Glycosides for Peripheral Neuropathic Pain: A Potential Medicinal Components
Abstract
1. Introduction
2. Epidemiology of Neuropathic Pain
3. Pharmacological Treatment of Neuropathic Pain
4. Synopsis of Glycosides
5. The Antinociceptive Effect of Glycosides in a Rodent Model of Neuropathic Pain
5.1. Hesperidin
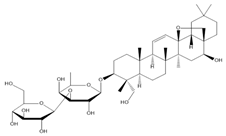
5.2. Saikosaponins
5.3. Isoorientin
5.4. Liquiritin
5.5. Albiflorin
5.6. Salidroside
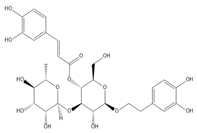
5.7. Morroniside
5.8. Verbascoside
5.9. Paeoniflorin
5.10. Diosmin
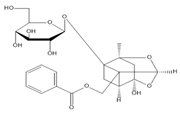
5.11. Geniposide
6. Antinociceptive Mechanisms Underlying the Action of Glycosides on Neuropathic Pain
6.1. Glycosides and Oxidative Stress
6.2. Glycosides and Transcriptional Regulation
6.2.1. Glycosides and Pro-Inflammatory Cytokines
6.2.2. Glycosides and the NOD-Like Receptor Protein 3 (NLRP3) Inflammasome
6.3. Glycosides and Ion Channels
6.3.1. Glycosides and P2X Receptors
6.3.2. Glycosides and Transient Receptor Potential Ankyrin 1 (TRPA1)
6.4. Glycosides and Membrane Receptors
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IASP | International Association for the Study of Pain |
| CNS | Central nervous system |
| PNS | Peripheral nervous system |
| HIV | Human immunodeficiency virus |
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| NeuPSIG | Special Interest Group on Neuropathic Pain |
| LNP | Localized neuropathic pain |
| PWT | Paw withdrawal threshold |
| PWL | Paw withdrawal latencies |
| CCI | Chronic constriction injury |
| SNL | Spinal nerve ligation |
| TWL | Tail withdrawal latencies |
| STZ | streptozotocin |
| H&E staining | Hematoxylin-eosin staining |
| Cmax | Mean peak concentration |
| Tmax | Mean peak time |
| T1/2 | Half-life |
| NLRP3 | NOD-like receptor protein 3 |
| MCC950 | A selective inhibitor of the NLRP3 inflammasome |
| ZDF | Zucker diabetic fatty |
| T2DM | Type 2 diabetes mellitus |
| HPLC-MS | High Performance Liquid Chromatography-Mass Spectrometry |
| ROS | Reactive oxygen species |
| MIA | Monoiodoacetate |
| AUC | Area under curve |
| PTX | Paclitaxel |
| TNF-α | Tumor Necrosis Factor-Alpha |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| UHPLC-MS | ultra-high-performance liquid chromatography tandem mass spectrometry |
| MMPs | Matrix metalloproteinases |
| T-AOC | Total antioxidant capacity |
| CAT | Catalase |
| T-SOD | Total superoxide dismutase |
| MAD | Malondialdehyde |
| NF-κB | Nuclear factor kappa B |
| Keap1 | Kelch-like ECH associated protein 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ATP | Adenosine triphosphate |
| P2X7R | P2X receptor 7 |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TRP | Transient receptor potential |
| HPS | High-throughput screening |
| GPCR | G-protein-coupled receptor |
| GRP160 | G-protein-coupled receptor 160 |
| GPR34 | G-protein-coupled receptor 34 |
| GPR40 | G-protein-coupled receptor 40 |
| GLP-1R | Glucagon-like peptide-1 receptor |
| CTAP | μ-Opioid receptor antagonist |
| POMC | β-Endorphin precursor proopiomelanocortin |
| C3G | Cyanidin-3-o-glucoside |
| RNS | Reactive nitrogen species |
| TRPM2 | Transient receptor potential melastatin 2 |
| TRPM3 | Transient receptor potential melastatin 3 |
| TRPM4 | Transient receptor potential melastatin 4 |
| TRPC3 | Transient receptor potential channel canonical 3 |
| TRPC4 | Transient receptor potential channel canonical 4 |
| TRPV1 | Transient receptor potential vanilloid type 1 |
| TRPV4 | Transient receptor potential vanilloid type 4 |
| RCS | Reactive carbonyl species |
| SP | Substance P |
| CGRP | Calcitonin gene-related peptide |
References
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2007, 70, 1630–1635. [Google Scholar] [CrossRef]
- Boyd, A.; Bleakley, C.; Hurley, D.A.; Gill, C.; Hannon-Fletcher, M.; Bell, P.; McDonough, S. Herbal medicinal products or preparations for neuropathic pain. Cochrane Database Syst. Rev. 2019, 4, CD010528. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.; Kuner, R.; Jensen, T. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- YB, T. Effect of Bone Mesenchymal Stromal Cells on Neuropathic Pain and Its Mechanism; Shandong University: Jinan, China, 2020. [Google Scholar]
- Haanpää, M.; Backonja, M.; Bennett, M.; Bouhassira, D.; Cruccu, G.; Hansson, P.; Jensen, T.; Kauppila, T.; Rice, A.; Smith, B.; et al. Assessment of neuropathic pain in primary care. Am. J. Med. 2009, 122, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Garcia-Larrea, L.; Cruccu, G. Reappraising neuropathic pain in humans--how symptoms help disclose mechanisms. Nat. Rev. Neurol. 2013, 9, 572–582. [Google Scholar] [CrossRef]
- Calvo, M.; Dawes, J.M.; Bennett, D.L.H. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012, 11, 629–642. [Google Scholar] [CrossRef]
- Torrance, N.; Lawson, K.D.; Afolabi, E.; Bennett, M.I.; Serpell, M.G.; Dunn, K.M.; Smith, B.H. Estimating the burden of disease in chronic pain with and without neuropathic characteristics: Does the choice between the EQ-5D and SF-6D matter? Pain 2014, 155, 1996–2004. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- Attal, N.; Lanteri-Minet, M.; Laurent, B.; Fermanian, J.; Bouhassira, D. The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain 2011, 152, 2836–2843. [Google Scholar] [CrossRef]
- Wiffen, P.; Collins, S.; McQuay, H.; Carroll, D.; Jadad, A.; Moore, A. Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst. Rev. 2005, 2000, CD001133. [Google Scholar] [CrossRef]
- Metcalfe, A.; Williams, J.; McChesney, J.; Patten, S.; Jetté, N. Use of complementary and alternative medicine by those with a chronic disease and the general population—Results of a national population based survey. BMC Complement. Altern. Med. 2010, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Basu, A. In Vitro and In Vivo Effects of Flavonoids on Peripheral Neuropathic Pain. Molecules 2020, 25, 1171. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Santos, C.; Freitas Filho, J.; Menezes, P.; Freitas, J. Polyacetylene Glycosides: Isolation, Biological Activities and Synthesis. Chem. Rec. 2021. [Google Scholar] [CrossRef]
- Tian, X.; Li, M.; Lin, T.; Qiu, Y.; Zhu, Y.; Li, X.; Tao, W.; Wang, P.; Ren, X.; Chen, L. A review on the structure and pharmacological activity of phenylethanoid glycosides. Eur. J. Med. Chem. 2021, 209, 112563. [Google Scholar] [CrossRef]
- Khan, H.; Pervaiz, A.; Intagliata, S.; Das, N.; Nagulapalli Venkata, K.C.; Atanasov, A.G.; Najda, A.; Nabavi, S.M.; Wang, D.; Pittalà, V.; et al. The analgesic potential of glycosides derived from medicinal plants. DARU J. Pharm. Sci. 2020, 28, 387–401. [Google Scholar] [CrossRef]
- Sa, K.N.; Moreira, L.; Baptista, A.F.; Yeng, L.T.; Teixeira, M.J.; Galhardoni, R.; de Andrade, D.C. Prevalence of chronic pain in developing countries: Systematic review and meta-analysis. Pain Rep. 2019, 4, e779. [Google Scholar] [CrossRef]
- King, S.; Chambers, C.; Huguet, A.; MacNevin, R.; McGrath, P.; Parker, L.; MacDonald, A. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011, 152, 2729–2738. [Google Scholar] [CrossRef]
- Hechler, T.; Dobe, M.; Zernikow, B. Commentary: A worldwide call for multimodal inpatient treatment for children and adolescents suffering from chronic pain and pain-related disability. J. Pediatr. Psychol. 2010, 35, 138–140. [Google Scholar] [CrossRef]
- Huguet, A.; Miró, J. The severity of chronic pediatric pain: An epidemiological study. J. Pain 2008, 9, 226–236. [Google Scholar] [CrossRef]
- De Toledo, I.; Conti Réus, J.; Fernandes, M.; Porporatti, A.; Peres, M.; Takaschima, A.; Linhares, M.; Guerra, E.; De Luca Canto, G. Prevalence of trigeminal neuralgia: A systematic review. J. Am. Dent. Assoc. 2016, 147, 570–576.e572. [Google Scholar] [CrossRef]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- van Hecke, O.; Torrance, N.; Smith, B. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013, 111, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Stingl, J.C. Prevalence and comorbidity of chronic pain in the German general population. J. Psychiatr. Res. 2012, 46, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Torrance, N.; Smith, B.; Bennett, M.; Lee, A. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J. Pain 2006, 7, 281–289. [Google Scholar] [CrossRef]
- Bouhassira, D.; Lantéri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef]
- VanDenKerkhof, E.G.; Mann, E.G.; Torrance, N.; Smith, B.H.; Johnson, A.; Gilron, I. An Epidemiological Study of Neuropathic Pain Symptoms in Canadian Adults. Pain Res. Manag. 2016, 2016, 9815750. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis Primers 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Padua, L.; Devigili, G.; Rota, E.; Tamburin, S.; Eleopra, R.; Cruccu, G.; Truini, A. Prevalence of Neuropathic Pain in Patients with Traumatic Brachial Plexus Injury: A Multicenter Prospective Hospital-Based Study. Pain Med. 2017, 18, 2428–2432. [Google Scholar] [CrossRef][Green Version]
- Lechner, B.; Chow, S.; Chow, R.; Zhang, L.; Tsao, M.; Danjoux, C.; Barnes, E.; DeAngelis, C.; Vuong, S.; Ganesh, V.; et al. The incidence of neuropathic pain in bone metastases patients referred for palliative radiotherapy. Radiother. Oncol. 2016, 118, 557–561. [Google Scholar] [CrossRef]
- Orhurhu, M.S.; Chu, R.; Claus, L.; Roberts, J.; Salisu, B.; Urits, I.; Orhurhu, E.; Viswanath, O.; Kaye, A.D.; Kaye, A.J.; et al. Neuropathic Pain and Sickle Cell Disease: A Review of Pharmacologic Management. Curr. Pain Headache Rep. 2020, 24, 52. [Google Scholar] [CrossRef]
- Yanaizumi, R.; Nagamine, Y.; Harada, S.; Kojima, K.; Tazawa, T.; Goto, T. Prevalence of neuropathic pain in terminally ill patients with cancer admitted to a general ward: A prospective observational study. J. Int. Med. Res. 2021, 49, 0300060520987726. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.; Häuser, W.; Cohen, S.; Fitzcharles, M. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain 2020, 161, 1694–1697. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Attal, N.; Martinez, V.; Bouhassira, D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021, 6, e884. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Li, C.Y.; Zhou, L.; Ao, L.Y.; Fang, W.R.; Li, Y.M. Research progress of mechanisms and drug therapy for neuropathic pain. Life Sci. 2017, 190, 68–77. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Boyle, J.; Eriksson, M.E.; Gribble, L.; Gouni, R.; Johnsen, S.; Coppini, D.V.; Kerr, D. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: Impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care 2012, 35, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.; Voute, M.; Macian, N.; Ganry, H.; Pereira, B. Effectiveness and safety of 5% lidocaine-medicated plaster on localized neuropathic pain after knee surgery: A randomized, double-blind controlled trial. Pain 2019, 160, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Marshman, L.A.G.; Plummer, D.; Downs, E. Effect of Gabapentin vs Pregabalin on Pain Intensity in Adults with Chronic Sciatica: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Nalamachu, S.; Morley-Forster, P. Diagnosing and managing postherpetic neuralgia. Drugs Aging 2012, 29, 863–869. [Google Scholar] [CrossRef]
- Henningfield, J.E.; Ashworth, J.B.; Gerlach, K.K.; Simone, B.; Schnoll, S.H. The nexus of opioids, pain, and addiction: Challenges and solutions. Prev. Med. 2019, 128, 105852. [Google Scholar] [CrossRef]
- Doostmohammadi, M.; Rahimi, H.R. ADME and toxicity considerations for tramadol: From basic research to clinical implications. Expert Opin. Drug Metab. Toxicol. 2020, 16, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Xinyaohui. Available online: http://www.xinyaohui.com/news/201510/29/7237.html (accessed on 1 December 2021).
- Lu, Y.; Hou, S.; Chen, T. Advances in the study of vincristine: An anticancer ingredient from Catharanthus roseus. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2003, 28, 1006–1009. [Google Scholar]
- Wu, X.; Xie, H.; Long, X.; Zhang, J.; Huang, T.; Hao, X.; Zhang, Y. Chemical constituents of Catharanthus roseus. J. Chin. Pharm. Sci. 2017, 52, 631–636. [Google Scholar]
- Yuan, Y.; Jiang, T.; Zhou, X.; Liu, Y. Discovery and development of artemisinin. Chin. Sci. Bull. 2017, 62, 1914–1927. [Google Scholar] [CrossRef]
- Li, S.-H.; Li, L.; Yang, R.-N.; Liang, S.-D. Compounds of traditional Chinese medicine and neuropathic pain. Chin. J. Nat. Med. 2020, 18, 28–35. [Google Scholar] [CrossRef]
- Cheeke, P.R. Glycosides: Naturally Occurring; ENCYCLOPEDIA OF LIFE SCIENCE; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2001. [Google Scholar]
- Yu, B.; Sun, J.; Yang, X. Assembly of naturally occurring glycosides, evolved tactics, and glycosylation methods. Acc. Chem. Res. 2012, 45, 1227–1236. [Google Scholar] [CrossRef]
- Weymouth-Wilson, A.C. The role of carbohydrates in biologically active natural products. Nat. Prod. Rep. 1997, 14, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.H.; Wang, C.Q.; Liu, J.H.; Li, X.W.; Wang, X.; Shang, M.Y.; Cai, S.Q.; Zhu, S.; Komatsu, K. Comparative studies of saponins in 1-3-year-old main roots, fibrous roots, and rhizomes of Panax notoginseng, and identification of different parts and growth-year samples. J. Nat. Med. 2013, 67, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Mao, L.-N.; Liu, C.-P.; Sun, Y.-H.; Jiang, B.; Zhang, W.; Li, J.-X. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci. Rep. 2016, 6, 19266. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, H.; Xu, D.; Yin, Q.; Cheng, L.; Wang, L.; Song, S.; Zhang, M. Attenuation of neuropathic pain by saikosaponin a in a rat model of chronic constriction injury. Neurochem. Res. 2014, 39, 2136–2142. [Google Scholar] [CrossRef]
- Ribeiro, M.H.L. Glycosides. In Biotechnology of Bioactive Compounds; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 301–344. [Google Scholar] [CrossRef]
- Zuo, A.; Dong, H.; Yu, Y.; Shu, Q.; Zheng, L.; Yu, X.; Cao, S. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, W.; Wang, C.; Wu, L.; Zhang, J. Paeoniflorin attenuates the neuroinflammatory response in a rat model of chronic constriction injury. Mol. Med. Rep. 2017, 15, 3179–3185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiao, J.; Wang, B.; Bai, M.; Shen, J.; Cheng, Y. Paeoniflorin Ameliorates Fructose-Induced Insulin Resistance and Hepatic Steatosis by Activating LKB1/AMPK and AKT Pathways. Nutrients 2018, 10, 1024. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Ren, Y.; Wu, X.; Liu, Y.; Wang, T.; Li, Y.; Cong, Y.; Guo, Y. Neuroprotective effects of salidroside on ageing hippocampal neurons and naturally ageing mice via the PI3K/Akt/TERT pathway. Phytother. Res. PTR 2021, 35, 5767–5780. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.L.; Cui, R.; Shao, A.M.; Wu, Z.M. Salidroside Ameliorates Diabetic Neuropathic Pain in Rats by Inhibiting Neuroinflammation. J. Mol. Neurosci. 2017, 63, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet. Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Sonza, A.; Sanada, L.; Oliveira, L.; Bernardo-Filho, M.; Sá-Caputo, D.; Zaro, M.; Achaval, M. Whole-body vibration mediates mechanical hypersensitivity through Aβ-fiber and C-fiber thermal sensation in a chronic pain model. Exp. Biol. Med. (Maywood N. J.) 2021, 246, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef]
- Zheng, R.; Fu, Y.; Zhu, J.; Xu, J.; Xiang, Q.; Chen, L.; Zhong, H.; Li, J.; Yu, C. Long-term low-dose morphine for patients with moderate cancer pain is predominant factor effecting clinically meaningful pain reduction. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer 2018, 26, 4115–4120. [Google Scholar] [CrossRef] [PubMed]
- Sandkühler, J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 2009, 89, 707–758. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Liu, L.; Fan, Y.; Wang, M.; Li, L.; Zou, L.; Yuan, H.; Shi, L.; Yang, R.; Liang, S.; et al. Role of hesperidin in P2X3 receptor-mediated neuropathic pain in the dorsal root ganglia. Int. J. Neurosci. 2019, 129, 784–793. [Google Scholar] [CrossRef]
- Carballo-Villalobos, A.I.; Gonzalez-Trujano, M.E.; Alvarado-Vazquez, N.; Lopez-Munoz, F.J. Pro-inflammatory cytokines involvement in the hesperidin antihyperalgesic effects at peripheral and central levels in a neuropathic pain model. Inflammopharmacology 2017, 25, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Visnagri, A.; Kandhare, A.D.; Chakravarty, S.; Ghosh, P.; Bodhankar, S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 2014, 52, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Luo, J.; Xu, Y.; Sun, X.; Yang, S.; Yang, M. Ultra-high performance supercritical fluid chromatography method for separation and quantitation of saikosaponins in herbal medicine. J. Pharm. Biomed. Anal. 2021, 199, 114039. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Villalobos, A.I.; Gonzalez-Trujano, M.E.; Pellicer, F.; Alvarado-Vasquez, N.; Lopez-Munoz, F.J. Central and peripheral anti-hyperalgesic effects of diosmin in a neuropathic pain model in rats. Biomed. Pharmacother. 2018, 97, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, M.M.; Rossaneis, A.C.; Fattori, V.; Longhi-Balbinot, D.T.; Freitas, A.; Cunha, F.Q.; Alves-Filho, J.C.; Cunha, T.M.; Casagrande, R.; Verri, W.A., Jr. Diosmin reduces chronic constriction injury-induced neuropathic pain in mice. Chem. Biol. Interact 2017, 273, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Poureshagh, E.; Hosseinzadeh, H. The Effect of Verbascoside in Neuropathic Pain Induced by Chronic Constriction Injury in Rats. Phytother. Res. 2016, 30, 128–135. [Google Scholar] [CrossRef]
- Lee, G.; Choi, J.; Nam, Y.J.; Song, M.J.; Kim, J.K.; Kim, W.J.; Kim, P.; Lee, J.S.; Kim, S.; No, K.T.; et al. Identification and characterization of saikosaponins as antagonists of transient receptor potential A1 channel. Phytother. Res. 2020, 34, 788–795. [Google Scholar] [CrossRef]
- Andoh, T.; Kobayashi, N.; Uta, D.; Kuraishi, Y. Prophylactic topical paeoniflorin prevents mechanical allodynia caused by paclitaxel in mice through adenosine A1 receptors. Phytomedicine 2017, 25, 1–7. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, J.; Ma, S.; Zhou, J. Paeoniflorin attenuates chronic constriction injury-induced neuropathic pain by suppressing spinal NLRP3 inflammasome activation. Inflammopharmacology 2020, 28, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
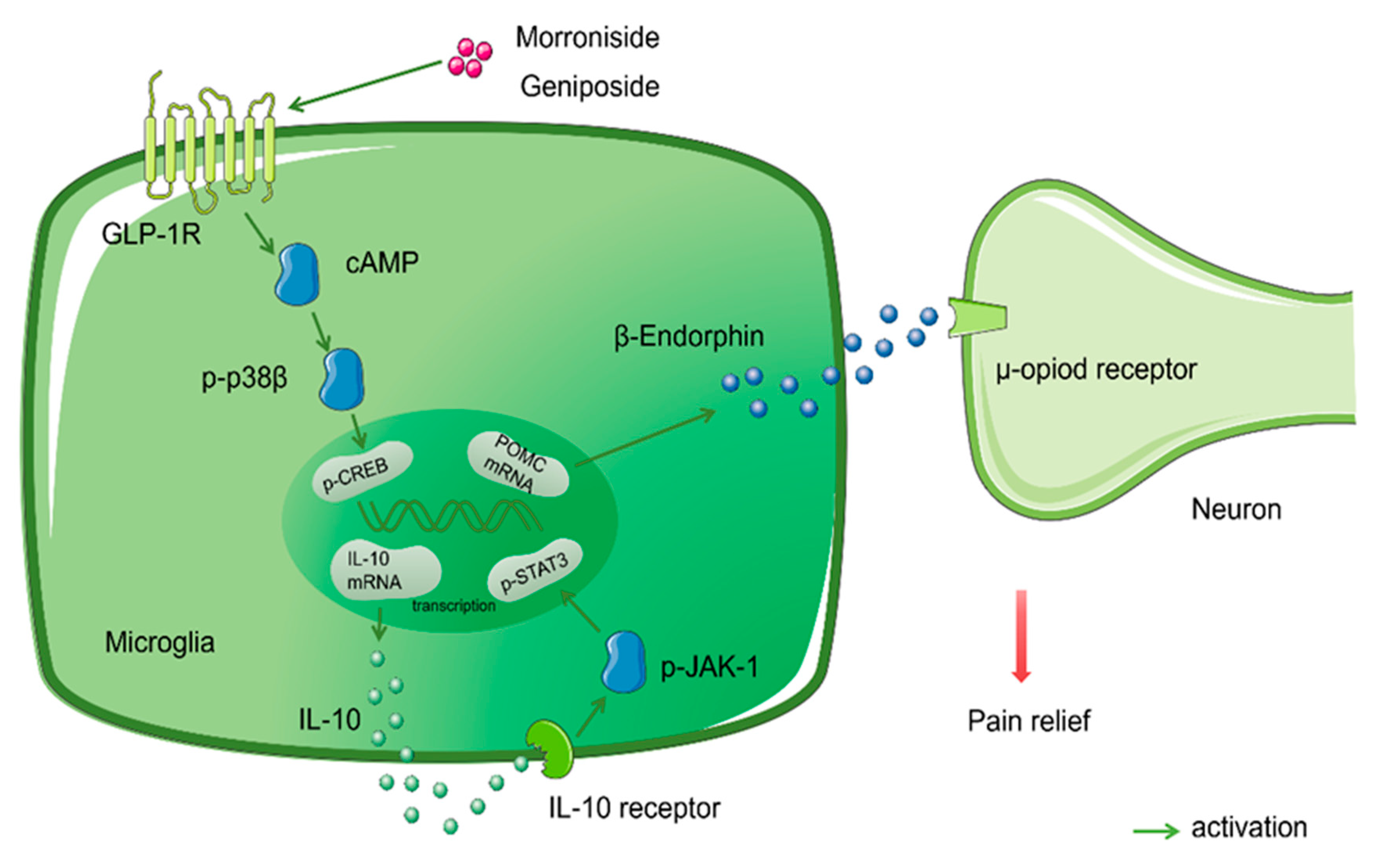
- Xu, M.; Wu, H.Y.; Liu, H.; Gong, N.; Wang, Y.R.; Wang, Y.X. Morroniside, a secoiridoid glycoside from Cornus officinalis, attenuates neuropathic pain by activation of spinal glucagon-like peptide-1 receptors. Br. J. Pharmacol. 2017, 174, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, H.; Mao, X.; Li, X.; Wang, Y. The GLP-1 receptor herbal agonist morroniside attenuates neuropathic pain via spinal microglial expression of IL-10 and beta-endorphin. Biochem. Biophys. Res. Commun. 2020, 530, 494–499. [Google Scholar] [CrossRef]
- Zhang, M.T.; Wang, B.; Jia, Y.N.; Liu, N.; Ma, P.S.; Gong, S.S.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Neuroprotective effect of liquiritin against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Biomed. Pharmacother. 2017, 95, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, Q.; Bian, F.; Zhao, Y.; Ma, W.; Zhang, Y.; Lu, W.; Lei, P.; Zhang, L.; Hao, X.; et al. Salidroside alleviates diabetic neuropathic pain through regulation of the AMPK-NLRP3 inflammasome axis. Toxicol. Appl. Pharmacol. 2021, 416, 115468. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, N.; Zhu, C.; Ma, L.; Yang, J.; Du, J.; Zhang, W.; Sun, T.; Niu, J.; Yu, J. Antinociceptive effect of isoorientin against neuropathic pain induced by the chronic constriction injury of the sciatic nerve in mice. Int. Immunopharmacol. 2019, 75, 105753. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, J.; Ma, S.; Zhang, J.; Zhou, J. Albiflorin Attenuates Mood Disorders Under Neuropathic Pain State by Suppressing the Hippocampal NLRP3 Inflammasome Activation During Chronic Constriction Injury. Int. J. Neuropsychopharmacol. 2021, 24, 64–76. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z. Analgesic effect of geniposide on diabetic neuropathic pain in rats. Northwest Pharm. J. 2018, 33, 206–210. [Google Scholar]
- Anilkumar, K.; Reddy, G.V.; Azad, R.; Yarla, N.S.; Dharmapuri, G.; Srivastava, A.; Kamal, M.A.; Pallu, R. Evaluation of Anti-Inflammatory Properties of Isoorientin Isolated from Tubers of Pueraria tuberosa. Oxid. Med. Cell Longev. 2017, 2017, 5498054. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Xiao, H.; Wu, W.; Wang, Y.; Liu, X. MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem. Toxicol. 2013, 53, 62–68. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Wong, Z.C.; Wang, C.; Fung, A.H.; Wong, E.O.; Chan, G.K.; Dong, T.T.; Chen, Y.; Tsim, K.W. Isoorientin derived from Gentiana veitchiorum Hemsl. flowers inhibits melanogenesis by down-regulating MITF-induced tyrosinase expression. Phytomedicine 2019, 57, 129–136. [Google Scholar] [CrossRef]
- Wang, H.; Shan, H.; Lu, H. Preparative Separation and Purification of Liquiritin and Glycyrrhizic Acid from Glycyrrhiza uralensis Fisch by High-Speed Countercurrent Chromatography. J. Chromatogr. Sci. 2020, 58, 823–830. [Google Scholar] [CrossRef]
- Zhai, K.F.; Duan, H.; Cui, C.Y.; Cao, Y.Y.; Si, J.L.; Yang, H.J.; Wang, Y.C.; Cao, W.G.; Gao, G.Z.; Wei, Z.J. Liquiritin from Glycyrrhiza uralensis Attenuating Rheumatoid Arthritis via Reducing Inflammation, Suppressing Angiogenesis, and Inhibiting MAPK Signaling Pathway. J. Agric. Food Chem. 2019, 67, 2856–2864. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, Y.Q.; Wang, X.; Li, X.F.; Xie, Z. An artificial intelligence system reveals liquiritin inhibits SARS-CoV-2 by mimicking type I interferon. BioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Hu, X.; Chen, L.; Qiu, Z.; Zhao, N.; Yu, Z.; Sun, S.; Xu, Y.; Guo, Y.; et al. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J. Ethnopharmacol. 2016, 179, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fu, J.; Ma, S.; Peng, R.; Yu, J.; Cong, L.; Pan, L.; Zhang, Z.; Tian, H.; Che, C.; et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics 2018, 8, 5945–5959. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zou, L.; Xie, J.; Xie, W.; Wen, S.; Xie, Q.; Gao, Y.; Li, G.; Zhang, C.; Xu, C.; et al. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol. Brain 2016, 9, 44. [Google Scholar] [CrossRef]
- Brussee, V.; Guo, G.; Dong, Y.; Cheng, C.; Martinez, J.A.; Smith, D.; Glazner, G.W.; Fernyhough, P.; Zochodne, D.W. Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes 2008, 57, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Kouitcheu Mabeku, L.B.; Noundjeu Ngamga, M.L.; Leundji, H. Helicobacter pylori infection, a risk factor for Type 2 diabetes mellitus: A hospital-based cross-sectional study among dyspeptic patients in Douala-Cameroon. Sci. Rep. 2020, 10, 12141. [Google Scholar] [CrossRef]
- Yu, H.; Yao, S.; Zhou, C.; Fu, F.; Luo, H.; Du, W.; Jin, H.; Tong, P.; Chen, D.; Wu, C.; et al. Morroniside attenuates apoptosis and pyroptosis of chondrocytes and ameliorates osteoarthritic development by inhibiting NF-κB signaling. J. Ethnopharmacol. 2021, 266, 113447. [Google Scholar] [CrossRef]
- Harris, C.S.; Asim, M.; Saleem, A.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A. Characterizing the cytoprotective activity of Sarracenia purpurea L., a medicinal plant that inhibits glucotoxicity in PC12 cells. BMC Complement. Altern. Med. 2012, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tan, F.; Zhu, J.; Yue, C.; Li, Q. Separation, purification and quantification of verbascoside from Penstemon barbatus (Cav.) Roth. Food Chem. 2012, 135, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Isacchi, B.; Iacopi, R.; Bergonzi, M.C.; Ghelardini, C.; Galeotti, N.; Norcini, M.; Vivoli, E.; Vincieri, F.F.; Bilia, A.R. Antihyperalgesic activity of verbascoside in two models of neuropathic pain. J. Pharm. Pharm. 2011, 63, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Seo, K.; Ryu, H.; Yuk, H.; Park, H.; Lim, Y.; Ahn, K.; Oh, S. Anti-inflammatory effect of stem bark of Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and LPS-induced murine model of acute lung injury. J. Ethnopharmacol. 2018, 210, 23–30. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Rossi, R.; Vitari, F.; Carollo, V.; Deponti, D.; Corino, C.; Domeneghini, C. Changes in nitrosative stress biomarkers in swine intestine following dietary intervention with verbascoside. Histol. Histopathol. 2013, 28, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, L.; Zhu, X.; Zhang, K.; Huang, B.; Zhang, J.; Zhang, Y.; Zhu, L.; Zhou, B.; Zhou, F. Protective effect of paeoniflorin on Abeta25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol. Neurobiol. 2014, 34, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.Y.; Yang, Y.P.; Luo, W.F.; Mao, C.J.; Han, R.; Sun, X.; Cheng, J.; Liu, C.F. Paeoniflorin, a potent natural compound, protects PC12 cells from MPP+ and acidic damage via autophagic pathway. J. Ethnopharmacol. 2010, 131, 122–129. [Google Scholar] [CrossRef]
- Hu, M.; Chen, C.; Liu, J.; Cai, L.; Shao, J.; Chen, Z.; Lin, L.; Zheng, T.; Ding, X.; Li, Z. The melanogenic effects and underlying mechanism of paeoniflorin in human melanocytes and vitiligo mice. Fitoterapia. 2020, 140, 104416. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Vinothkumar, V.; Murali, R. Antidiabetic Efficacy of Citrus Fruits with Special Allusion to Flavone Glycosides. In Bioactive Food as Dietary Interventions for Diabetes; Academic Press: Cambridge, MA, USA, 2019; pp. 335–346. [Google Scholar] [CrossRef]
- Wang, M.; Yang, L.; Chen, Z.; Dai, L.; Xi, C.; Wu, X.; Wu, G.; Wang, Y.; Hu, J. Geniposide ameliorates chronic unpredictable mild stress induced depression-like behavior through inhibition of ceramide-PP2A signaling via the PI3K/Akt/GSK3β axis. Psychopharmacology 2021, 238, 2789–2800. [Google Scholar] [CrossRef]
- Gong, N.; Fan, H.; Ma, A.N.; Xiao, Q.; Wang, Y.X. Geniposide and its iridoid analogs exhibit antinociception by acting at the spinal GLP-1 receptors. Neuropharmacology 2014, 84, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Materazzi, S.; Benemei, S.; Geppetti, P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev. Physiol. Biochem. Pharm. 2014, 167, 1–43. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Avramut, M. Matrix metalloproteinases in neuropathic pain and migraine: Friends, enemies, and therapeutic targets. Pain Res. Treat. 2012, 2012, 952906. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Makuch, W.; Przewlocka, B.; Mika, J. Minocycline prevents dynorphin-induced neurotoxicity during neuropathic pain in rats. Neuropharmacology 2014, 86, 301–310. [Google Scholar] [CrossRef]
- Jurga, A.M.; Piotrowska, A.; Makuch, W.; Przewlocka, B.; Mika, J. Blockade of P2X4 Receptors Inhibits Neuropathic Pain-Related Behavior by Preventing MMP-9 Activation and, Consequently, Pronociceptive Interleukin Release in a Rat Model. Front. Pharm. 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharm. 2013, 716, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Kang, J.; Unabia, G.C.; Hulsebosch, C.E. Spatial and temporal activation of spinal glial cells: Role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp. Neurol. 2012, 234, 362–372. [Google Scholar] [CrossRef]
- Segal, J.P.; Tresidder, K.A.; Bhatt, C.; Gilron, I.; Ghasemlou, N. Circadian control of pain and neuroinflammation. J. Neurosci. Res. 2018, 96, 1002–1020. [Google Scholar] [CrossRef]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef]
- O’Brien, W.; Pham, L.; Symons, G.; Monif, M.; Shultz, S.; McDonald, S. The NLRP3 inflammasome in traumatic brain injury: Potential as a biomarker and therapeutic target. J. Neuroinflamm. 2020, 17, 104. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, Z.; Linghu, K.; Liu, J.; Gan, L.; Lin, L. Small molecule-driven SIRT3-autophagy-mediated NLRP3 inflammasome inhibition ameliorates inflammatory crosstalk between macrophages and adipocytes. Br. J. Pharmacol. 2020, 177, 4645–4665. [Google Scholar] [CrossRef]
- Sun, X.; Cao, L.; Ge, J.; Ge, J.; Yang, X.; Du, B.; Song, J. The NLRP3-related inflammasome modulates pain behavior in a rat model of trigeminal neuropathic pain. Life Sci. 2021, 277, 119489. [Google Scholar] [CrossRef] [PubMed]
- Arruri, V.; Gundu, C.; Kalvala, A.; Sherkhane, B.; Khatri, D.; Singh, S. Carvacrol abates NLRP3 inflammasome activation by augmenting Keap1/Nrf-2/p62 directed autophagy and mitochondrial quality control in neuropathic pain. Nutr. Neurosci. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, J. P2X3-Containing Receptors as Targets for the Treatment of Chronic Pain. Neurother. J. Am. Soc. Exp. NeuroTher. 2020, 17, 826–838. [Google Scholar] [CrossRef]
- Drill, M.; Jones, N.C.; Hunn, M.; O’Brien, T.J.; Monif, M. Antagonism of the ATP-gated P2X7 receptor: A potential therapeutic strategy for cancer. Purinergic Signal. 2021, 17, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; Ase, A.R.; Seguela, P. P2X receptor channels in chronic pain pathways. Br. J. Pharm. 2018, 175, 2219–2230. [Google Scholar] [CrossRef]
- Sperlágh, B.; Illes, P. P2X7 receptor: An emerging target in central nervous system diseases. Trends Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef]
- Cortright, D.N.; Krause, J.E.; Broom, D.C. TRP channels and pain. Biochim. Biophys. Acta 2007, 1772, 978–988. [Google Scholar] [CrossRef]
- Jordt, S.; Bautista, D.; Chuang, H.; McKemy, D.; Zygmunt, P.; Högestätt, E.; Meng, I.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Isami, K.; Nakamura, S.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol. Pain 2012, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Bae, C.; Wang, J.; Lee, K.; Hankerd, K.; Kim, H.; Chung, J.; La, J. Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol. Pain 2019, 15, 1744806919840098. [Google Scholar] [CrossRef] [PubMed]
- Boiko, N.; Medrano, G.; Montano, E.; Jiang, N.; Williams, C.R.; Madungwe, N.B.; Bopassa, J.C.; Kim, C.C.; Parrish, J.Z.; Hargreaves, K.M.; et al. TrpA1 activation in peripheral sensory neurons underlies the ionic basis of pain hypersensitivity in response to vinca alkaloids. PLoS ONE 2017, 12, e0186888. [Google Scholar] [CrossRef]
- Yosten, G.; Harada, C.; Haddock, C.; Giancotti, L.; Kolar, G.; Patel, R.; Guo, C.; Chen, Z.; Zhang, J.; Doyle, T.; et al. GPR160 de-orphanization reveals critical roles in neuropathic pain in rodents. J. Clin. Investig. 2020, 130, 2587–2592. [Google Scholar] [CrossRef]
- Sayo, A.; Konishi, H.; Kobayashi, M.; Kano, K.; Kobayashi, H.; Hibi, H.; Aoki, J.; Kiyama, H. GPR34 in spinal microglia exacerbates neuropathic pain in mice. J. Neuroinflamm. 2019, 16, 82. [Google Scholar] [CrossRef]
- Karki, P.; Kurihara, T.; Nakamachi, T.; Watanabe, J.; Asada, T.; Oyoshi, T.; Shioda, S.; Yoshimura, M.; Arita, K.; Miyata, A. Attenuation of inflammatory and neuropathic pain behaviors in mice through activation of free fatty acid receptor GPR40. Mol. Pain 2015, 11, 6. [Google Scholar] [CrossRef]
- Mao, X.; Wu, H.; Tang, X.; Ali, U.; Liu, H.; Wang, Y. Activation of GPR40 produces mechanical antiallodynia via the spinal glial interleukin-10/β-endorphin pathway. J. Neuroinflamm. 2019, 16, 84. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Atabaki, R.; Roohbakhsh, A. The role of orphan G protein-coupled receptors in the modulation of pain: A review. Life Sci. 2018, 212, 59–69. [Google Scholar] [CrossRef]
- Gong, N.; Xiao, Q.; Zhu, B.; Zhang, C.Y.; Wang, Y.C.; Fan, H.; Ma, A.N.; Wang, Y.X. Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J. Neurosci. 2014, 34, 5322–5334. [Google Scholar] [CrossRef]
- Liu, J.; Yin, F.; Zheng, X.; Jing, J.; Hu, Y. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem. Int. 2007, 51, 361–369. [Google Scholar] [CrossRef]
- Zhu, B.; Gong, N.; Fan, H.; Peng, C.; Ding, X.; Jiang, Y.; Wang, Y. Lamiophlomis rotata, an orally available Tibetan herbal painkiller, specifically reduces pain hypersensitivity states through the activation of spinal glucagon-like peptide-1 receptors. Anesthesiology 2014, 121, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Roger, B.; Papin, J.; Vacher, P.; Raoux, M.; Mulot, A.; Dubois, M.; Kerr-Conte, J.; Voy, B.; Pattou, F.; Charpentier, G.; et al. Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells. Diabetologia 2011, 54, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.; Zippin, J.; Kamenetsky, M.; Buck, J.; Levin, L. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J. Gen. Physiol. 2008, 132, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. Recent advances in understanding the role of glucagon-like peptide 1. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Wu, H.; Tang, X.; Mao, X.; Wang, Y. Autocrine Interleukin-10 Mediates Glucagon-Like Peptide-1 Receptor-Induced Spinal Microglial β-Endorphin Expression. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 11701–11714. [Google Scholar] [CrossRef]
- Alavi, S.; Ebrahimi Shahmabadi, H. GLP-1 peptide analogs for targeting pancreatic beta cells. Drug Discov. Today 2021, 26, 1936–1943. [Google Scholar] [CrossRef]
- Geng, X.; Wang, F.; Tian, D.; Huang, L.; Streator, E.; Zhu, J.; Kurihara, H.; He, R.; Yao, X.; Zhang, Y.; et al. Cardiac glycosides inhibit cancer through Na/K-ATPase-dependent cell death induction. Biochem. Pharmacol. 2020, 182, 114226. [Google Scholar] [CrossRef]
- Patel, S. Plant-derived cardiac glycosides: Role in heart ailments and cancer management. Biomed. Pharmacother. = Biomed. Pharmacother. 2016, 84, 1036–1041. [Google Scholar] [CrossRef]
- Xue, X.; Deng, Y.; Wang, J.; Zhou, M.; Liao, L.; Wang, C.; Peng, C.; Li, Y. Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L. with good effect of alleviating atherosclerosis. Phytomed. Int. J. Phytother. Phytopharm. 2021, 91, 153694. [Google Scholar] [CrossRef]
- Dey, S.; Jayaraman, N. Glycosidic bond hydrolysis in septanosides: A comparison of mono-, di-, and 2-chloro-2-deoxy-septanosides. Carbohydr. Res. 2014, 399, 49–56. [Google Scholar] [CrossRef]
- He, W.J. Study on the Interaction of Milk Protein with Cyanidin-3-O-Glucoside and Its Effect on Pigment Stability; Jianggnan University: Wuxi, China, 2018. [Google Scholar]
- Yu, H.; Xu, J.; Su, J.; Lu, W.; Lin, G. Synthesis of novel salidroside esters by lipase-mediated acylation with various functional acyl groups. J. Biosci. Bioeng. 2008, 106, 65–68. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Fernyhough, P. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium 2008, 44, 112–122. [Google Scholar] [CrossRef]
- Simon, F.; Varela, D.; Cabello-Verrugio, C. Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell. Signal. 2013, 25, 1614–1624. [Google Scholar] [CrossRef]
- Bubolz, A.; Mendoza, S.; Zheng, X.; Zinkevich, N.; Li, R.; Gutterman, D.; Zhang, D. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: Role of Ca2+ entry and mitochondrial ROS signaling. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H634–H642. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M.; Ciğ, B.; Ozgül, C. Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: Role of TRPV1 channels. Neuroscience 2013, 242, 151–160. [Google Scholar] [CrossRef]
- Trevisan, G.; Benemei, S.; Materazzi, S.; De Logu, F.; De Siena, G.; Fusi, C.; Fortes Rossato, M.; Coppi, E.; Marone, I.M.; Ferreira, J.; et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 2016, 139, 1361–1377. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A.; Bäumert, H.; Rettinger, J.; Eichele, A.; Lambrecht, G.; Mutschler, E.; Schmalzing, G. P2X1 and P2X3 receptors form stable trimers: A novel structural motif of ligand-gated ion channels. EMBO J. 1998, 17, 3016–3028. [Google Scholar] [CrossRef]
- North, R. P2X receptors. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2016, 371, 20150427. [Google Scholar] [CrossRef] [PubMed]
- Suurvali, J.; Boudinot, P.; Kanellopoulos, J.; Ruutel Boudinot, S. P2X4: A fast and sensitive purinergic receptor. Biomed. J. 2017, 40, 245–256. [Google Scholar] [CrossRef] [PubMed]

| Grade | Drug | Neuropathic Pain Conditions | Major Side-Effects | Strength of Recommendation |
|---|---|---|---|---|
| First-line drug | ||||
| Serotonin-noradrenaline reuptake inhibitors duloxetine and venlafaxine | All | Nausea | Strong | |
| Tricyclic antidepressants | All | Sedation, anticholinergic effects | Strong | |
| Pregabalin, gabapentin, gabapentin extended release or enacarbil | All | Sedation, dizziness, peripheral oedema | Strong | |
| Second-line drug | ||||
| Tramadol | All | Nausea/vomiting, constipation, dizziness | Weak | |
| Capsaicin 8% patches | Peripheral | local pain, oedema, and erythema | Weak | |
| Lidocaine patches | Peripheral | Local erythema, rash | Weak | |
| Third-line drug | ||||
| Strong opioids | All | Nausea/vomiting, constipation, dizziness | Weak | |
| Botulinum toxin A | Peripheral | local pain | Weak |
 |  |  |  |
| Diosmin | Verbascoside | Hesperidin | Saikosaponin D |
 |  |  |  |
| Saikosaponin A | Paeoniflorin | Morroniside | Liquiritin |
 |  |  | 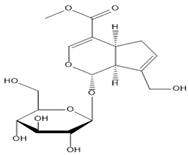 |
| Salidroside | Isoorientin | Albiflorin | Geniposide |
| Name | Pharmacological Activities | Animals | Model | Dose mg/kg (Route of Administration) | Nociceptive Tests | Mechanism | Refences | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randall–Selitto Paw Pressure Test | Von Frey Filament Test | Radiant Heat Test | Hot Plate Test | Tail Immersion Test | Acetone Drop Test | Cold Plate Test | Rotarod Test | Spontaneous Exploratory Test | Walking Test | Open-Field Test | Elevated Plus Maze Test | Forced Swim Test | Electrophysiology Examination | |||||||
| Diosmin | Antinociceptive, anti-inflammatory | Male Wistar rats | CCI | 10, 100, 316.2, 562.3 or 1000 mg/kg, i.p. | - | ↓ Mechanical hyperalgesia | ↓ Thermal hyperalgesia | - | - | → Locomotor activity | - | ↓ pro-inflammatory cytokines released | [67] | |||||||
| Male Swiss mice | CCI | 10 mg/kg, i.p. | - | ↓ Mechanical hyperalgesia | - | ↓ Thermal hyperalgesia | - | - | - | - | ↑ NO/cGMP/PKG/KATP channel signaling pathway, ↓ pro-inflammatory cytokines released | [68] | ||||||||
| Verbascoside | Antinociceptive, Antioxidant, | Adult male Wistar rats | CCI | 50, 100, and 200 mg/kg, i.p. | ↓ mechanical allodynia | ↓ heat hyperalgesia | ↓ cold allodynia | ↓ Oxidative stress | [69] | |||||||||||
| Hesperidin | Antinociceptive, antidiabetic | Male SD rats | CCI | 50 mg/kg, i.p. | - | ↓ Mechanical hyperalgesia | ↓ Thermal hyperalgesia | - | - | - | ↓ P2X3 receptor | [70] | ||||||||
| Male Wistar rats | CCI | 100 mg/kg, i.p. | - | ↓ Mechanical hyperalgesia | ↓ Thermal hyperalgesia | - | - | - | ↓ Pro-inflammatory cytokines released | [71] | ||||||||||
| Adult SD rats | STZ-induced diabetic neuropathy | Hesperidin: 25, 50, 100 mg/kg, p.o. Insulin: 10 IU/kg, s.c. | ↓ Mechanical hyperalgesia | ↓ Mechano-tactile allodynia | - | ↓ Thermal hyperalgesia | - | ↑ MNCV, ↑ SNCV | ↓ Glycated hemoglobin, AR activity, oxidonitrosative stress, neural calcium, and pro-inflammatory cytokines | [72] | ||||||||||
| Saikosaponin D | Antinociceptive | Male ICR mice | VCR-induced neuropathic pain | 5,20 mg/kg, i.p. | - | ↓ Mechanical hypersensitivity | - | - | - | - | - | - | - | ↓ Activation of TRPA1 channel | [73] | |||||
| Saikosaponin A | Antinociceptive, anti-inflammatory | Adult SD rats | CCI | 6.25, 12.50 and 25.00 mg/kg, i.p. | - | ↓ Mechanical allodynia | ↓ Thermal hyperalgesia | - | - | - | - | - | - | ↓ Activation of p38 MAPK and NF-κB signaling pathway | [55] | |||||
| Paeoniflorin | Antinociceptive, anti-inflammatory | Male Wistar rats | CCI | 50 mg/kg, i.p. | - | ↓ Mechanical allodynia | ↓ Thermal hyperalgesia | - | - | - | - | - | - | ↓ Activation of p38 MAPK and NF-κB signaling pathway | [58] | |||||
| Male C57BL/6NCr mice | PTX-induced neuropathic pain | 0.1 and 1%, applied topically (20 μL) | - | ↓ Mechanical allodynia | - | - | - | - | - | - | ↑ The activation of adenosine A1 receptor | [74] | ||||||||
| SD rats | CCI | Paeoniflorin: 25, 50, 100 mg/kg, i.p. MCC950:10 mg/kg, i.p. ML385:500 pmol/5 μL, i.t. | - | ↓ Mechanical allodynia | ↓ Thermal hyperalgesia | - | - | - | - | - | - | ↓ Spinal NLRP3 infammasome activation | [75] | |||||||
| Morroniside | Antinociceptive | Adult male Wistar rats | SNL | 30, 100, 300, 600 mg/kg, p.o. 3, 10, 30, 100, 300 μg, i.t. | ↓ Mechanical allodynia | ↓ Thermal hyperalgesia | No sedation or motor side effects | ↑ Activation of GLP-1 receptor | [76] | |||||||||||
| Wistar 1-day-old neonatal rat pups; male adult rat | SNL | 300 μg, i.t. | ↓ Mechanical allodynia | ↑ GLP-1R/ (IL-10/β-Endorphin antinociceptive pathway. | [77] | |||||||||||||||
| liquiritin | Antinociceptive, anti-inflammatory, neuroprotective | ICR mice | CCI | 30, 60, 120 mg/kg, i.p. | ↓ Mechanical allodynia | ↓ Thermal hyperalgesia | ↓ Cold allodynia | → Motor coordination | → Exploratory behavior | ↑ Sciatic function index | ↑ MNCV, ↑ MNCP | ↓ Pro-inflammatory cytokines, ↑ Anti- inflflammatory cytokine | [78] | |||||||
| Salidroside | Antinociceptive, anti-inflammation, stress reduce, | Lean rats | Zucker diabetic fatty (ZDF) rats | 25, 50, 100 mg/kg, p.o. | ↓ Mechanical hyperalgesia | ↓ Thermal hyperalgesia | ↑ SNCV | ↓ Pro-inflammatory cytokines, ↓ P2X7 receptor | [61] | |||||||||||
| Male SD rats | T2DM rat model | 50, 100 mg/kg, p.o. | - | ↓ Mechanical allodynia | ↓ Thermal hyperalgesia | ↑ AMPK activation, ↓ NLRP3 inflammasome activation | [79] | |||||||||||||
| Isoorientin | Antinociceptive, Antioxidant, | Male ICR mice | CCI | 7.5, 15, and 30 mg/kg/day, p.o. | - | ↓ Mechanical allodynia | ↓ thermal hyperalgesia | - | ↓ cold allodynia | ↑ SNCV, ↑ SNAP | ↑ T-AOC, T-SOD, CAT, MDA ↓ Pro-inflammatory cytokines | [80] | ||||||||
| Albiflorin | Antinociceptive, anti-anxiety and anti-depressant anti- inflammatory, neuroprotective, | Adult male SD rats | CCI | 50 mg/kg, i.p. | ↓ Mechanical allodynia | ↓ Anxiety-like behavior | ↓ Anxiety-like behavior | ↓ Depression-like behavior | ↓ NLRP3 inflammasome activity | [81] | ||||||||||
| Geniposide | Antinociceptive, anti-inflammatory, | Adult male SD rats | STZ-induced diabetic neuropathy | 1, 10, 100 mg/kg, i.p. | ↓ Mechanical allodynia | ↓ Pro-inflammatory cytokines, | [82] | |||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.-M.; Li, Y.-X.; Liu, S.; Zhu, C.-H.; Lan, X.-B.; Du, J.; Ma, L.; Yang, J.-M.; Zheng, P.; Yu, J.-Q.; et al. Glycosides for Peripheral Neuropathic Pain: A Potential Medicinal Components. Molecules 2022, 27, 255. https://doi.org/10.3390/molecules27010255
Tian M-M, Li Y-X, Liu S, Zhu C-H, Lan X-B, Du J, Ma L, Yang J-M, Zheng P, Yu J-Q, et al. Glycosides for Peripheral Neuropathic Pain: A Potential Medicinal Components. Molecules. 2022; 27(1):255. https://doi.org/10.3390/molecules27010255
Chicago/Turabian StyleTian, Miao-Miao, Yu-Xiang Li, Shan Liu, Chun-Hao Zhu, Xiao-Bing Lan, Juan Du, Lin Ma, Jia-Mei Yang, Ping Zheng, Jian-Qiang Yu, and et al. 2022. "Glycosides for Peripheral Neuropathic Pain: A Potential Medicinal Components" Molecules 27, no. 1: 255. https://doi.org/10.3390/molecules27010255
APA StyleTian, M.-M., Li, Y.-X., Liu, S., Zhu, C.-H., Lan, X.-B., Du, J., Ma, L., Yang, J.-M., Zheng, P., Yu, J.-Q., & Liu, N. (2022). Glycosides for Peripheral Neuropathic Pain: A Potential Medicinal Components. Molecules, 27(1), 255. https://doi.org/10.3390/molecules27010255





