The Effects of Formulation on Imidacloprid Dissipation in Grapes and Vine Leaves and on Required Pre-Harvest Intervals under Lebanese Climatic Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. SL-and WDG-Imidacloprid Dissipation Kinetics
2.2. Matrix and Residue Levels
2.3. Formulation and Residues Level
3. Materials and Methods
3.1. Chemicals and Reagents
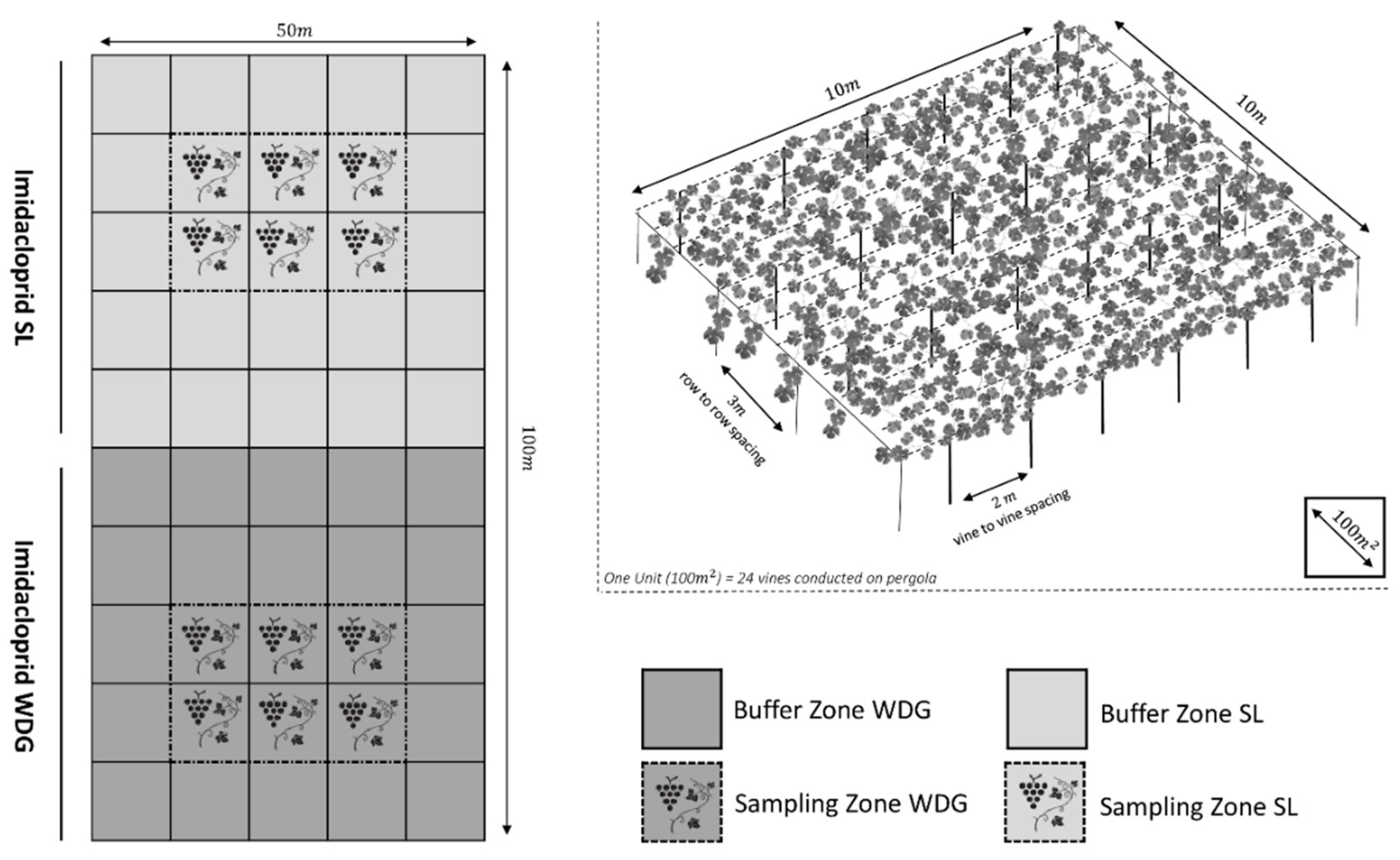
3.2. Site Location and Specification
3.3. Pesticide Application and Sampling
Sampling Procedure for Grapes and Vine Leaves
3.4. Residue Extraction and Clean-Up
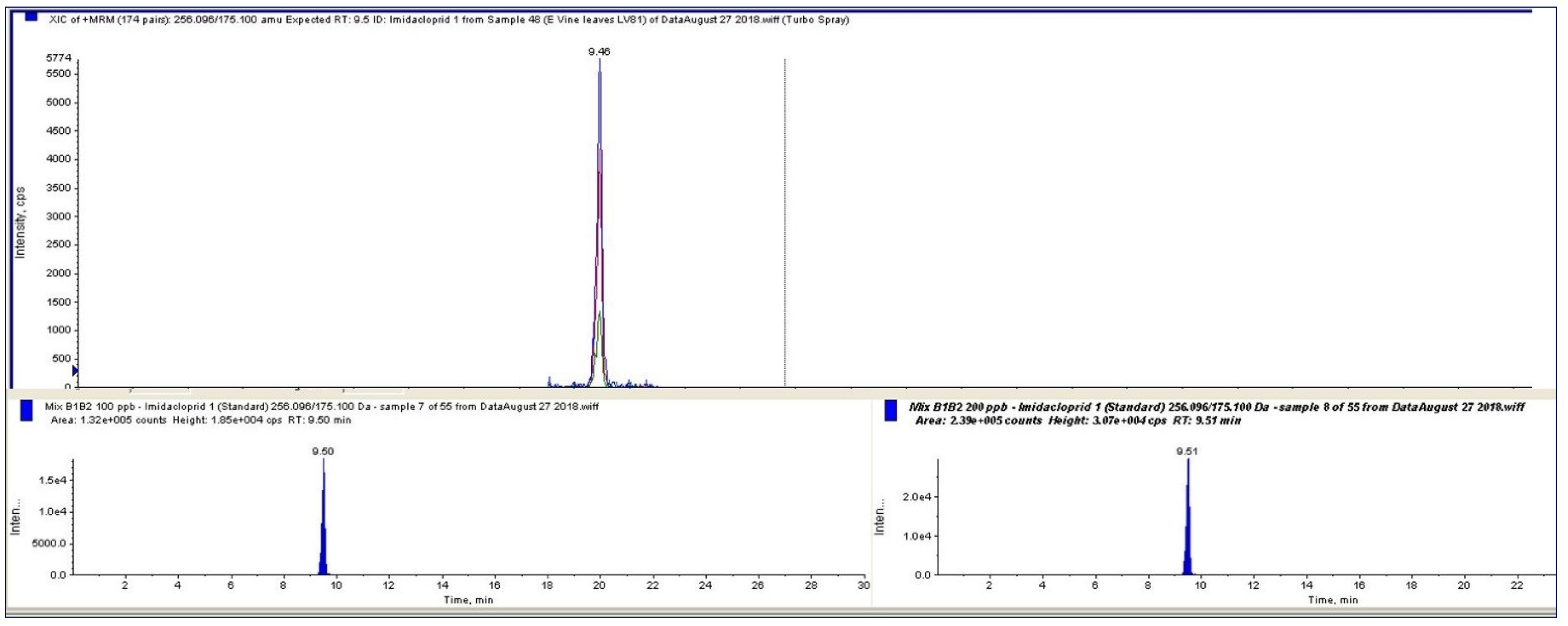
3.5. Instrumentation and LC-MS/MS Analytical Conditions
3.6. Method Validation for Grapes and Vine Leaves
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sannakki, S.; Rajpurohit, V.; Nargund, V.; Kulkarni, P. Diagnosis and classification of grape leaf diseases using neural networks. In Proceedings of the Fourth International Conference on Computing, Communications and Networking Technologies (ICCCNT), Tiruchengode, India, 4–6 July 2013; pp. 5–8, ISBN 978-1-4799-3926-8. [Google Scholar]
- Lacerda, D.S.; Costa, P.C.; Funchal, C.; Dani, C.; Gomez, R. Benefits of Vine Leaf on Different Biological Systems. Grape Wine Biotechnol. 2016, 19, 125–143. [Google Scholar] [CrossRef]
- Maestroni, B.M.; Ghanem, I.; Correll, R.; Alnaser, A.A.; Islam, M.; Cesio, V.; Heinzen, H.; Cannavan, A. Required Withholding Period for Vine Leaves Following Spraying with Pesticide. J. Health Environ. Res. 2018, 4, 140. [Google Scholar] [CrossRef]
- Mdh, P.; Mw, A.; Sn, A. Determination of Pre-Harvest Interval for Quinalphos, Malathion, Diazinon and Cypermethrin in Major Vegetables. J. Environ. Anal. Toxicol. 2018, 8, 100163. [Google Scholar] [CrossRef]
- EU Pesticides Database (v.2.1) Details. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/products/?event=details&p=163 (accessed on 4 April 2021).
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis vinifera L. Varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Hayar, S.; Zeitoun, R.; Maestroni, B.M. Validation of a Rapid Multiresidue Method for the Determination of Pesticide Residues in Vine Leaves. Comparison of the Results According to the Different Conservation Methods. Molecules 2021, 26, 1176. [Google Scholar] [CrossRef] [PubMed]
- Hayar, S.; Al Hadi, J.; Merhi, A.; Majed, L.; Saba Ayoun, L. L’effet de 3 modes de conservation sur la dissipation des résidus de pesticides dans les feuilles de vigne Vitis vinifera. In Proceedings of the 47ème Congrès du Groupe Français des Pesticides: Regards Transfrontaliers sur les Pesticides, Nancy, France, 15 May 2017; pp. 65–75. [Google Scholar]
- Sandikly, N.; Hayar, S.; Millet, M. Monitoring Survey of Pesticide Residues in Table Grapes and Risk Assessment to Human in Lebanon. In Proceedings of the 49ème Congrès du Groupe Français des Pesticides, Montpellier, France, 21 May 2019. [Google Scholar]
- Maclachlan, D.J.; Hamilton, D. A New Tool for the Evaluation of Crop Residue Trial Data (Day-Zero-plus Decline). Food Addit. Contam. Part A 2010, 27, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; El-Sayed, W.; Ahmed, N. The Relationship Between Different Formulation Types And The Residue Levels Of Pesticides On Tomato Fruits. Res. J. Agric. Biol. Sci. 2013, 9, 8–16. [Google Scholar]
- Alister, C.; Araya, M.; Becerra, K.; Saavedra, J.; Kogan, M. Preharvest Interval Periods and Their Relation to Fruit Growth Stages and Pesticide Formulations. Food Chem. 2017, 221, 548–554. [Google Scholar] [CrossRef]
- Cress, D. Factors Affecting Pesticide Behavior and Breakdown. Available online: https://bookstore.ksre.ksu.edu/pubs/MF958.pdf (accessed on 17 April 2021).
- Ad Meijs, W.H.M. New Type of Formulations and New Application Techniques; Consequences for the Authorisation of Pesticides. Environmentalist 2008, 28, 5–8. [Google Scholar] [CrossRef][Green Version]
- Cabras, P.; Gennari, M.; Meloni, M.; Cabitza, F.; Cubeddu, M. Pesticide Residues in Lettuce. 2. Influence of Formulations. J. Agric. Food Chem. 1989, 37, 1405–1407. [Google Scholar] [CrossRef]
- Buzzetti, K. Role of the Formulation in the Efficacy and Dissipation of Agricultural Insecticides. Insectic. Agric. Toxicol. 2018, 43–64. Available online: https://www.intechopen.com/chapters/58195 (accessed on 19 October 2021).
- Montemurro, N.; Grieco, F.; Lacertosa, G.; Visconti, A. Chlorpyrifos Decline Curves and Residue Levels from Different Commercial Formulations Applied to Oranges. J. Agric. Food Chem. 2002, 50, 5975–5980. [Google Scholar] [CrossRef] [PubMed]
- Abou Zeid, M.; Jammoul, A.; Melki, K.; Abou-Jawda, Y.; Kallassy, M. Dissipation of 3 Commercial Formulations of Penconazole 10% EC on Greenhouse Tomatoes in Lebanon. Res. J. Chem. Environ. 2020, 13, 2230. [Google Scholar]
- Mohapatra, S.; Ahuja, A.; Sharma, D.; Manthirachalam, D.; Prakash, G.; Kumar, S. Residue Study of Imidacloprid in Grapes (Vitis vinifera L.) and Soil. Qual. Assur. Saf. Crops Foods 2011, 3, 24–27. [Google Scholar] [CrossRef]
- EU. Commission Implementing Regulation (EU) 2020/1643 of 5 November 2020 Amending Implementing Regulation (EU) No 540/2011 as Regards the Approval Periods of the Active Substances Calcium Phosphide, Denathonium Benzoate, Haloxyfop-P, Imidacloprid, Pencycuron and Zeta-Cypermethrin (Text with EEA Relevance); EU: Brussels, Belgium, 2020; Volume 370. [Google Scholar]
- EU Pesticides Database (v.2.2) Pesticide Residue(s) and Maximum Residue Levels (Mg/Kg). Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=details&pest_res_ids=326&product_ids=&v=1&e=search.pr (accessed on 19 October 2021).
- Edwards, C.A. Factors that affect the persistence of pesticides in plants and soils. Pure Appl. Chem. 1975, 42, 39–56. [Google Scholar] [CrossRef]
- Lichiheb, N.; Bedos, C.; Personne, E.; Benoit, P.; Bergheaud, V.; Fanucci, O.; Bouhlel, J.; Barriuso, E. Measuring Leaf Penetration and Volatilization of Chlorothalonil and Epoxiconazole Applied on Wheat Leaves in a Laboratory-Scale Experiment. J. Environ. Qual. 2015, 44, 1782–1790. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Possingham, J.; Chambers, T.; Radler, F.; Grncarevic, M. Cuticular Transpiration and Wax Structure and Composition of Leaves and Fruit of Vitis vinifera. Aust. J. Biol. Sci. 1967, 20, 1149. [Google Scholar] [CrossRef]
- Chowdhury, A.; Jepson, P.; Ford, M.; Frampton, G. The Role of Cuticular Waxes and Surface Roughness in Determining the Insecticidal Efficacy of Deltamethrin and Dimethoate Applied as Emulsifiable Concentrates to Leaf Surfaces. Int. J. Pest Manag. 2005, 51, 253–263. [Google Scholar] [CrossRef]
- Baur, P.; Marzouk, H.; Schönherr, J.; Bauer, H. Mobilities of Organic Compounds in Plant Cuticles as Affected by Structure and Molar Volumes of Chemicals and Plant Species. Planta 1996, 199, 404–412. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Bletsou, A.A.; Hanafi, A.H.; Dasenaki, M.E.; Thomaidis, N.S. Development of Specific LC-ESI-MS/MS Methods to Determine Bifenthrin, Lufenuron, and Iprodione Residue Levels in Green Beans, Peas, and Chili Peppers Under Egyptian Field Conditions. Food Anal. Methods 2013, 6, 1099–1112. [Google Scholar] [CrossRef]
- Abdallah, O. Dissipation Behavior of Chlorfenapyr and Difenoconazole Residues in/on Grapes (Vitis vinifera L.). Nat. Sci. 2014, 12, 49–54. [Google Scholar]
- Hanafi, A.; Dasenaki, M.; Bletsou, A.; Thomaidis, N.S. Dissipation Rate Study and Pre-Harvest Intervals Calculation of Imidacloprid and Oxamyl in Exported Egyptian Green Beans and Chili Peppers after Pestigation Treatment. Food Chem. 2018, 240, 1047–1054. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Hatfield, J.M.; Jaudzems, V.G.; White, R.G.; Keller, M. Grape Berry Cv. Shiraz Epicuticular Wax and Transpiration during Ripening and Preharvest Weight Loss. Am. J. Enol. Vitic. 2004, 55, 121–127. [Google Scholar]
- Arora, P.K.; Jyot, G.; Singh, B.; Battu, R.S.; Singh, B.; Aulakh, P.S. Persistence of Imidacloprid on Grape Leaves, Grape Berries and Soil. Bull. Environ. Contam. Toxicol. 2009, 82, 239–242. [Google Scholar] [CrossRef]
- Karthik, P.; Venugopal, S.; Datchina Murthy, K.; Lokesh, S.; Karthik, G.; Sharmila, U.; Paramasivam, M.; Senguttuvan, K.; Gunasekaran, K.; Kuttalam, S. Bioefficacy, Phytotoxicity, Safety to Natural Enemies and Residue Dynamics of Imidacloprid 70 WG in Okra (Abelmoschus esculenta (L) Moench) under Open Field Conditions. Crop Prot. 2015, 71, 88–94. [Google Scholar] [CrossRef]
- Oliva, J.; Cermeño, S.; Cámara, M.A.; Martínez, G.; Barba, A. Disappearance of Six Pesticides in Fresh and Processed Zucchini, Bioavailability and Health Risk Assessment. Food Chem. 2017, 229, 172–177. [Google Scholar] [CrossRef]
- Scholz, K.; Reinhard, F. Photolysis of Imidacloprid (NTN 33893) on the Leaf Surface of Tomato Plants. Pestic. Sci. 1999, 55, 652–654. [Google Scholar] [CrossRef]
- Dikshit, A.K.; Pachauri, D.C.; Jindal, T. Maximum Residue Limit and Risk Assessment of Beta-Cyfluthrin and Imidacloprid on Tomato (Lycopersicon esculentum Mill). Bull. Environ. Contam. Toxicol. 2003, 70, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- OECD Guidance Document on Crop Field Trials. Available online: https://read.oecd-ilibrary.org/environment/guidance-document-on-crop-field-trials_2794fdd6-en (accessed on 19 October 2021).
- Food and Agriculture Organization of the United Nations Guidelines on Good Practice for Ground Application of Pesticides. Available online: http://www.fao.org/3/y2767e/y2767e00.htm (accessed on 19 March 2021).
- FAO. Recommended Methods of Sampling for the Determination of Pesticide Residues for Compliance With MRLS: CAC/GL 33-1999. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B33-1999%252FCXG_033e.pdf (accessed on 19 March 2021).
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Guidance SANTE/12682/2019—Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. Available online: https://www.accredia.it/en/documento/guidance-sante-12682-2019-guidance-document-on-analytical-quality-control-and-method-validation-procedures-for-pesticides-residues-analysis-in-food-and-feed/ (accessed on 19 October 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
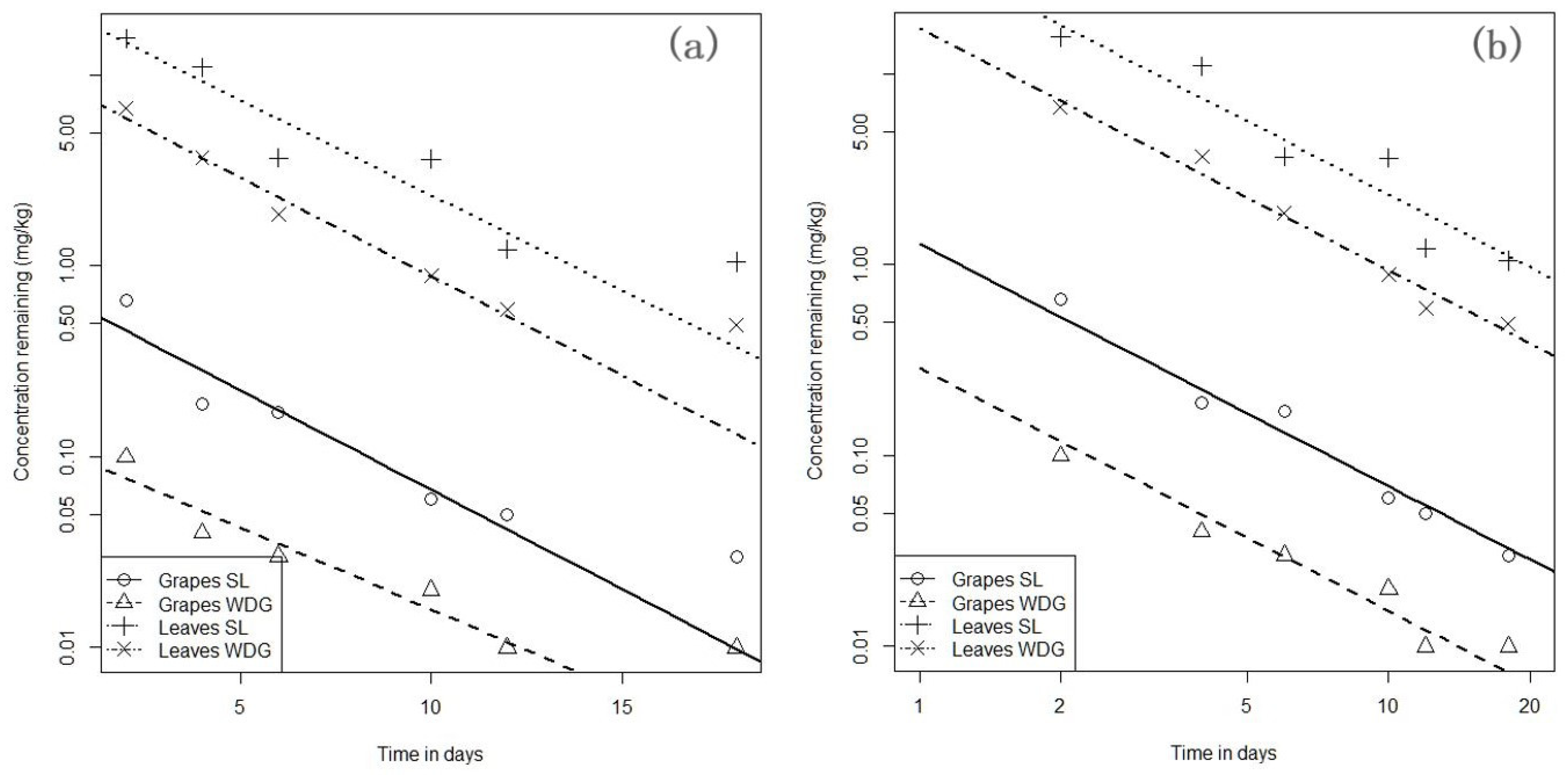
| Grapes | ||||||||
| Imidacloprid Formulation | Regression Equation | Slope (k) | Intercept (b) | DT50 (Days) | PHI (Days) | MRL (mg kg−1) | ||
| EU 2021 | EU 2022 | EU 2021 | EU 2022 | |||||
| SL | −1.269 (±0.068) | 0.249 | 0.546 | 0.196 | 0.477 | 1 | 0.7 | |
| WDG | −1.269 (±0.068) | −1.249 | 0.546 | −0.984 | −0.703 | 1 | 0.7 | |
| Vine Leaves | ||||||||
| Imidacloprid Formulation | Regression Equation | Slope(k) | Intercept (b) | DT50 (Days) | PHI (Days) | MRL (mg kg−1) | ||
| EU 2021 | EU 2022 | EU 2021 | EU 2022 | |||||
| SL | −1.269 (±0.068) | 3.774 | 0.546 | 2.428 | 6.603 | 2 | 0.01 * | |
| WDG | −1.269 (±0.068) | 2.855 | 0.546 | 1.704 | 5.879 | 2 | 0.01 * | |
| Imidacloprid Formulation | Grapes | |||||
| Mean Concentration (±SD) in mg kg−1 | ||||||
| T2 | T4 | T6 | T10 | T12 | T18 | |
| SL * | 0.66 (±0.031) a (0) b | 0.19 (±0.020) (64.1) | 0.17 (±0.012) (67.9) | 0.06 (±0.003) (88.7) | 0.05 (±0.006) (90.5) | 0.03 (±0.008) (94.3) |
| WDG ** | 0.10 (±0.004) (0) | 0.04 (±0.008) (50) | 0.03 (±0.001) (62.5) | 0.02 (±0.018) (75) | 0.01 (±0.001) (87.5) | 0.01 (±0.003) (87.5) |
| Imidacloprid Formulation | Vine Leaves | |||||
| Mean Concentration (±SD) in mg kg−1 | ||||||
| T2 | T4 | T6 | T10 | T12 | T18 | |
| SL | 15.60 (±0.960) (8.77) | 11.00 (±0.780) (35.7) | 3.69 (±0.510) (78.4) | 3.64 (±0.501) (78.7) | 1.22 (±0.300) (92.8) | 1.05 (±0.230) (93.8) |
| WDG | 6.71 (±0.148) (0) | 3.68 (±0.580) (42.5) | 1.87 (±0.019) (70.8) | 0.89 (±0.090) (86.1) | 0.59 (±0.210) (90.8) | 0.49 (±0.111) (92.3) |
| Trade Name | Active Substance (%) Formulation Type | Recommended Dose (L ha−1–Kg ha−1) | PHI (Days) | Supplier Country | Importer |
|---|---|---|---|---|---|
| Pilarking® Plus | Imidacloprid 70% WDG | 0.3 | 14 | Zhejiang Hisun Chemical Co., LTD Zhejiang, China | Rmaily Trading Est. |
| Diclean | Imidacloprid 20% SL | 0.35 | 14 | Hailir Pesticides and Chemicals Group Co., LTD, Chengyang, China | National Development and General Trading Co. |
| Condition | Content | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument: | Model AB Sciex 3200 QTRAP LC-MS/MS SYSTEM | |||||||||||
| Column: | C18 column, Phenomenex Analytical Synergi, 150 × 2 mm, 2.5 μm particle size | |||||||||||
| Column Flow: | Gradient elution program at 0.4 mL·min−1 | |||||||||||
| Source temperature: | 500 °C–5000 v | |||||||||||
| Ion Spray- Potential: | Electron Spray Ionization, | |||||||||||
| Mode: | Positive Mode | |||||||||||
| Molecule | RT (min) | Precursor ion (m/z) | Transition Q1 (m/z) | DP | CE | CXP | Transition Q2 (m/z) | DP | CE | CXP | LOD | LOQ |
| (Volts) | (Volts) | (ng/g) | ||||||||||
| Imidacloprid | 9.47 | 256 | 209 | 51 | 21 | 7 | 175.0 | 46 | 25 | 7 | 1.93 | 6.45 |
| Matrix | Level of Spiking (mg kg−1) | Recovery Mean (RM%) | Repeatability (RSDr%) | Reproducibility (RSDRW%) |
|---|---|---|---|---|
| Grapes | 0.01 | 96.5 | 16.6 | 12.1 |
| Vine leaves | 92.0 | 17.0 | 19.0 | |
| Grapes | 0.05 | 92.6 | 13.3 | 9.5 |
| Vine leaves | 84.0 | 7.0 | 8.0 | |
| Grapes | 0.1 | 98.5 | 1.2 | 2.3 |
| Vine leaves | 82.0 | 11.0 | 13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majed, L.; Hayar, S.; Zeitoun, R.; Maestroni, B.M.; Dousset, S. The Effects of Formulation on Imidacloprid Dissipation in Grapes and Vine Leaves and on Required Pre-Harvest Intervals under Lebanese Climatic Conditions. Molecules 2022, 27, 252. https://doi.org/10.3390/molecules27010252
Majed L, Hayar S, Zeitoun R, Maestroni BM, Dousset S. The Effects of Formulation on Imidacloprid Dissipation in Grapes and Vine Leaves and on Required Pre-Harvest Intervals under Lebanese Climatic Conditions. Molecules. 2022; 27(1):252. https://doi.org/10.3390/molecules27010252
Chicago/Turabian StyleMajed, Liliane, Salem Hayar, Rawan Zeitoun, Britt Marianna Maestroni, and Sylvie Dousset. 2022. "The Effects of Formulation on Imidacloprid Dissipation in Grapes and Vine Leaves and on Required Pre-Harvest Intervals under Lebanese Climatic Conditions" Molecules 27, no. 1: 252. https://doi.org/10.3390/molecules27010252
APA StyleMajed, L., Hayar, S., Zeitoun, R., Maestroni, B. M., & Dousset, S. (2022). The Effects of Formulation on Imidacloprid Dissipation in Grapes and Vine Leaves and on Required Pre-Harvest Intervals under Lebanese Climatic Conditions. Molecules, 27(1), 252. https://doi.org/10.3390/molecules27010252







