Single-Step Hydrothermal Synthesis of Biochar from H3PO4-Activated Lettuce Waste for Efficient Adsorption of Cd(II) in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
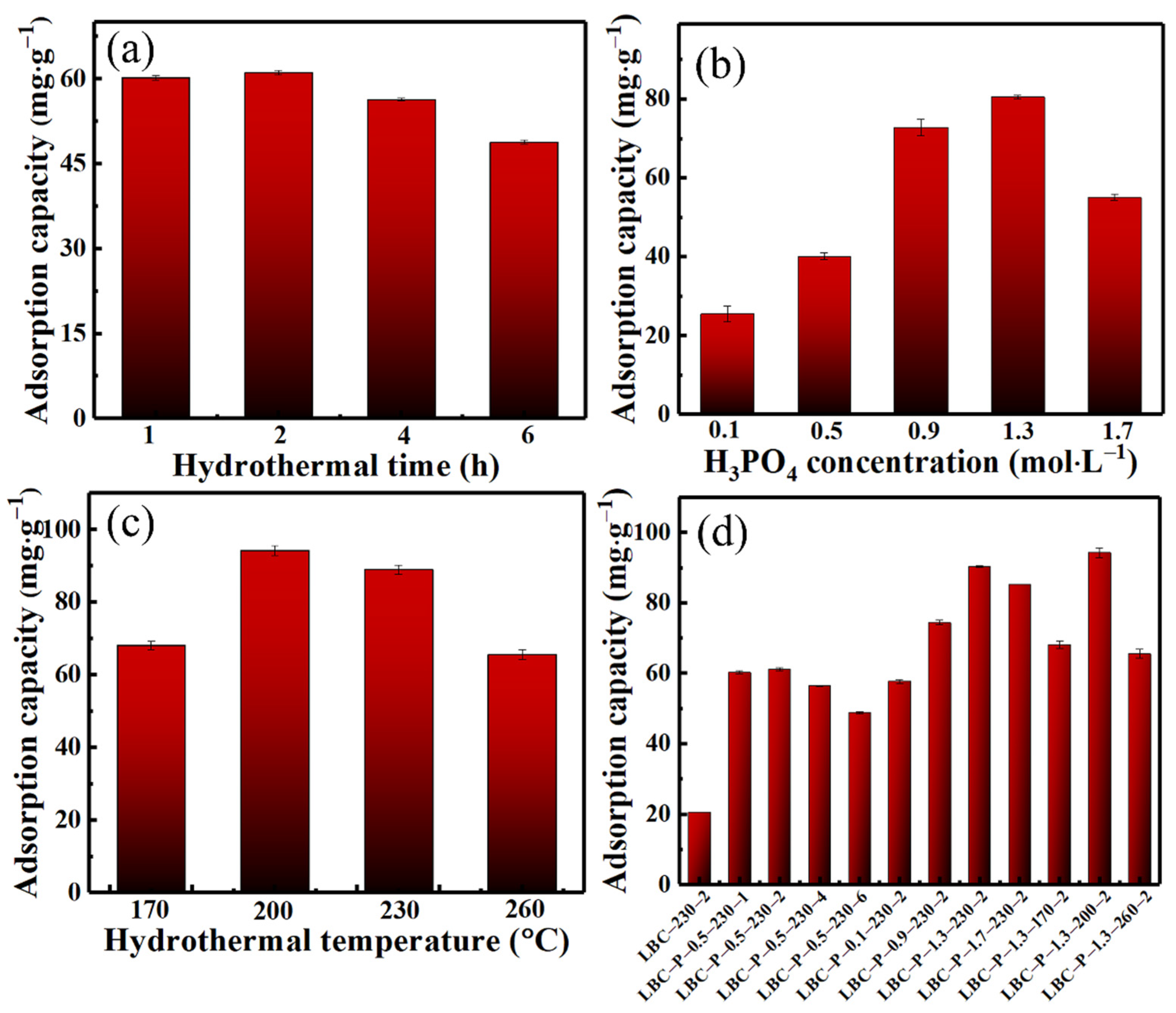
2.1. Determination of Optimal Hydrothermal Conditions of H3PO4-Activated Biochar
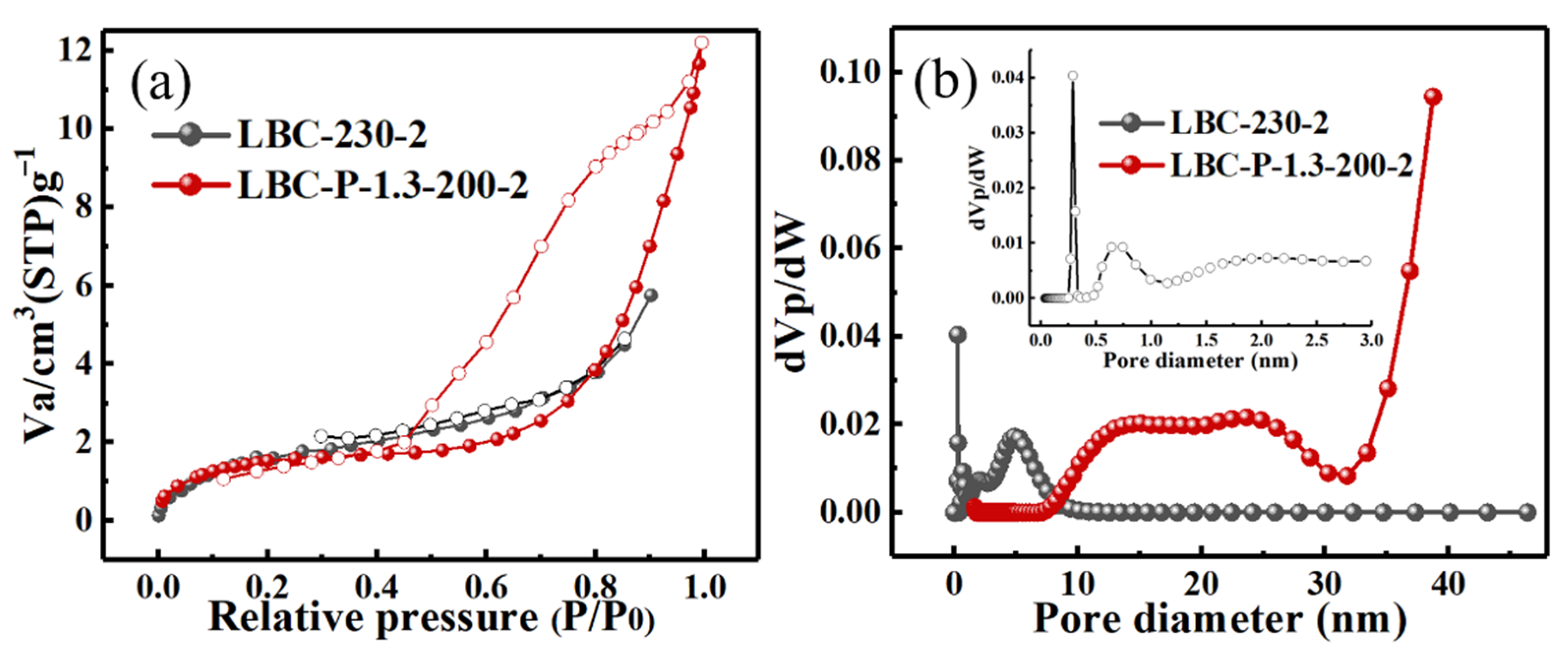
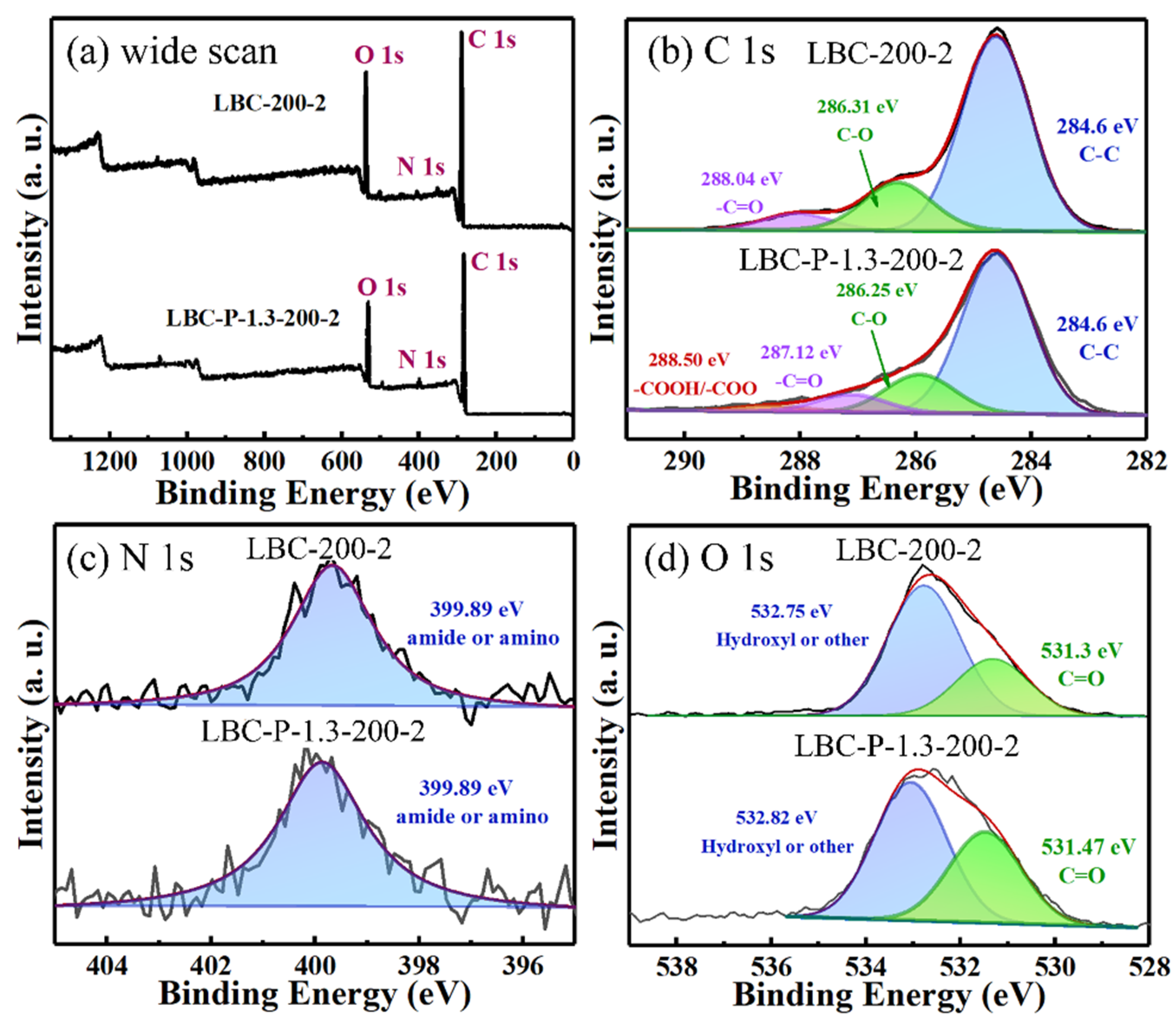
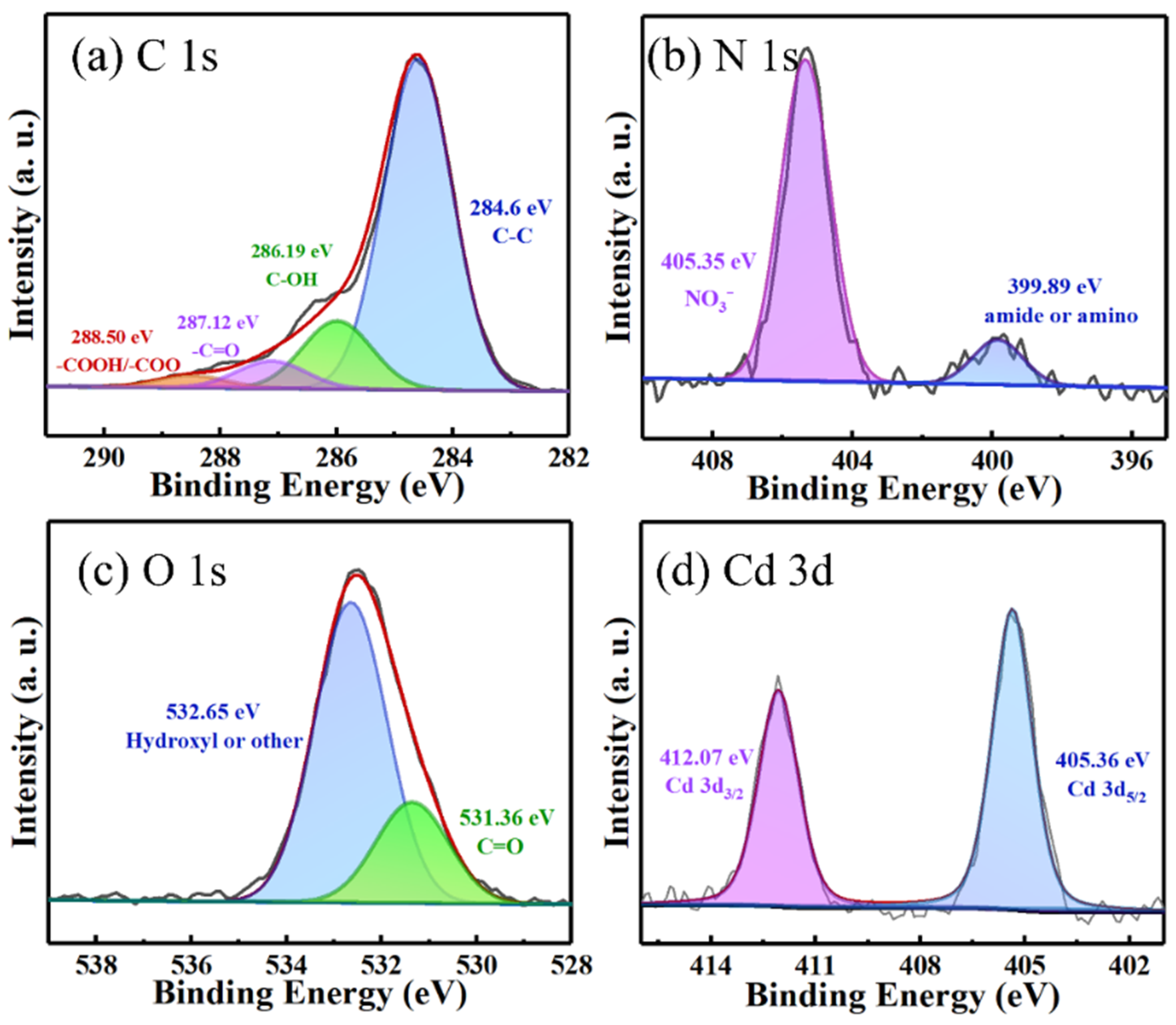
2.2. Characterization of H3PO4-Activated Biochar
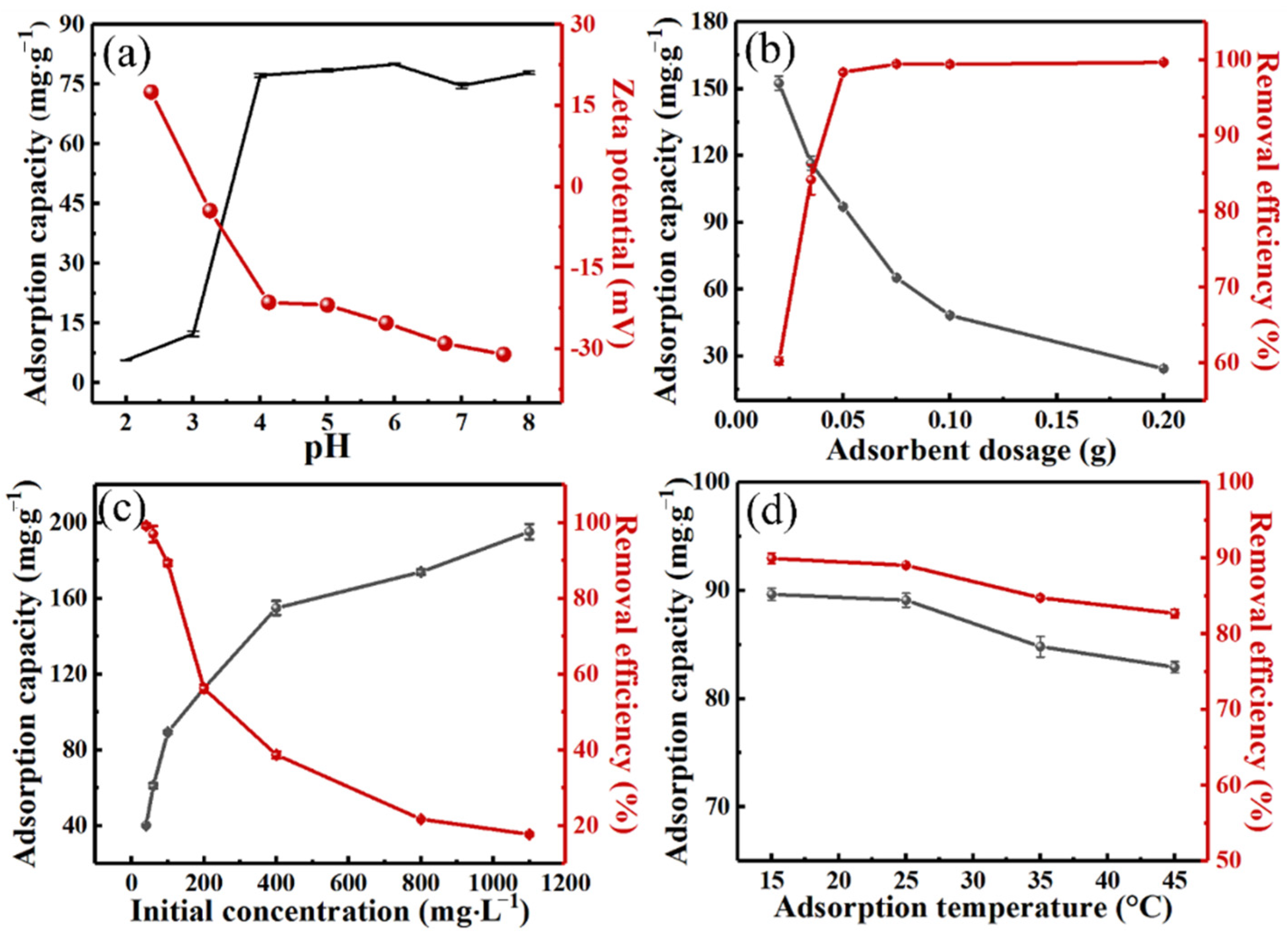
2.3. Effect of Environmental Factors on Adsorption Performance
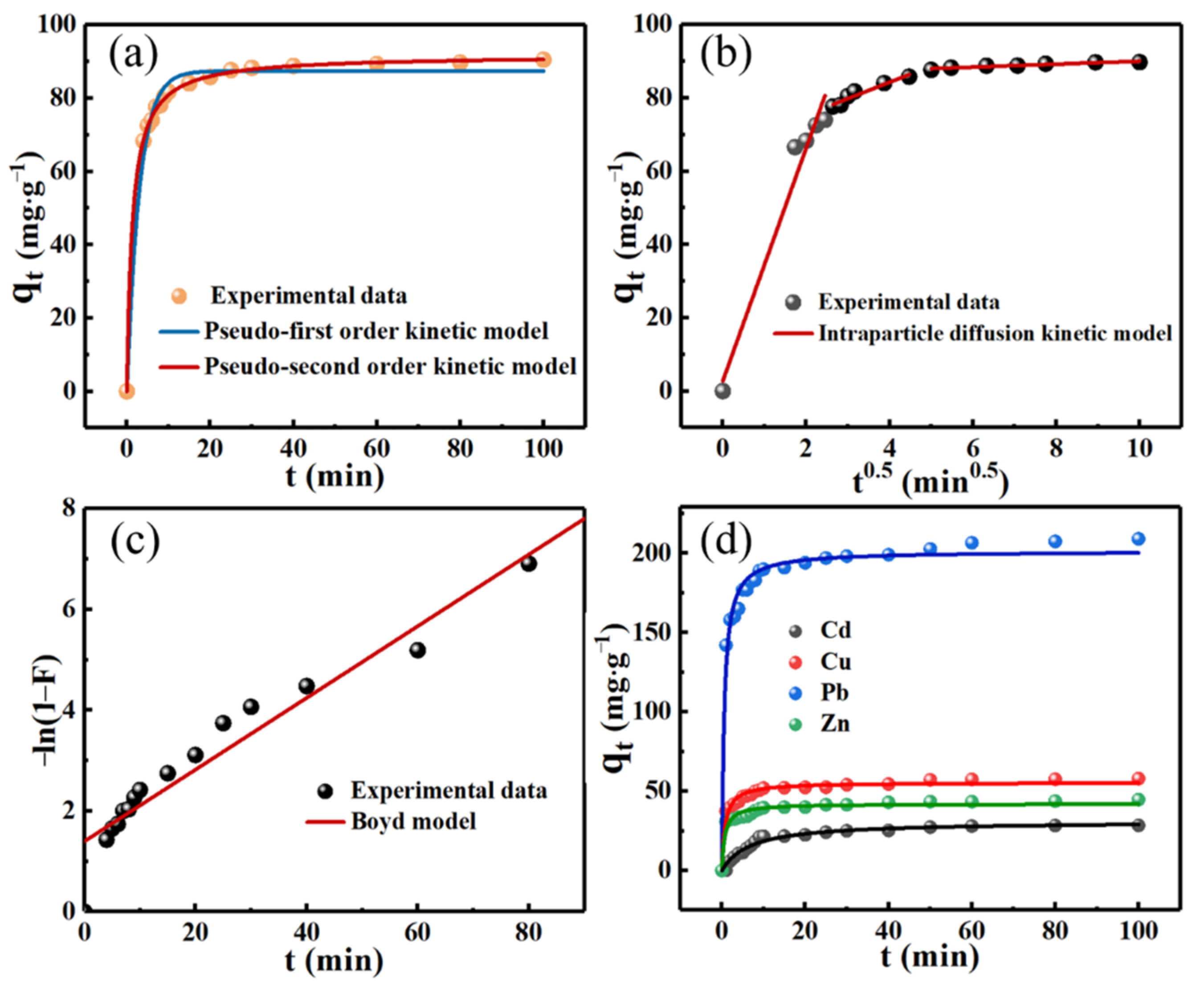
2.4. Adsorption Kinetics
2.5. Adsorption Isotherms
2.6. Adsorption Thermodynamics
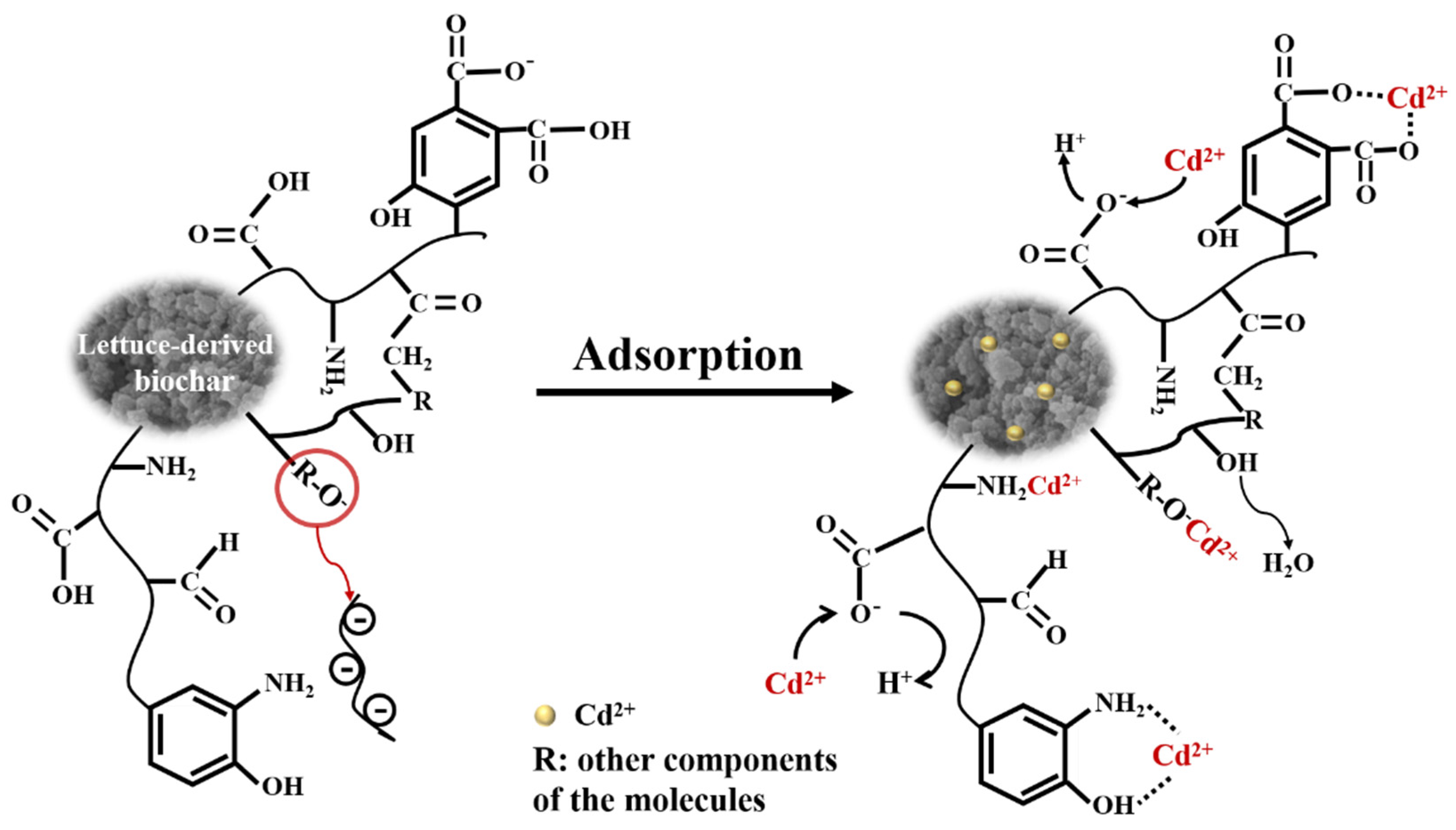
2.7. The Postulated Adsorption Mechanisms
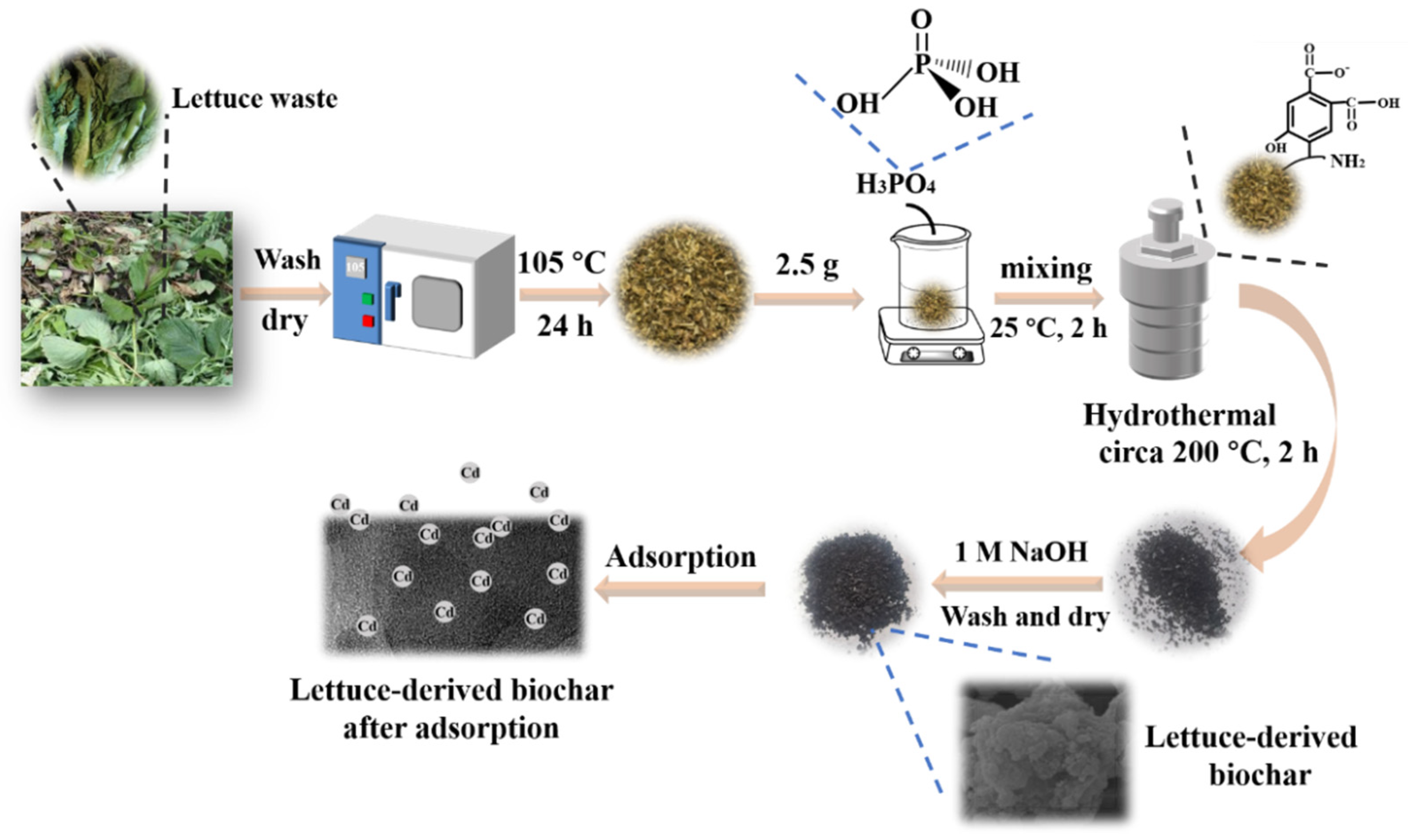
3. Materials and Methods
3.1. Materials
3.2. Hydrothermal Synthesis of Biochar without Activator Addition
3.3. Hydrothermal Synthesis of Biochar with H3PO4 Activating Agent
3.4. Characterization
3.5. Batch Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fu, L.; Yan, Z.; Zhao, Q.; Yang, H. Novel 2D nanosheets with potential applications in heavy metal purification: A review. Adv. Mater. Interfaces 2018, 5, 1801094. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, Z.; Peng, C.; Chai, L.-Y.; Kuroda, K.; Okido, M.; Song, Y.-X. New insights into the interaction between heavy metals and struvite: Struvite as platform for heterogeneous nucleation of heavy metal hydroxide. Chem. Eng. J. 2019, 365, 60–69. [Google Scholar] [CrossRef]
- Xu, X.; Hu, X.; Wang, T.; Sun, M.; Wang, L.; Zhang, L. Non-inverted U-shaped challenges to regional sustainability: The health risk of soil heavy metals in coastal China. J. Clean. Prod. 2021, 279, 123746. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Zhang, T.C.; Xiang, G.; Wang, X.; Pehkonen, S.; Yuan, S. A magnetic γ-Fe2O3@PANI@TiO2 core–shell nanocomposite for arsenic removal via a coupled visible-light-induced photocatalytic oxidation–adsorption process. Nanoscale Adv. 2020, 2, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, D.; Wang, Y.; Zhang, T.C.; Xiang, G.; Zhang, Y.; Yuan, S. Core–shell structured magnetic γ-Fe2O3@PANI nanocomposites for enhanced As(V) adsorption. Ind. Eng. Chem. Res. 2020, 59, 7554–7563. [Google Scholar] [CrossRef]
- Ouyang, L.; Wang, Y.; Zhang, P.; Wang, X.; Yuan, S. Heterostructured MWCNTs@PANI@TiO2 nanocomposites for enhanced adsorption of As(III) from aqueous solution: Adsorption and photocatalytic oxidation behaviors. Ind. Eng. Chem. Res. 2020, 59, 11743–11756. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, J.; Yu, G.; Núñez-Delgado, A.; Kornaros, M. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Li, G.; Gao, B.; Yue, Q.; Li, X. Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresour. Technol. 2016, 217, 239–244. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New notion of biochar: A review on the mechanism of biochar applications in advanced oxidation processes. Chem. Eng. J. 2021, 416. [Google Scholar] [CrossRef]
- Egirani, D.E.; Poyi, N.R.; Shehata, N. Preparation and characterization of powdered and granular activated carbon from Palmae biomass for cadmium removal. Int. J. Environ. Sci. Technol. 2020, 17, 2443–2454. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Amro, A.N.; Abhary, M.K.; Shaikh, M.M.; Ali, S. Removal of lead and cadmium ions from aqueous solution by adsorption on a low-cost phragmites biomass. Processes 2019, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Chen, H.; Feng, Q.; Yao, D.; Chen, H.; Wang, H.; Zhou, Z.; Li, H.; Tian, Y.; Lu, X. Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod. 2017, 165, 221–230. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental advances in biomass autothermal/oxidative pyrolysis: A review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Li, Q.; Faramarzi, A.; Zhang, S.; Wang, Y.; Hu, X.; Gholizadeh, M. Progress in catalytic pyrolysis of municipal solid waste. Energy Convers. Manag. 2020, 226. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K. Catalytic pyrolysis of biomass and polymer wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wang, M.; Jiang, E.; Wang, D.; Zhang, K.; Ren, Y.; Jiang, Y. Pyrolysis of torrefied biomass. Trends Biotechnol. 2018, 36, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Jayaraman, S.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal pre-treatment for mesoporous carbon synthesis: Enhancement of chemical activation. J. Mater. Chem. A 2014, 2, 520–528. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.E.; Ledesma, B.; Roman, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Sanguansri, L.; Fox, E.M.; Cobiac, L.; Cole, M.B. Recovery of wasted fruit and vegetables for improving sustainable diets. Trends Food Sci. Technol. 2020, 95, 75–85. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. Binary doping of nitrogen and phosphorus into porous carbon: A novel difunctional material for enhancing CO2 capture and supercapacitance. J. Mater. Sci. Technol. 2022, 99, 73–81. [Google Scholar] [CrossRef]
- Kumar, S.; Gawande, M.B.; Kopp, J.; Kment, S.; Varma, R.S.; Zbořil, R. P-and F-co-doped carbon nitride nanocatalysts for photocatalytic CO2 reduction and thermocatalytic furanics synthesis from sugars. ChemSusChem 2020, 13, 5231–5238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Zhang, T.C.; Xiang, G.; Wang, X.; Yuan, S. Removal of trace arsenite through simultaneous photocatalytic oxidation and adsorption by magnetic Fe3O4@PpPDA@TiO2 core–shell nanoparticles. ACS Appl. Nano. Mater. 2020, 3, 8495–8504. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Yuan, S. rGO/N-porous carbon composites for enhanced CO2 capture and energy storage performances. J. Alloy. Compd. 2021, 857, 157534. [Google Scholar] [CrossRef]
- Tiryaki, B.; Yagmur, E.; Banford, A.; Aktas, Z. Comparison of activated carbon produced from natural biomass and equivalent chemical compositions. J. Anal. Appl. Pyrolysis 2014, 105, 276–283. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Yuan, S. N,S-containing polycondensate-derived porous carbon materials for superior CO2 adsorption and supercapacitor. Appl. Surf. Sci. 2021, 562, 150128. [Google Scholar] [CrossRef]
- Kan, Y.; Yue, Q.; Liu, S.; Gao, B. Effects of Cu and CuO on the preparation of activated carbon from waste circuit boards by H3PO4 activation. Chem. Eng. J. 2018, 331, 93–101. [Google Scholar] [CrossRef]
- Ewecharoen, A.; Thiravetyan, P.; Nakbanpote, W. Comparison of nickel adsorption from electroplating rinse water by coir pith and modified coir pith. Chem. Eng. J. 2008, 137, 181–188. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhang, T.C.; Xiang, G.; Wang, X.; Yuan, S. One-pot synthesis of a magnetic TiO2/PTh/γ-Fe2O3 heterojunction nanocomposite for removing trace arsenite via simultaneous photocatalytic oxidation and adsorption. Ind. Eng. Chem. Res. 2020, 60, 528–540. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, P.; Yang, Z.; Lv, L.; Tang, S.; Liang, B. Successive grafting of poly (hydroxyethyl methacrylate) brushes and melamine onto chitosan microspheres for effective Cu(II) uptake. Int. J. Biol. Macromol. 2018, 109, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, B.Y.; Tang, X.; Yue, Q.Y.; Zhong, Q.Q.; Li, Q. Characteristics of cellulosic amine-crosslinked copolymer and its sorption properties for Cr(VI) from aqueous solutions. J. Hazard. Mater. 2011, 189, 420–426. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Senthil Kumar, P.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Temkin, M.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physiochim. USSR 1940, 12, 217–222. [Google Scholar]
- Cerofolini, G.; Re, N. The mathematical theory of adsorption on non-ideal surfaces. La Riv. Del Nuovo Cim. Ser. 3 1993, 16, 1–63. [Google Scholar] [CrossRef]
- Langmuir, I. Chemical Reactions at Low Pressures. Iv. The cleanup of nitrogen by a heated molybdenum filament.1. J. Am. Chem. Soc. 1919, 41, 167–194. [Google Scholar] [CrossRef]
- Yadav, S.; Srivastava, V.; Banerjee, S.; Weng, C.-H.; Sharma, Y.C. Adsorption characteristics of modified sand for the removal of hexavalent chromium ions from aqueous solutions: Kinetic, thermodynamic and equilibrium studies. Catena 2013, 100, 120–127. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Mohan, D.; Singh, K.P. Single-and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—an agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Yin, G.; Song, X.; Tao, L.; Sarkar, B.; Sarmah, A.K.; Zhang, W.; Lin, Q.; Xiao, R.; Liu, Q.; Wang, H. Novel Fe-Mn binary oxide-biochar as an adsorbent for removing Cd (II) from aqueous solutions. Chem. Eng. J. 2020, 389, 124465. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Sillanpää, M. Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. Chem. Eng. J. 2013, 223, 40–47. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Kang, Y.; Zhang, J. Biomass-derived carbon sorbents for Cd(II) removal: Activation and adsorption mechanism. ACS Sustain. Chem. Eng. 2017, 5, 4103–4109. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Kula, I.; Uğurlu, M.; Karaoğlu, H.; Çelik, A. Adsorption of Cd(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Bioresour. Technol. 2008, 99, 492–501. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Yu, Z.; Zeng, G.; Luo, Y.; Jiang, L.; Yang, Z.; Qian, Y.; Wu, H. Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]
- Azouaou, N.; Sadaoui, Z.; Djaafri, A.; Mokaddem, H. Adsorption of cadmium from aqueous solution onto untreated coffee grounds: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2010, 184, 126–134. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, J.; Yang, Z.; Tang, S.; Liang, B.; Pehkonen, S.O. Click functionalization of poly (glycidyl methacrylate) microspheres with triazole-4-carboxylic acid for the effective adsorption of Pb(ii) ions. New J. Chem. 2017, 41, 6475–6488. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S.; Zamani, S.; Akbari, S.; Salehi, M. Optimization and modeling of aqueous Cr(VI) adsorption onto activated carbon prepared from sugar beet bagasse agricultural waste by application of response surface methodology. Surf. Interfaces 2020, 18. [Google Scholar] [CrossRef]
- Juang, R.-S.; Chung, J.-Y. Equilibrium sorption of heavy metals and phosphate from single- and binary-sorbate solutions on goethite. J. Colloid Interface Sci. 2004, 275, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.B.; Yuan, S.; Cai, X.; Xiang, G.; Zhang, Y.X.; Pehkonen, S.; Liu, X.Y. Magnetic nickel chrysotile nanotubes tethered with pH-sensitive poly(methacrylic acid) brushes for Cu(II) adsorption. J. Mol. Liq. 2019, 276, 611–623. [Google Scholar] [CrossRef]



| Samples | Models | Model Parameters | ||||
| kf (min−1) | ks (g∙mg−1·min−1) | qmax (mg∙g−1) | R2 | |||
| LBC-P-1.3-200-2 | PFO | 0.331 | − | 87.40 | 0.988 | |
| PSO | − | 0.008 | 91.75 | 0.999 | ||
| Samples | Models | Model Parameters | ||||
| kid (mg·g−1·min−0.5) | Cid (mg∙g−1) | kf (min−1) | R2 | |||
| LBC-P-1.3-200-2 | 1 | 31.76 | 2.808 | − | 0.955 | |
| Weber–Morris | 2 | 4.443 | 66.49 | − | 0.910 | |
| 3 | 0.406 | 85.95 | − | 0.905 | ||
| Boyd | − | − | 0.071 | 0.950 | ||
| Isotherms | Parameters | Temperature (K) | |||
|---|---|---|---|---|---|
| 288.15 | 298.15 | 308.15 | 318.15 | ||
| Langmuir | qm (mg∙g−1) | 171.5 | 173.2 | 142.6 | 115.4 |
| KL (L∙mg−1) | 0.092 | 0.062 | 0.076 | 2.290 | |
| R2 | 0.612 | 0.560 | 0.398 | 0.615 | |
| Freundlich | KF (mg1−1/n∙L1/n∙g−1) | 60.08 | 65.19 | 58.84 | 55.46 |
| 1/n | 0.170 | 0.153 | 0.139 | 0.133 | |
| R2 | 0.976 | 0.945 | 0.929 | 0.920 | |
| Temkin | KT (L∙mg−1) | 77.07 | 633.1 | 452.1 | 246.5 |
| bt | 150.9 | 193.7 | 232.8 | 247.3 | |
| R2 | 0.911 | 0.847 | 0.914 | 0.878 | |
| Sips | qm | 195.9 | 195.8 | 159.1 | 144.3 |
| Ks | 0.199 | 0.028 | 0.038 | 0.094 | |
| nS | 0.082 | 0.008 | 0.009 | 0.023 | |
| R2 | 0.989 | 0.987 | 0.971 | 0.965 | |
| Adsorbents | Heavy Metals | Reaction Temperature (°C) | qm (mg∙g−1) | Ref. | |
|---|---|---|---|---|---|
| Raw Materials | Activating Agents | ||||
| Lettuce waste | − | Cd(II) | 25 | 20.5 | This work |
| Lettuce waste | H3PO4 | Cd(II) | 25 | 198.5 | This work |
| Bagasse pitch | H2SO4 | Cd(II) | 25 | 38.3 | [44] |
| Chicken feather | H3PO4 | Cd(II) | 25 | 7.8 | [45] |
| Pennisetum sp. straw | KMnO4, Fe(NO3)3 | Cd(II) | 25 | 95.2 | [46] |
| Microfibrillated cellulose | Succinic anhydride | Cd(II) | 25 | 236.1 | [47] |
| Phragmites australis | Urea phosphate | Cd(II) | 30 | 40.7 | [48] |
| Apricot stones | H2SO4 | Cd(II) | 25 | 33.6 | [49] |
| Olive stone | ZnCl2 | Cd(II) | 30 | 1.7 | [50] |
| Swine manure | MnO2 | Cd(II) | 25 | 45.8 | [51] |
| Coffee grounds | - | Cd(II) | 20 | 15.7 | [52] |
| C0 (mg∙L−1) | ΔH° (kJ∙mol−1) | ΔS° (J∙mol−1∙K−1) | ΔG° (kJ∙mol−1) | |||
|---|---|---|---|---|---|---|
| 288.15 K | 298.15 K | 308.15 K | 318.15 K | |||
| 100 | −18.88 | −46.97 | −5.35 | −4.88 | −4.41 | −3.94 |
| 200 | −14.93 | −39.44 | −3.57 | −3.17 | −2.78 | −2.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhang, T.C.; Ouyang, L.; Yuan, S. Single-Step Hydrothermal Synthesis of Biochar from H3PO4-Activated Lettuce Waste for Efficient Adsorption of Cd(II) in Aqueous Solution. Molecules 2022, 27, 269. https://doi.org/10.3390/molecules27010269
Chen Q, Zhang TC, Ouyang L, Yuan S. Single-Step Hydrothermal Synthesis of Biochar from H3PO4-Activated Lettuce Waste for Efficient Adsorption of Cd(II) in Aqueous Solution. Molecules. 2022; 27(1):269. https://doi.org/10.3390/molecules27010269
Chicago/Turabian StyleChen, Quyun, Tian C. Zhang, Like Ouyang, and Shaojun Yuan. 2022. "Single-Step Hydrothermal Synthesis of Biochar from H3PO4-Activated Lettuce Waste for Efficient Adsorption of Cd(II) in Aqueous Solution" Molecules 27, no. 1: 269. https://doi.org/10.3390/molecules27010269








