Polysaccharides Release in a Laboratory-Scale Batch Hydrothermal Pretreatment of Wheat Straw under Rigorous Isothermal Operation
Abstract
:1. Introduction
2. Results and Discussion
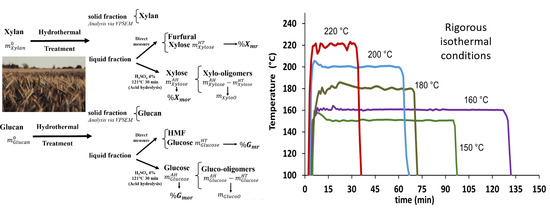
2.1. Characterization of Temperature Behavior for HP Experiments
2.2. Analysis of pH Values in the HP Experiments
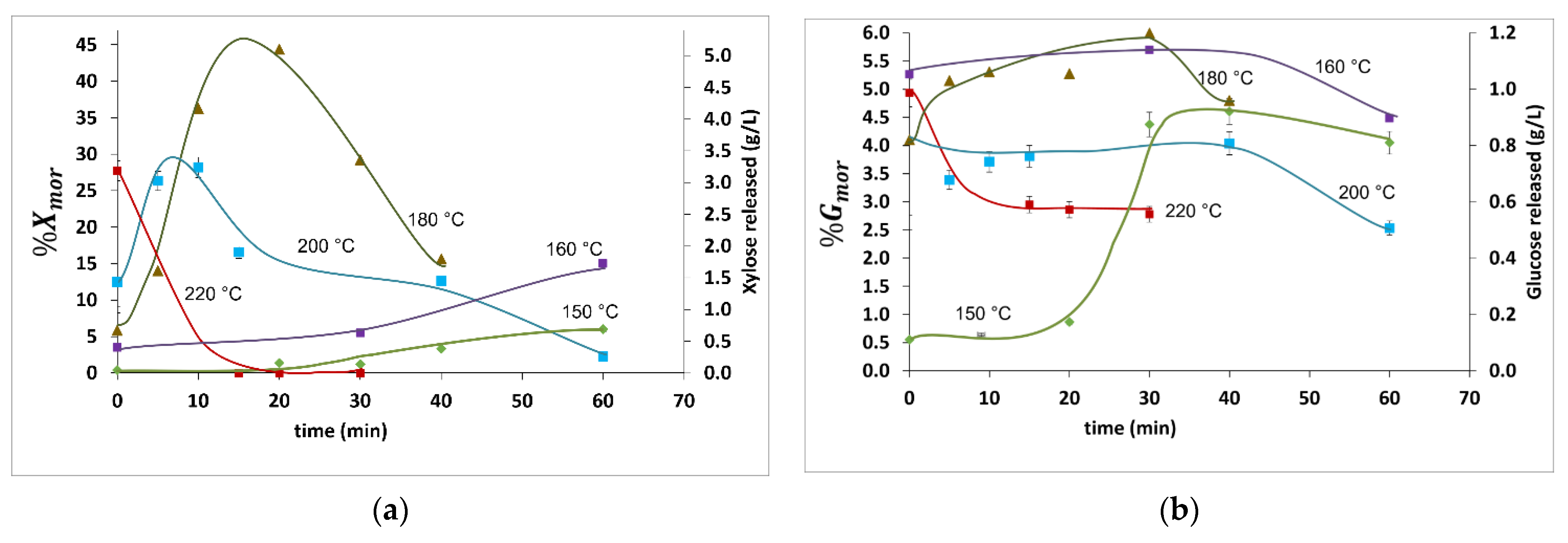
2.3. Analysis and Discussion of Xylan and Glucan Release Results
3. Materials and Methods
3.1. Wheat Straw Characterization
3.2. Experimental HP Runs
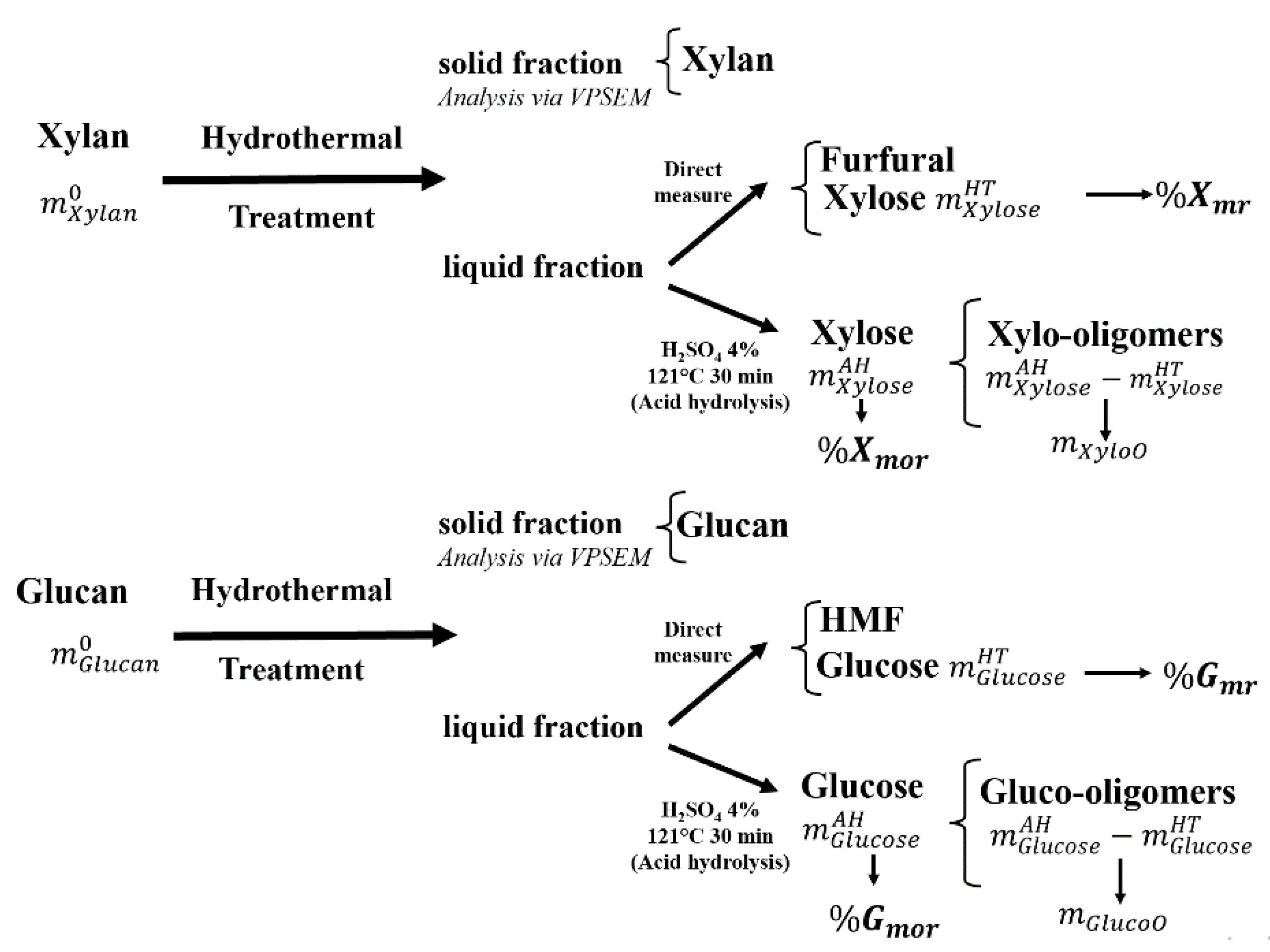
3.3. Analysis on Xylan and Glucan Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ilanidis, D.; Stagge, S.; Jonsson, L.J.; Martin, C. Hydrothermal pretreatment of wheat straw: Effects of temperature and acidity on byproduct formation and inhibition of enzymatic hydrolysis and ethanolic fermentation. Agronomy 2021, 11, 487. [Google Scholar] [CrossRef]
- Mandavgane, S.A.; Kulkarni, B.D. Wheat Straw Valorization: Material Balance and Biorefinery Approach. In Biorefinery Production Technologies for Chemicals and Energy; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 371–381. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Liu, D.; Zhao, X. Pretreatment of lignocellulosic biomass for efficient enzymatic saccharification of cellulose. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–65. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.-N.; Sanchez, A.; Rocha, G.J.; Romani, A.; Castro, E.; Torres, A.; Rodriguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, F.; Parra, C.; Sanchez, A. Role of Steam Explosion on Enzymatic Digestibility, Xylan Extraction, and Lignin Release of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 5234–5240. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, K.; Wang, K.; Ma, J.; Yang, H.; Chen, J. The effects of time and temperature in hydrothermal pretreatment on the enzymatic efficiency of wheat straw. BioRes. 2018, 13, 5193–5203. [Google Scholar]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5- hydroxymethylfurfural production-a review. Biomass Conv. Bioref. 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Freitas, C.; Carmona, E.; Brienzo, M. Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100184. [Google Scholar] [CrossRef]
- Capetti, C.C.; Vacilotto, M.M.; Dabul, A.N.G.; Veiga, A.G.; Arnoldi, V.O.; Polikarpov, I. Recent advances in the enzymatic production and applications of xylooligosaccharides. World J. Microb. Biotechnol. 2021, 37, 169. [Google Scholar] [CrossRef]
- Sidiras, D.; Batzias, F.; Ranjan, R.; Tsapatsis, M. Simulation and optimization of batch autohydrolysis of wheat straw to monosaccharides and oligosaccharides. Bioresour. Technol. 2011, 102, 10486–10492. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Vicente, A.A.; Teixeira, J.A. Kinetic modeling of enzymatic saccharification using wheat straw pretreated under autohydrolysis and organosolv process. Ind. Crops. Prod. 2012, 36, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Silva-Fernandes, T.; Duarte, L.C.; Carvalheiro, F.; Marques, S.; LoureiroDias, M.C.; Fonseca, C.; Girio, F. Biorefining strategy for maximal monosaccharide recovery from three different feedstocks: Eucalyptus residues, wheat straw and olive tree pruning. Bioresour. Technol. 2015, 183, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossberg, C.; Steffien, D.; Bremer, M.; Koenig, S.; Carvalheiro, F.; Duarte, L.C.; Moniz, P.; Hoernicke, M.; Bertau, M.; Fischer, S. Pulp properties resulting from different pretreatments of wheat straw and their influence on enzymatic hydrolysis rate. Bioresour. Technol. 2014, 169, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prunescu, R.M.; Blanke, M.; Jakobsen, J.G.; Sin, G. Dynamic modeling and validation of a biomass hydrothermal pretreatment process-a demonstration scale study. AIChE J. 2015, 61, 4235–4250. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, F.; Sanchez, A.; Amaya-Delgado, L. Xylooligosaccharides production from lignocellulosic biomass using a pilot-scale pretreatment continuous tubular reactor: Modelling and experimental validation. Ind. Crops Prod. 2019, 134, 62–70. [Google Scholar] [CrossRef]
- Makishima, S.; Mizuno, M.; Sato, N.; Shinji, K.; Suzuki, M.; Nozaki, K.; Takahashi, F.; Kanda, T.; Amano, Y. Development of continuous flow type hydrothermal reactor for hemicellulose fraction recovery from corncob. Bioresour. Technol. 2009, 100, 2842–2848. [Google Scholar] [CrossRef] [Green Version]
- Sievers, D.A.; Stickel, J.J. Modeling residence-time distribution in horizontal screw hydrolysis reactors. Chem. Eng. Sci. 2018, 175, 396–404. [Google Scholar] [CrossRef]
- Jaramillo, I.; Sanchez, A. Mass Flow Dynamic Modeling and Residence Time Control of a Continuous Tubular Reactor for Biomass Pretreatment. ACS Sustain. Chem. Eng. 2018, 6, 8570–8577. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Tian, R.; Bian, J.; Peng, F. Hydrothermal pretreatment for the production of oligosaccharides: A review. Bioresour. Technol. 2022, 343, 126075. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Galbe, M.; Garrote, G.; Ramirez-Gutierrez, D.M.; Ximenes, E.; Sun, S.N.; Lachos-Perez, D.; Rodríguez-Jasso, R.M.; Sun, R.C.; Yang, B.; et al. Severity factor kinetic model as a strategic parameter of hydrothermal processing (steam explosion and liquid hot water) for biomass fractionation under biorefinery concept. Bioresour. Technol. 2021, 342, 125961. [Google Scholar] [CrossRef]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Effects of operational conditions on auto-catalyzed and sulfuric-acid-catalyzed hydrothermal pretreatment of sugarcane bagasse at different severity factor. Ind. Crops Prod. 2021, 159, 113077. [Google Scholar] [CrossRef]
- Yu, A.; Zhang, B.; Yu, F.; Xu, G.; Song, A. A real explosion: The requirement of steam explosion pretreatment. Bioresour. Technol. 2012, 121, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Häring, H.; Smirnova, I. Design of an industrial autohydrolysis pretreatment plant for annual lignocellulose. Biomass Conv. Bioref. 2019, 11, 2293–2310. [Google Scholar] [CrossRef]
- Zhang, B.; von Keitz, M.; Valentas, K. Thermal Effects on Hydrothermal Biomass Liquefaction. In Biotechnology for Fuels and Chemicals; Adney, W.S., McMillan, J.D., Mielenz, J., Klasson, K.T., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 511–518. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Trajano, H.L.; DeMartini, J.D.; Studer, M.H.; Wyman, C.E. Comparison of the effectiveness of a fluidized sand bath and a steam chamber for reactor heating. Ind. Eng. Chem. Res. 2013, 52, 4932–4938. [Google Scholar] [CrossRef]
- Shi, J.; Pu, Y.; Yang, B.; Ragauskas, A.; Wyman, C.E. Comparison of microwaves to fluidized sand baths for heating tubular reactors for hydrothermal and dilute acid batch pretreatment of corn stover. Bioresour. Technol. 2011, 102, 5952–5961. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Girio, F.M. Wheat straw autohydrolysis: Process optimization and products characterization. Appl. Biochem. Biotechnol. 2008, 153, 84–93. [Google Scholar] [CrossRef]
- Pérez Pimienta, J.A.; Papa, G.; Gladden, J.M.; Simmons, B.A.; Sanchez, A. The effect of continuous tubular reactor technologies on the pretreatment of lignocellulosic biomass at pilot-scale for bioethanol production. RSC Adv. 2020, 10, 8147–18159. [Google Scholar] [CrossRef]
- Gonzalez-Rios, J.A.; Valle-Pérez, A.U.; Amaya-Delgado, L. A quick fed-batch saccharification strategy of wheat straw at high solid loadings improving lignocellulosic ethanol productivity. Biomass Conv. Bioref. 2021, 1–13. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Magaña, G.; Rodriguez, F.; Leon-Rodriguez, A.D.; Sanchez, A. Co-production of ethanol-hydrogen by genetically engineered Escherichia coli in sustainable biorefineries for lignocellulosic ethanol production. Chem. Eng. J. 2021, 406, 126829. [Google Scholar] [CrossRef]
- Pérez Pimienta, J.A.; Papa, G.; Rodriguez, A.; Barcelos, C.A.; Liang, L.; Stavila, V.; Sanchez, A.; Gladden, J.M.; Simmons, B.A. Pilot-scale hydrothermal pretreatment and optimized saccharification enables bisabolene production from multiple feedstocks. Green Chem. 2019, 21, 3152–3164. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of extractives in biomass. In Technical Report; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Rojas-Rejon, O.A.; Sanchez, A. The impact of particle size and initial solid loading on thermochemical pretreatment of wheat straw for improving sugar recovery. Bioprocess. Biosyst. Eng. 2014, 37, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wyman, C.E. Characterization of the degree of polymerization of xylooligomers produced by flowthrough hydrolysis of pure xylan and corn stover with water. Bioresour. Technol. 2014, 99, 5756–5762. [Google Scholar] [CrossRef]


| Reaction Temperatures (°C) | Reaction Times (min) | Mean Heating Times (min), Rates (°C/min) | Mean Cooling Times (min), Rates (°C/min) |
|---|---|---|---|
| 150 | 0, 20, 30, 40, 60 | 3, 43 | 3, −17 |
| 160 | 0, 30, 60 | 5, 28 | 4, −15 |
| 180 | 0, 5, 10, 20, 30, 40 | 6, 27 | 4, −20 |
| 200 | 0, 5, 10, 15, 40, 60 | 6, 30 | 3, −33 |
| 220 | 0, 15, 20, 30 | 6, 34 | 3, −40 |
| Reaction Temperature (°C) | Reaction Time (min) | %Xmr | %Gmr | pH | ||
|---|---|---|---|---|---|---|
| 150 | 0 | 0.010 | 0.01 | 0.40/0.00 | 0.60/0.10 | 5.3 |
| 150 | 20 | 0.020 | 0.93 | 1.4/0.20 | 0.90/0.20 | 4.8 |
| 150 | 30 | 0.030 | 1.5 | 1.2/0.10 | 5.3/1.1 | 4.8 |
| 150 | 40 | 0.030 | 0.84 | 3.3/0.40 | 4.6/0.90 | 4.8 |
| 150 | 60 | 0.13 | 0.87 | 6.0/0.70 | 4.1/0.81 | 4.6 |
| 160 | 0 | 0.010 | 1.3 | 3.5/0.40 | 5.3/1.1 | 5.1 |
| 160 | 30 | 0.020 | 1.5 | 5.5/0.60 | 5.5/1.2 | 4.4 |
| 160 | 60 | 0.080 | 2.0 | 15/1.7 | 4.5/0.90 | 4.5 |
| 180 | 0 | 0.010 | 0.010 | 5.9/0.70 | 4.1/0.80 | 4.7 |
| 180 | 5 | 0.19 | 0.010 | 14/1.6 | 5.2/1.0 | 4.4 |
| 180 | 10 | 0.34 | 1.3 | 36/4.0 | 5.3/1.1 | 4.4 |
| 180 | 20 | 1.8 | 1.7 | 43/4.8 | 5.3/1.1 | 4.0 |
| 180 | 30 | 2.7 | 1.6 | 29/3.3 | 5.5/1.2 | 4.1 |
| 180 | 40 | 3.5 | 1.8 | 16/1.7 | 4.8/1.0 | 4.1 |
| 200 | 0 | 0.19 | 0.010 | 13/1.4 | 4.1/0.80 | 4.4 |
| 200 | 5 | 1.7 | 1.5 | 26/2.9 | 3.4/0.70 | 4.1 |
| 200 | 10 | 13 | 2.7 | 28/3.1 | 3.7/0.70 | 3.8 |
| 200 | 15 | 8.0 | 1.9 | 17/1.8 | 3.8/0.80 | 3.7 |
| 200 | 40 | 4.5 | 2.5 | 13/1.4 | 4.0/0.80 | 3.8 |
| 200 | 60 | 0.37 | 2.8 | 2.3/0.3 | 2.5/0.50 | 3.5 |
| 220 | 0 | 5.3 | 1.4 | 28/3.1 | 4.9/1.0 | 4.0 |
| 220 | 15 | 0.12 | 2.6 | 0.20/0.00 | 3.0/0.60 | 3.4 |
| 220 | 20 | 0.090 | 1.9 | 0.20/0.00 | 2.9/0.60 | 3.3 |
| 220 | 30 | 0.11 | 1.7 | 0.00/0.00 | 2.8/0.60 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, F.; Aguilar-Garnica, E.; Santiago-Toribio, A.; Sánchez, A. Polysaccharides Release in a Laboratory-Scale Batch Hydrothermal Pretreatment of Wheat Straw under Rigorous Isothermal Operation. Molecules 2022, 27, 26. https://doi.org/10.3390/molecules27010026
Rodríguez F, Aguilar-Garnica E, Santiago-Toribio A, Sánchez A. Polysaccharides Release in a Laboratory-Scale Batch Hydrothermal Pretreatment of Wheat Straw under Rigorous Isothermal Operation. Molecules. 2022; 27(1):26. https://doi.org/10.3390/molecules27010026
Chicago/Turabian StyleRodríguez, Felicia, Efrén Aguilar-Garnica, Adrián Santiago-Toribio, and Arturo Sánchez. 2022. "Polysaccharides Release in a Laboratory-Scale Batch Hydrothermal Pretreatment of Wheat Straw under Rigorous Isothermal Operation" Molecules 27, no. 1: 26. https://doi.org/10.3390/molecules27010026
APA StyleRodríguez, F., Aguilar-Garnica, E., Santiago-Toribio, A., & Sánchez, A. (2022). Polysaccharides Release in a Laboratory-Scale Batch Hydrothermal Pretreatment of Wheat Straw under Rigorous Isothermal Operation. Molecules, 27(1), 26. https://doi.org/10.3390/molecules27010026