–6898. [Google Scholar] [CrossRef]
- Miao, W.; Yu, C.; Hao, E.; Jiao, L. Functionalized BODIPYs as Fluorescent Molecular Rotors for Viscosity Detection. Front. Chem. 2019, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ogle, M.M.; Smith McWilliams, A.D.; Ware, M.J.; Curley, S.A.; Corr, S.J.; Martí, A.A. Sensing Temperature in Vitro and in Cells Using a BODIPY Molecular Probe. J. Phys. Chem. B 2019, 123, 7282–7289. [Google Scholar] [CrossRef]
- Vyšniauskas, A.; Cornell, B.; Sherin, P.S.; Maleckaitė, K.; Kubánková, M.; Izquierdo, M.A.; Vu, T.T.; Volkova, Y.A.; Budynina, E.M.; Molteni, C.; et al. Cyclopropyl Substituents Transform the Viscosity-Sensitive BODIPY Molecular Rotor into a Temperature Sensor. ACS Sens. 2021, 6, 2158–2167. [Google Scholar] [CrossRef]
- Steinmark, I.E.; James, A.L.; Chung, P.H.; Morton, P.E.; Parsons, M.; Dreiss, C.A.; Lorenz, C.D.; Yahioglu, G.; Suhling, K. Targeted fluorescence lifetime probes reveal responsive organelle viscosity and membrane fluidity. PLoS ONE 2019, 14, e0211165. [Google Scholar] [CrossRef]
- Arai, S.; Lee, S.C.; Zhai, D.; Suzuki, M.; Chang, Y.T. A molecular fluorescent probe for targeted visualization of temperature at the endoplasmic reticulum. Sci. Rep. 2014, 4, 2–7. [Google Scholar] [CrossRef]
- Nölle, J.M.; Jüngst, C.; Zumbusch, A.; Wöll, D. Monitoring of viscosity changes during free radical polymerization using fluorescence lifetime measurements. Polym. Chem. 2014, 5, 2700–2703. [Google Scholar] [CrossRef] [Green Version]
- Barja, B.C.; Chesta, C.A.; Atvars, T.D.; Aramendía, P.F. Fluorescent polymer coatings with tuneable sensitive range for remote temperature sensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 13–16. [Google Scholar] [CrossRef]
- Hosny, N.A.; Fitzgerald, C.; Vyšniauskas, A.; Athanasiadis, A.; Berkemeier, T.; Uygur, N.; Pöschl, U.; Shiraiwa, M.; Kalberer, M.; Pope, F.D.; et al. Direct imaging of changes in aerosol particle viscosity upon hydration and chemical aging. Chem. Sci. 2016, 7, 1357–1367. [Google Scholar] [CrossRef] [Green Version]
- Munson, C.A.; Baker, G.A.; Baker, S.N.; Bright, F.V. Effects of subzero temperatures on fluorescent probes sequestered within aerosol-OT reverse micelles. Langmuir 2004, 20, 1551–1557. [Google Scholar] [CrossRef]
- Olšinová, M.; Jurkiewicz, P.; Pozník, M.; Šachl, R.; Prausová, T.; Hof, M.; Kozmík, V.; Teplý, F.; Svoboda, J.; Cebecauer, M. Di- and tri-oxalkyl derivatives of a boron dipyrromethene (BODIPY) rotor dye in lipid bilayers. Phys. Chem. Chem. Phys. 2014, 16, 10688–10697. [Google Scholar] [CrossRef]
- Colom, A.; Derivery, E.; Soleimanpour, S.; Tomba, C.; Molin, M.D.; Sakai, N.; González-Gaitán, M.; Matile, S.; Roux, A. A fluorescent membrane tension probe. Nat. Chem. 2018, 10, 1118–1125. [Google Scholar] [CrossRef]
- Hassoun, S.; Karam, P. Fluorescent-Based Thermal Sensing in Lipid Membranes. Langmuir 2020, 36, 1221–1226. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [Green Version]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suhling, K. Molecular rotor measures viscosity of live cells via fluorescence lifetime imaging. J. Am. Chem. Soc. 2008, 130, 6672–6673. [Google Scholar] [CrossRef] [Green Version]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Heo, J.; Woo, H.C.; Lee, J.A.; Seo, Y.H.; Lee, C.L.; Kim, S.; Kwon, O.P. Fluorescent Molecular Rotors for Viscosity Sensors. Chem. A Eur. J. 2018, 24, 13706–13718. [Google Scholar] [CrossRef]
- Vyšniauskas, A.; Kuimova, M.K. A twisted tale: Measuring viscosity and temperature of microenvironments using molecular rotors. Int. Rev. Phys. Chem. 2018, 37, 259–285. [Google Scholar] [CrossRef]
- Kuimova, M.K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 2012, 14, 12671–12686. [Google Scholar] [CrossRef]
- Levitt, J.A.; Chung, P.H.; Kuimova, M.K.; Yahioglu, G.; Wang, Y.; Qu, J.; Suhling, K. Fluorescence anisotropy of molecular rotors. Chemphyschem 2011, 12, 662–672. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Mu, H.; Zhu, T.; Zhang, Z.; Du, J.; Peng, X. D-PET-controlled “off-on” Polarity-sensitive Probes for Reporting Local Hydrophilicity within Lysosomes. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Vu, T.T.; Méallet-Renault, R.; Clavier, G.; Trofimov, B.A.; Kuimova, M.K. Tuning BODIPY molecular rotors into the red: Sensitivity to viscosity: Vs. temperature. J. Mater. Chem. C 2016, 4, 2828–2833. [Google Scholar] [CrossRef] [Green Version]
- Vyšniauskas, A.; López-Duarte, I.; Duchemin, N.; Vu, T.T.; Wu, Y.; Budynina, E.M.; Volkova, Y.A.; Peña Cabrera, E.; Ramírez-Ornelas, D.E.; Kuimova, M.K. Exploring viscosity, polarity and temperature sensitivity of BODIPY-based molecular rotors. Phys. Chem. Chem. Phys. 2017, 19, 25252–25259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichler, J.; Knof, J.; Lenz, H. Measurements on the depth of penetration of light (0.35–1.0 μm) in tissue. Radiat. Environ. Biophys. 1977, 14, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging: Progress continues in the development of smaller, more penetrable probes for biological imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Alamudi, S.H.; Su, D.; Lee, K.J.; Lee, J.Y.; Belmonte-Vázquez, J.L.; Park, H.S.; Peña-Cabrera, E.; Chang, Y.T. A palette of background-free tame fluorescent probes for intracellular multi-color labelling in live cells. Chem. Sci. 2018, 9, 2376–2383. [Google Scholar] [CrossRef] [Green Version]
- Maleckaite, K.; Dodonova, J.; Toliautas, S.; Zilenaite, R.; Jurgutis, D.; Karabanovas, V.; Tumkevicius, S.; Vysniauskas, A. Designing a Red-Emitting Viscosity Sensitive BODIPY Fluorophore for Intracellular Viscosity Imaging. Chem. A Eur. J. 2021. [Google Scholar] [CrossRef]
- Le Guennic, B.; Jacquemin, D. Taking Up the Cyanine Challenge with Quantum Tools. Acc. Chem. Res. 2015, 48, 530–537. [Google Scholar] [CrossRef]
- Berraud-Pache, R.; Neese, F.; Bistoni, G.; Izsák, R. Unveiling the Photophysical Properties of Boron-dipyrromethene Dyes Using a New Accurate Excited State Coupled Cluster Method. J. Chem. Theory Comput. 2020, 16, 564–575. [Google Scholar] [CrossRef]
- Momeni, M.R.; Brown, A. Why do TD-DFT excitation energies of BODIPY/aza-BODIPY families largely deviate from experiment? Answers from electron correlated and multireference methods. J. Chem. Theory Comput. 2015, 11, 2619–2632. [Google Scholar] [CrossRef]
- Prlj, A.; Vannay, L.; Corminboeuf, C. Fluorescence Quenching in BODIPY Dyes: The Role of Intramolecular Interactions and Charge Transfer. Helv. Chim. Acta 2017, 100, 1–9. [Google Scholar] [CrossRef]
- Eshuis, H.; Van Voorhis, T. The influence of initial conditions on charge transfer dynamics. Phys. Chem. Chem. Phys. 2009, 11, 10293–10298. [Google Scholar] [CrossRef]
- Lin, Z.; Kohn, A.W.; Van Voorhis, T. Toward Prediction of Nonradiative Decay Pathways in Organic Compounds II: Two Internal Conversion Channels in BODIPYs. J. Phys. Chem. C 2020, 124, 3925–3938. [Google Scholar] [CrossRef]
- Liu, X.; Chi, W.; Qiao, Q.; Kokate, S.V.; Cabrera, E.P.; Xu, Z.; Liu, X.; Chang, Y.T. Molecular Mechanism of Viscosity Sensitivity in BODIPY Rotors and Application to Motion-Based Fluorescent Sensors. ACS Sens. 2020, 5, 731–739. [Google Scholar] [CrossRef]
- Toliautas, S.; Dodonova, J.; Žvirblis, A.; Čiplys, I.; Polita, A.; Devižis, A.; Tumkevičius, S.; Šulskus, J.; Vyšniauskas, A. Enhancing the Viscosity-Sensitive Range of a BODIPY Molecular Rotor by Two Orders of Magnitude. Chem. A Eur. J. 2019, 25, 10342–10349. [Google Scholar] [CrossRef]
- Sazanovich, I.V.; Kirmaier, C.; Hindin, E.; Yu, L.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Structural Control of the Excited-State Dynamics of Bis (dipyrrinato) zinc Complexes: Self-Assembling Chromophores for Light-Harvesting Architectures. J. Am. Chem. Soc. 2004, 1, 2664–2665. [Google Scholar] [CrossRef]
- Reichardt, C. Solvent and Solvent Effects in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2003; pp. 1–599. [Google Scholar]
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [CrossRef]
- Förster, T.; Hoffmann, G. Effect of viscosity on the fluorescence quantum yield of some dye systems. Z. Fur Phys. Chem. 1971, 75, 63–76. [Google Scholar] [CrossRef]
- Chung, P.H.; Levitt, J.A.; Kuimova, M.K.; Yahioglu, G.; Suhling, K. Mapping intracellular viscosity by advanced fluorescence imaging of molecular rotors in living cells. Multiphot. Microsc. Biomed. Sci. XI 2011, 7903, 790323. [Google Scholar] [CrossRef]
- Polita, A.; Toliautas, S.; Žvirblis, R.; Vyšniauskas, A. The effect of solvent polarity and macromolecular crowding on the viscosity sensitivity of a molecular rotor BODIPY-C10. Phys. Chem. Chem. Phys. 2020, 22, 8296–8303. [Google Scholar] [CrossRef]
- Becker, W. The bh TCSPC Handbook; Becker et Hickl: Berlin, Germany, 2010; pp. 1–566. [Google Scholar]
- Xue, K.; Wang, C.; Wang, J.; Lv, S.; Hao, B.; Zhu, C.; Tang, B.Z. A Sensitive and Reliable Organic Fluorescent Nanothermometer for Noninvasive Temperature Sensing. J. Am. Chem. Soc. 2021, 143, 14147–14157. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, C.; Schubert, U.S.; Hoogenboom, R. Aqueous polymeric sensors based on temperature-induced polymer phase transitions and solvatochromic dyes. Chem. Commun. 2011, 47, 8750–8765. [Google Scholar] [CrossRef]
- Brannon, J.H.; Magde, D. Absolute quantum yield determination by thermal blooming. Fluorescein. J. Phys. Chem. 1978, 82, 705–709. [Google Scholar] [CrossRef]
- Wall, K.P.; Dillon, R.; Knowles, M.K. Fluorescence quantum yield measurements of fluorescent proteins: A laboratory experiment for a biochemistry or molecular biophysics laboratory course. Biochem. Mol. Biol. Educ. 2015, 43, 52–59. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Parr, R.G. Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
- Momeni, M.R.; Brown, A. A Local CC2 and TDA-DFT Double Hybrid Study on BODIPY/aza-BODIPY Dimers as Heavy Atom Free Triplet Photosensitizers for Photodynamic Therapy Applications. J. Phys. Chem. A 2016, 120, 2550–2560. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]




| Theoretical | Experiment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Derivative | , nm | , nm | , cm | , nm | , nm | , cm | , ns | QY, % | kr, 108 × s | knr, 108 × s |
| BODIPY-C10 | 423 | 443 | 1094 | 500 | 515 | 583 | 0.8 | 12 | 1.5 | 11 |
| BP-PH-8M | 465 | 489 | 1087 | 530 | 565 | 1169 | 4.3 | 87 | 2 | 0.3 |
| BP-PH-CF3 | 476 | 518 | 1703 | 575 | 610 | 998 | 4 | 57 | 1.5 | 1 |
| BP-PH | 485 | 535 | 1959 | 590 | 630 | 1076 | 3.1 | 28 | 0.9 | 2.3 |
| BP-PH-OMe | 510 | 576 | 2258 | 625 | 680 | 1294 | 1.6 | 12 | 0.8 | 5.5 |
| Derivative | [%/°C] | [%/f] | x |
|---|---|---|---|
| BODIPY-C10 | 0.9 | 157 | 0.21 |
| BP-PH-8M | 0.3 | 72 | 0.02 |
| BP-PH-CF3 | 0.5 | 116 | 0.04 |
| BP-PH | 0.6 | 141 | 0.05 |
| BP-PH-OMe | 0.4 | 265 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleckaitė, K.; Narkevičius, D.; Žilėnaitė, R.; Dodonova-Vaitkūnienė, J.; Toliautas, S.; Tumkevičius, S.; Vyšniauskas, A. Give or Take: Effects of Electron-Accepting/-Withdrawing Groups in Red-Fluorescent BODIPY Molecular Rotors. Molecules 2022, 27, 23. https://doi.org/10.3390/molecules27010023
Maleckaitė K, Narkevičius D, Žilėnaitė R, Dodonova-Vaitkūnienė J, Toliautas S, Tumkevičius S, Vyšniauskas A. Give or Take: Effects of Electron-Accepting/-Withdrawing Groups in Red-Fluorescent BODIPY Molecular Rotors. Molecules. 2022; 27(1):23. https://doi.org/10.3390/molecules27010023
Chicago/Turabian StyleMaleckaitė, Karolina, Domantas Narkevičius, Rugilė Žilėnaitė, Jelena Dodonova-Vaitkūnienė, Stepas Toliautas, Sigitas Tumkevičius, and Aurimas Vyšniauskas. 2022. "Give or Take: Effects of Electron-Accepting/-Withdrawing Groups in Red-Fluorescent BODIPY Molecular Rotors" Molecules 27, no. 1: 23. https://doi.org/10.3390/molecules27010023
APA StyleMaleckaitė, K., Narkevičius, D., Žilėnaitė, R., Dodonova-Vaitkūnienė, J., Toliautas, S., Tumkevičius, S., & Vyšniauskas, A. (2022). Give or Take: Effects of Electron-Accepting/-Withdrawing Groups in Red-Fluorescent BODIPY Molecular Rotors. Molecules, 27(1), 23. https://doi.org/10.3390/molecules27010023