Electrochemical Signal Substance for Multiplexed Immunosensing Interface Construction: A Mini Review
Abstract
:1. Introduction
2. Electrochemical Signal Substances for Multiplexed Immunoassay
2.1. Organic Molecules
2.2. Metal Ions
2.3. Metal Nanoparticles
2.4. Prussian Blue
2.5. Other Substance for Electrochemical Signal Generation
3. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ab1 | Coating antibody |
| Ab2 | Labeling antibodies |
| AFP α | α-fetoprotein |
| ALP | Alkaline phosphatase |
| BSA | Bovine Serum Albumin |
| CA | Carbohydrate antigen |
| CEA | Carcinoembryonic antigen |
| CGS-MB | Carboxyl graphene nanosheets-methylene blue |
| CGS-PB | Carboxyl graphene nanosheets-Prussian Blue |
| CNTs | Carbon nanotubes |
| CP | Chitosan-poly(acrylic acid) nanospheres |
| CYFRA 21-1 | Cytokeratin 19 fragments |
| DPV | Differential Pulse Voltammetry |
| GA | Glutaraldehyde |
| GCE | Glass carbon electrode |
| GOD | Glucose oxidase |
| GR | Graphene |
| GS | Graphene nanosheets |
| HCG | Human chorionic gonadotropin |
| IL-8 | Interleukin-8 |
| LSV | Linear-sweep stripping voltammetric |
| MB | Methylene blue |
| NPs | Nanoparticles |
| NSE | Neuron specific enolase |
| PDDA | Poly(diallyldimethylammonium chloride) |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PSA | prostate specific antigen |
| PTCA | 3,4,9,10-perylenetetracarboxylic acid |
| Pt@SBA-15 | Platinum nanoparticle-functionalized mesoporous silica |
| TB | Toluidine blue |
| THI | Thionine |
| SA | Sodium alginate |
| SPCEs | Screen-printed carbon electrodes |
| SWV | Square wave voltammograms |
References
- Pashayan, N.; Pharoah, P. The challenge of early detection in cancer. Science 2020, 368, 589–590. [Google Scholar] [CrossRef]
- Kalinich, M.; Haber, D.A. Cancer detection: Seeking signals in blood. Science 2018, 359, 866–867. [Google Scholar] [CrossRef]
- Brentnall, T.A. Progress in the Earlier Detection of Pancreatic Cancer. J. Clin. Oncol. 2016, 34, 1973–1974. [Google Scholar] [CrossRef]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X.Y. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [Green Version]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skladal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Munge, B.; Patel, V.; Jensen, G.; Bhirde, A.; Gong, J.D.; Kim, S.N.; Gillespie, J.; Gutkind, J.S.; Papadimitrakopoulos, F.; et al. Carbon nanotube amplification strategies for highly sensitive immunodetection of cancer biomarkers. J. Am. Chem. Soc. 2006, 128, 11199–11205. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Li, X.; An, S.; Zheng, D.; Pei, S.; Zheng, X.; Liu, Y.; Yao, Q.; Yang, M.; Dai, L. Highly sensitive and selective electrochemical immunosensors by substrate-enhanced electroless deposition of metal nanoparticles onto three-dimensional graphene@Ni foams. Sci. Bull. 2019, 64, 1272–1279. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Song, S.-Y.; Pretlow, T.G.; Abdul-Karim, F.W.; Kung, H.-J.; Dawson, D.V.; Park, W.-S.; Moon, Y.-W.; Tsai, M.-L.; Linehan, W.-M.; et al. Evidence of independent origin of multiple tumors from patients with prostate cancer. J. Natl. Cancer Inst. 1998, 90, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Che, G. A different method in diagnosis of Multiple Primary Lung Cancer. J. Thorac. Oncol. 2016, 11, 53–54. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yin, K.; Liu, S.Y.; Yan, L.X.; Su, J.; Wu, Y.L.; Zhang, X.C.; Zhong, W.Z.; Yang, X.N. Multiomics analysis reveals a distinct response mechanism in multiple primary lung adenocarcinoma after neoadjuvant immunotherapy. J. Immunother. Cancer 2021, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Han, H.; Ma, Z. Improved screen-printed carbon electrode for multiplexed label-free amperometric immuniosensor: Addressing its conductivity and reproducibility challenges. Biosens. Bioelectron. 2018, 101, 304–310. [Google Scholar] [CrossRef]
- Guo, W.; Ding, H.; Gu, C.; Liu, Y.; Jiang, X.; Su, B.; Shao, Y. Potential-resolved multicolor electrochemiluminescence for multiplex immunoassay in a single sample. J. Am. Chem. Soc. 2018, 140, 15904–15915. [Google Scholar] [CrossRef]
- Yang, S.M.; Lin, Q.; Zhang, H.; Yin, R.; Zhang, W.; Zhang, M.; Cui, Y. Dielectrophoresis assisted high-throughput detection system for multiplexed immunoassays. Biosens. Bioelectron. 2021, 180, 113148. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, T.; Guo, W.J.; Bai, Y.Y.; Pang, D.W.; Zhang, Z.L. Digital single virus immunoassay for ultrasensitive multiplex avian influenza virus detection based on fluorescent magnetic multifunctional nanospheres. ACS Appl. Mater. Interfaces 2019, 11, 5762–5770. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, J.; Zou, Y.; Gong, M.; Chen, H.; Hong, G.; Antaris, A.L.; Li, X.; Liu, C.-L.; Chen, C.; et al. Plasmonic micro-beads for fluorescence enhanced, multiplexed protein detection with flow cytometry. Chem. Sci. 2014, 5, 4070–4075. [Google Scholar] [CrossRef]
- Xu, S.; Feng, X.; Gao, T.; Liu, G.; Mao, Y.; Lin, J.; Yu, X.; Luo, X. Aptamer induced multicoloured Au NCs-MoS2 “switch on” fluorescence resonance energy transfer biosensor for dual color simultaneous detection of multiple tumor markers by single wavelength excitation. Anal. Chim. Acta 2017, 983, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Ge, S.; Yan, M.; Wang, S.; Yu, J.; Li, N.; Song, X. Multiplexed sandwich immunoassays using flow-injection electrochemiluminescence with designed substrate spatial-resolved technique for detection of tumor markers. Biosens. Bioelectron. 2013, 41, 684–690. [Google Scholar] [CrossRef]
- Lv, W.; Ye, H.; Yuan, Z.; Liu, X.; Chen, X.; Yang, W. Recent advances in electrochemiluminescence-based simultaneous detection of multiple targets. Trends Anal. Chem. 2020, 123, 115767. [Google Scholar] [CrossRef]
- Zhao, J.W.; Du, J.W.; Luo, J.H.; Chen, S.H.; Yuan, R. A novel potential-resolved electrochemiluminescence immunosensor for the simultaneous determination of brain natriuretic peptide and cardiac troponin I. Sens. Actuators B Chem. 2020, 311, 127934. [Google Scholar] [CrossRef]
- Dzgoev, A.; Mecklenburg, M.; Xie, B.; Miyabayashib, A.; Larsson, P.-O.; Danielsson, B. Optimization of a charge coupled device imaging enzyme linked immuno sorbent assay and supports for the simultaneous determination of multiple 2,4-D samples. Anal. Chim. Acta 1997, 347, 87–93. [Google Scholar] [CrossRef]
- Gao, L.; Yang, Q.; Wu, P.; Li, F. Recent advances in nanomaterial-enhanced enzyme-linked immunosorbent assays. Analyst 2020, 145, 4069–4078. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Wu, D.; Li, T.; Yin, Y.; Li, G. Improvement of enzyme-linked immunosorbent assay for the multicolor detection of biomarkers. Chem. Sci. 2016, 7, 3011–3016. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, J.; Zhao, X.; Chen, H.; Xu, H.; Bai, L.; Wang, W.; Yang, H.; Wei, D.; Yuan, B. Highly sensitive electrochemical immunosensor for the simultaneous detection of multiple tumor markers for signal amplification. Talanta 2021, 226, 122133. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; He, L.; Feng, Y.; Tan, H.; Chen, X.; Yang, W. Simultaneous detection of multiple neuroendocrine tumor markers in patient serum with an ultrasensitive and antifouling electrochemical immunosensor. Biosens. Bioelectron. 2021, 194, 113603. [Google Scholar] [CrossRef]
- Qi, H.; Ling, C.; Ma, Q.; Gao, Q.; Zhang, C. Sensitive electrochemical immunosensor array for the simultaneous detection of multiple tumor markers. Analyst 2012, 137, 393–399. [Google Scholar] [CrossRef]
- Monteleone, M.; Naccarato, A.; Sindona, G.; Tagarelli, A. A reliable and simple method for the assay of neuroendocrine tumor markers in human urine by solid-phase microextraction-gas chromatography-triple quadrupole mass spectrometry. Anal. Chim. Acta 2013, 759, 66–73. [Google Scholar] [CrossRef]
- Giesen, C.; Mairinger, T.; Khoury, L.; Waentig, L.; Jakubowski, N.; Panne, U. Multiplexed immunohistochemical detection of tumor markers in breast cancer tissue using laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 8177–8183. [Google Scholar] [CrossRef]
- Cao, X.; Han, Y.; Gao, C.; Huang, X.; Xu, Y.; Wang, N. PtAg nanowires: Facile synthesis and their applications as excellent oxygen reduction electrocatalysts for label-free electrochemical immunoassay. J. Mater. Chem. A 2013, 1, 14904–14909. [Google Scholar] [CrossRef]
- Cui, R.; Liu, C.; Shen, J.; Gao, D.; Zhu, J.-J.; Chen, H.-Y. Gold nanoparticle-colloidal carbon nanosphere hybrid material: Preparation, characterization, and application for an amplified electrochemical immunoassay. Adv. Funct. Mater. 2008, 18, 2197–2204. [Google Scholar] [CrossRef]
- Lu, D.; Lu, F.; Pang, G. A novel tetrahydrocannabinol electrochemical nano immunosensor based on horseradish peroxidase and double-layer gold nanoparticles. Molecules 2016, 21, 1377. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Jia, L.; Li, B.; Situ, B.; Liu, Q.; Wang, Q.; Gan, N. A sandwich HIV p24 amperometric immunosensor based on a direct gold electroplating-modified electrode. Molecules 2012, 17, 5988–6000. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.L.; Han, J.M.; Ma, J.; Ma, Z.F. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens. Bioelectron. 2013, 50, 356–361. [Google Scholar] [CrossRef]
- Shan, J.; Wang, L.Y.; Ma, Z.F. Novel metal-organic nanocomposites: Poly(methylene blue)-Au and its application for an ultrasensitive electrochemical immunosensing platform. Sens. Actuators B Chem. 2016, 237, 666–671. [Google Scholar] [CrossRef]
- Gao, Q.; Han, J.M.; Ma, Z.F. Polyamidoamine dendrimers-capped carbon dots/Au nanocrystal nanocomposites and its application for electrochemical immunosensor. Biosens. Bioelectron. 2013, 49, 323–328. [Google Scholar] [CrossRef]
- Li, W.X.; Ma, Z.F. Conductive catalytic redox hydrogel composed of aniline and vinyl-ferrocene for ultrasensitive detection of prostate specific antigen. Sens. Actuators B Chem. 2017, 248, 545–550. [Google Scholar] [CrossRef]
- Liu, Z.M.; Ma, Z.F. Fabrication of an ultrasensitive electrochemical immunosensor for CEA based on conducting long-chain polythiols. Biosens. Bioelectron. 2013, 46, 1–7. [Google Scholar] [CrossRef]
- Feng, J.J.; Chu, C.S.; Ma, Z.F. Fenton and Fenton-like catalysts for electrochemical immunoassay: A mini review. Electrochem. Commun. 2021, 125, 106970. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, Z.F. Dual-reaction triggered sensitivity amplification for ultrasensitive peptidecleavage based electrochemical detection of matrix metalloproteinase-7. Biosens. Bioelectron. 2018, 108, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.T.; Ma, Z.F. Amperometric glucose biosensor based on a triangular silver nanoprisms/chitosan composite film as immobilization matrix. Biosens. Bioelectron. 2010, 26, 1098–1103. [Google Scholar] [CrossRef]
- Wang, C.G.; Chen, Y.; Wang, T.T.; Ma, Z.F.; Su, Z.M. Biorecognition-driven self-assembly of gold nanorods: A rapid and sensitive approach toward antibody sensing. Chem. Mater. 2007, 19, 5809–5811. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhuo, Y.; Yuan, R.; Chai, Y.Q. An amplified electrochemical immunosensor based on in situ-produced 1-naphthol as electroactive substance and graphene oxide and Pt nanoparticles functionalized CeO2 nanocomposites as signal enhancer. Biosens. Bioelectron. 2015, 69, 321–327. [Google Scholar] [CrossRef]
- Zhang, N.N.; Xu, Y.; Ma, Z.F. Signal amplification based on tannic acid-assisted cyclic conversion of Fe(III)/Fe(II) for ultrasensitive electrochemical immunoassay of CA 12-5. Sens. Actuators B Chem. 2020, 317, 128244. [Google Scholar] [CrossRef]
- Wang, H.Q.; Ma, Z.F. Simultaneous detection of multiple tumor markers by label-free electrochemical immunoassay using chip-like glass carbon electrodes. Sens. Actuators B Chem. 2018, 256, 402–407. [Google Scholar] [CrossRef]
- Shan, J.; Ma, Z.F. Simultaneous detection of five biomarkers of lung cancer by electrochemical immunoassay. Microchim. Acta 2016, 183, 2889–2897. [Google Scholar] [CrossRef]
- Liang, X.Y.; Zhao, J.C.; Ma, Z.F. Improved binding induced self-assembled DNA to achieve ultrasensitive electrochemical proximity assay. Sens. Actuators B Chem. 2020, 304, 127278. [Google Scholar] [CrossRef]
- Qu, L.; Yang, L.; Li, Y.; Ren, X.; Wang, H.; Fan, D.; Wang, X.; Wei, Q.; Ju, H. Dual-signaling electrochemical ratiometric method for competitive immunoassay of CYFRA 21-1 Based on Urchin-like Fe3O4@PDA-Ag and Ni3Si2O5(OH)4-Au Absorbed Methylene Blue Nanotubes. ACS Appl. Mater. Interfaces 2021, 13, 5795–5802. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Wang, B.; Huang, J.; Qileng, A.; He, Z.; Lei, H.; Liu, W.; Liu, Y. Multiple amplified enzyme-free electrochemical immunosensor based on G-quadruplex/hemin functionalized mesoporous silica with redox-active intercalators for microcystin-LR detection. Biosens. Bioelectron. 2017, 98, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.J.; Yao, T.; Chu, C.S.; Ma, Z.F.; Han, H.L. Proton-responsive annunciator based on i-motif DNA structure modified metal organic frameworks for ameliorative construction of electrochemical immunosensing interface. J. Colloid Interface Sci. 2021, 608, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Biniaz, Z.; Mostafavi, A.; Shamspur, T.; Torkzadeh-Mahani, M.; Mohamadi, M. Electrochemical sandwich immunoassay for the prostate specific antigen using a polyclonal antibody conjugated to thionine and horseradish peroxidase. Microchim. Acta 2017, 184, 2731–2738. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, G.; Zhang, Y.; Zhu, M.; Wan, Y.; Shen, Y. Highly selective and sensitive electrochemical immunoassay of Cry1C using nanobody and pi-pi stacked graphene oxide/thionine assembly. Anal. Chem. 2016, 88, 9830–9836. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Zhang, Y.; Shen, G.; Xiang, H.; Long, X. A label-free electrochemical immunosensor based on a new polymer containing aldehyde and ferrocene groups. Talanta 2017, 164, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Rani, C.; Anand, A.V.; Ho, J.A. Disposable electrochemical immunosensor for carcinoembryonic antigen using ferrocene liposomes and MWCNT screen-printed electrode. Biosens. Bioelectron. 2009, 24, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Xu, B.Y.; Du, Y.; Xu, J.J.; Chen, H.Y. A branched electrode based electrochemical platform: Towards new label-free and reagentless simultaneous detection of two biomarkers. Chem. Commun. 2013, 49, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Salimi, A.; Hallaj, R. Magnetoimmunosensor for simultaneous electrochemical detection of carcinoembryonic antigen and α-fetoprotein using multifunctionalized Au nanotags. J. Electroanal. Chem. 2018, 811, 8–15. [Google Scholar] [CrossRef]
- Zhu, Q.; Chai, Y.; Yuan, R.; Zhuo, Y. Simultaneous detection of four biomarkers with one sensing surface based on redox probe tagging strategy. Anal. Chim. Acta 2013, 800, 22–28. [Google Scholar] [CrossRef]
- Wang, Z.F.; Liu, N.; Ma, Z.F. Platinum porous nanoparticles hybrid with metal ions as probes for simultaneous detection of multiplex cancer biomarkers. Biosens. Bioelectron. 2014, 53, 324–329. [Google Scholar] [CrossRef]
- Xu, T.; Jia, X.L.; Chen, X.; Ma, Z.F. Simultaneous electrochemical detection of multiple tumor markers using metal ions tagged immunocolloidal gold. Biosens. Bioelectron. 2014, 56, 174–179. [Google Scholar] [CrossRef]
- Li, L.; Wei, Y.; Zhang, S.; Chen, X.; Shao, T.; Feng, D. Electrochemical immunosensor based on metal ions functionalized CNSs@Au NPs nanocomposites as signal amplifier for simultaneous detection of triple tumor markers. J. Electroanal. Chem. 2021, 880, 114882. [Google Scholar] [CrossRef]
- Tang, Z.X.; Ma, Z.F. Ratiometric ultrasensitive electrochemical immunosensor based on redox substrate and immunoprobe. Sci. Rep. 2016, 6, 35440. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.F.; Liu, N. Design of immunoprobes for electrochemical multiplexed tumor marker detection. Expert Rev. Mol. Diagn. 2015, 15, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.H.; Yin, S.; Ma, Z.F. Ca2+-triggered pH-response sodium alginate hydrogel precipitation for amplified sandwich-type impedimetric immunosensor of tumor marker. ACS Sens. 2019, 4, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.F.; Feng, F.; Ma, Z.F. Metal ions doped chitosan-poly(acrylic acid) nanospheres: Synthesis and their application in simultaneously electrochemical detection of four markers of pancreatic cancer. Biosens. Bioelectron. 2016, 75, 148–154. [Google Scholar] [CrossRef]
- Putnin, T.; Ngamaroonchote, A.; Wiriyakun, N.; Ounnunkad, K.; Laocharoensuk, R. Dually functional polyethylenimine-coated gold nanoparticles: A versatile material for electrode modification and highly sensitive simultaneous determination of four tumor markers. Microchim. Acta 2019, 186, 305. [Google Scholar] [CrossRef]
- Siangproh, W.; Dungchai, W.; Rattanarat, P.; Chailapakul, O. Nanoparticle-based electrochemical detection in conventional and miniaturized systems and their bioanalytical applications: A review. Anal. Chim. Acta 2011, 690, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Brasiunas, B.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. Metal nanoparticle and quantum dot tags for signal amplification in electrochemical immunosensors for biomarker detection. Chemosensors 2021, 9, 85. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, Y.; He, P. DNA biosensors based on metal nanoparticles. J. Biomed. Nanotechnol. 2005, 1, 276–285. [Google Scholar] [CrossRef]
- Liu, L.; Chang, Y.; Xia, N.; Peng, P.; Zhang, L.; Jiang, M.; Zhang, J.; Liu, L. Simple, sensitive and label-free electrochemical detection of microRNAs based on the in situ formation of silver nanoparticles aggregates for signal amplification. Biosens. Bioelectron. 2017, 94, 235–242. [Google Scholar] [CrossRef]
- Xia, N.; Cheng, C.; Liu, L.; Peng, P.; Liu, C.; Chen, J. Electrochemical glycoprotein aptasensors based on the in-situ aggregation of silver nanoparticles induced by 4-mercaptophenyl boronic acid. Microchim. Acta 2017, 184, 4393–4400. [Google Scholar] [CrossRef]
- Liu, X.; Tseng, C.-L.; Lin, L.-Y.; Lee, C.-A.; Li, J.; Feng, L.; Song, L.; Li, X.; He, J.-H.; Sakthivel, R.; et al. Template-free synthesis of mesoporous Ce3NbO7/CeO2 hollow nanospheres for label-free electrochemical immunosensing of leptin. Sens. Actuators B Chem. 2021, 341, 130005. [Google Scholar] [CrossRef]
- Ge, S.; Yu, F.; Ge, L.; Yan, M.; Yu, J.; Chen, D. Disposable electrochemical immunosensor for simultaneous assay of a panel of breast cancer tumor markers. Analyst 2012, 137, 4727–4733. [Google Scholar] [CrossRef]
- Lai, G.; Wang, L.; Wu, J.; Ju, H.; Yan, F. Electrochemical stripping analysis of nanogold label-induced silver deposition for ultrasensitive multiplexed detection of tumor markers. Anal. Chim. Acta 2012, 721, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.K.; Woi, P.M. Current innovations of metal hexacyanoferrates-based nanocomposites toward electrochemical sensing: Materials selection and synthesis methods. Crit. Rev. Anal. Chem. 2020, 50, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Song, S.; Wang, D.; Zhang, H. Prussian blue analogs and their derived nanomaterials for electrochemical energy storage and electrocatalysis. Small Methods 2021, 5, 2001000. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimisation and applications of Prussian blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef]
- Dos Santos, P.L.; Katic, V.; Toledo, K.C.F.; Bonacin, J.A. Photochemical one-pot synthesis of reduced graphene oxide/Prussian blue nanocomposite for simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid. Sens. Actuators B Chem. 2018, 255, 2437–2447. [Google Scholar] [CrossRef]
- Sgobbi, L.F.; Razzino, C.A.; Machado, S.A.S. A disposable electrochemical sensor for simultaneous detection of sulfamethoxazole and trimethoprim antibiotics in urine based on multiwalled nanotubes decorated with Prussian blue nanocubes modified screen-printed electrode. Electrochim. Acta 2016, 191, 1010–1017. [Google Scholar] [CrossRef]
- Buleandra, M.; Rabinca, A.A.; Mihailciuc, C.; Balan, A.; Nichita, C.; Stamatin, I.; Ciucu, A.A. Screen-printed Prussian blue modified electrode for simultaneous detection of hydroquinone and catechol. Sens. Actuators B Chem. 2014, 203, 824–832. [Google Scholar] [CrossRef]
- Soleh, A.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Novel electrochemical sensor using a dual-working electrode system for the simultaneous determination of glucose, uric acid and dopamine. Microchem. J. 2020, 153, 104379. [Google Scholar] [CrossRef]
- Jia, X.L.; Liu, Z.M.; Liu, N.; Ma, Z.F. A label-free immunosensor based on graphene nanocomposites for simultaneous multiplexed electrochemical determination of tumor markers. Biosens. Bioelectron. 2014, 53, 160–166. [Google Scholar] [CrossRef]
- Habibi, M.M.; Mirhosseini, S.A.; Sajjadi, S.; Keihan, A.H. A novel label-free electrochemical immunesensor for ultrasensitive detection of LT toxin using prussian blue@gold nanoparticles composite as a signal amplification. Bioelectrochemistry 2021, 142, 107887. [Google Scholar] [CrossRef]
- Lai, G.S.; Yan, F.; Ju, H.X. Dual signal amplification of glucose oxidase-functionalized nanocomposites as a trace label for ultrasensitive simultaneous multiplexed electrochemical detection of tumor markers. Anal. Chem. 2009, 81, 9730–9736. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Han, X.; Hu, H.; Chang, M.; Ding, L.; Xiang, H.; Chen, Y.; Li, Y. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nat. Commun. 2021, 12, 2203. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Chen, Y.; Guo, L.; Wei, G. Biomimetic two-dimensional nanozymes: Synthesis, hybridization, functional tailoring, and biosensor applications. J. Mater. Chem. B 2020, 8, 10065–10086. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, X.; Ma, C.; Zhang, L.; Zhao, C.; Chen, S.; Li, K.; Zhang, M.; Mei, L.; Qi, Y.; et al. Enzyme-free sandwich-type electrochemical immunosensor for CEA detection based on the cooperation of an Ag/g-C3N4-modified electrode and Au@SiO2/Cu2O with core-shell structure. Bioelectrochemistry 2021, 142, 107931. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Deng, W.; Su, Y.; Zhu, X.; Peng, C.; Hu, H.; Peng, H.; Song, S.; Fan, C. Carbon nanotube-based ultrasensitive multiplexing electrochemical immunosensor for cancer biomarkers. Biosens. Bioelectron. 2011, 30, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Mao, K.; Li, H.; Wu, D.; Zhang, Y.; Du, B.; Wei, Q. Ultrasensitive multiplexed immunosensors for the simultaneous determination of endocrine disrupting compounds using Pt@SBA-15 as a non-enzymatic label. J. Mater. Chem. B 2013, 1, 5137–5142. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, L.; Zhang, Y.; Yang, H.; Ma, C.; Ge, S.; Yan, M.; Yu, J.; Song, X. Multiplexed enzyme-free electrochemical immunosensor based on ZnO nanorods modified reduced graphene oxide-paper electrode and silver deposition-induced signal amplification strategy. Biosens. Bioelectron. 2015, 71, 30–36. [Google Scholar] [CrossRef]

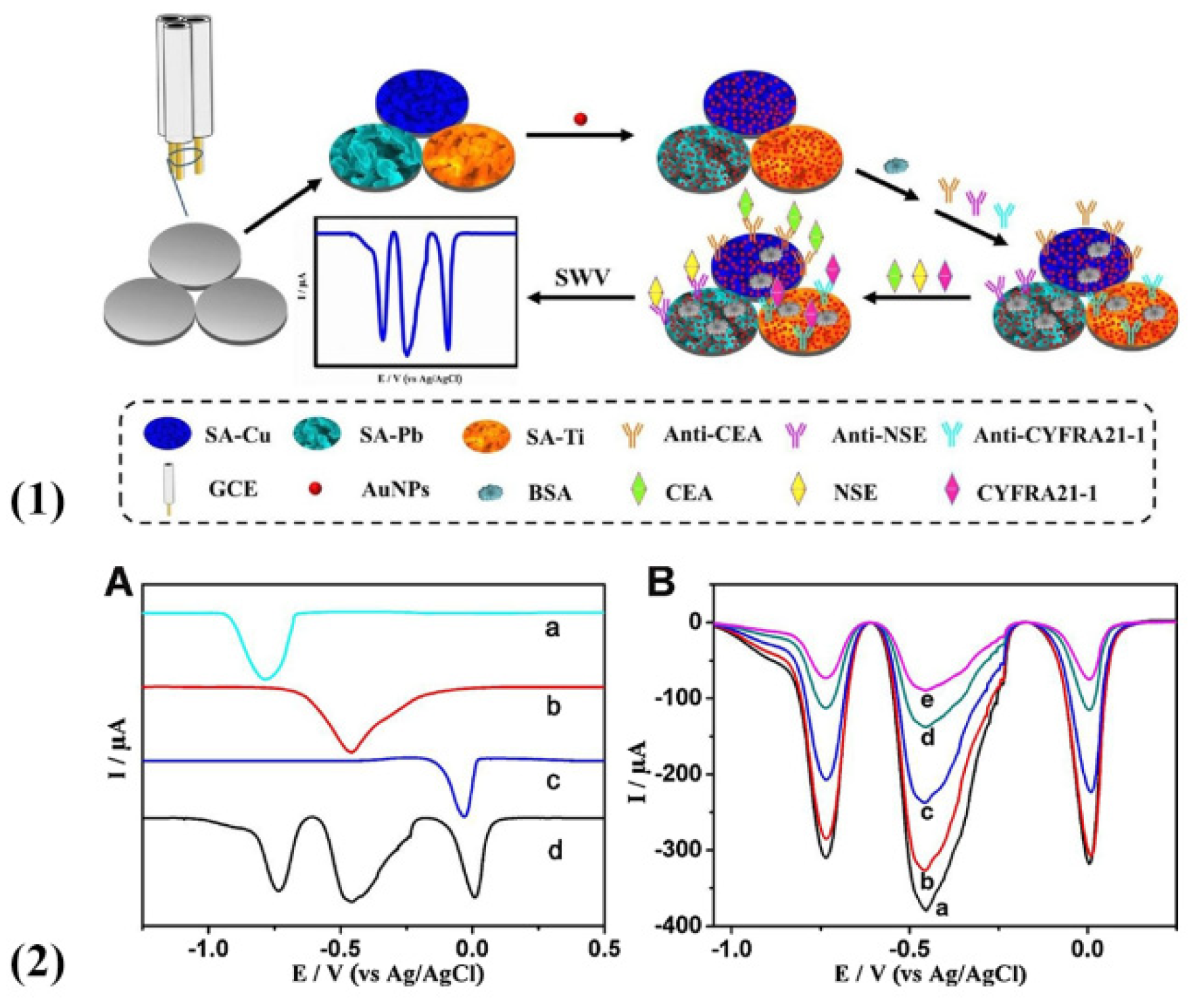
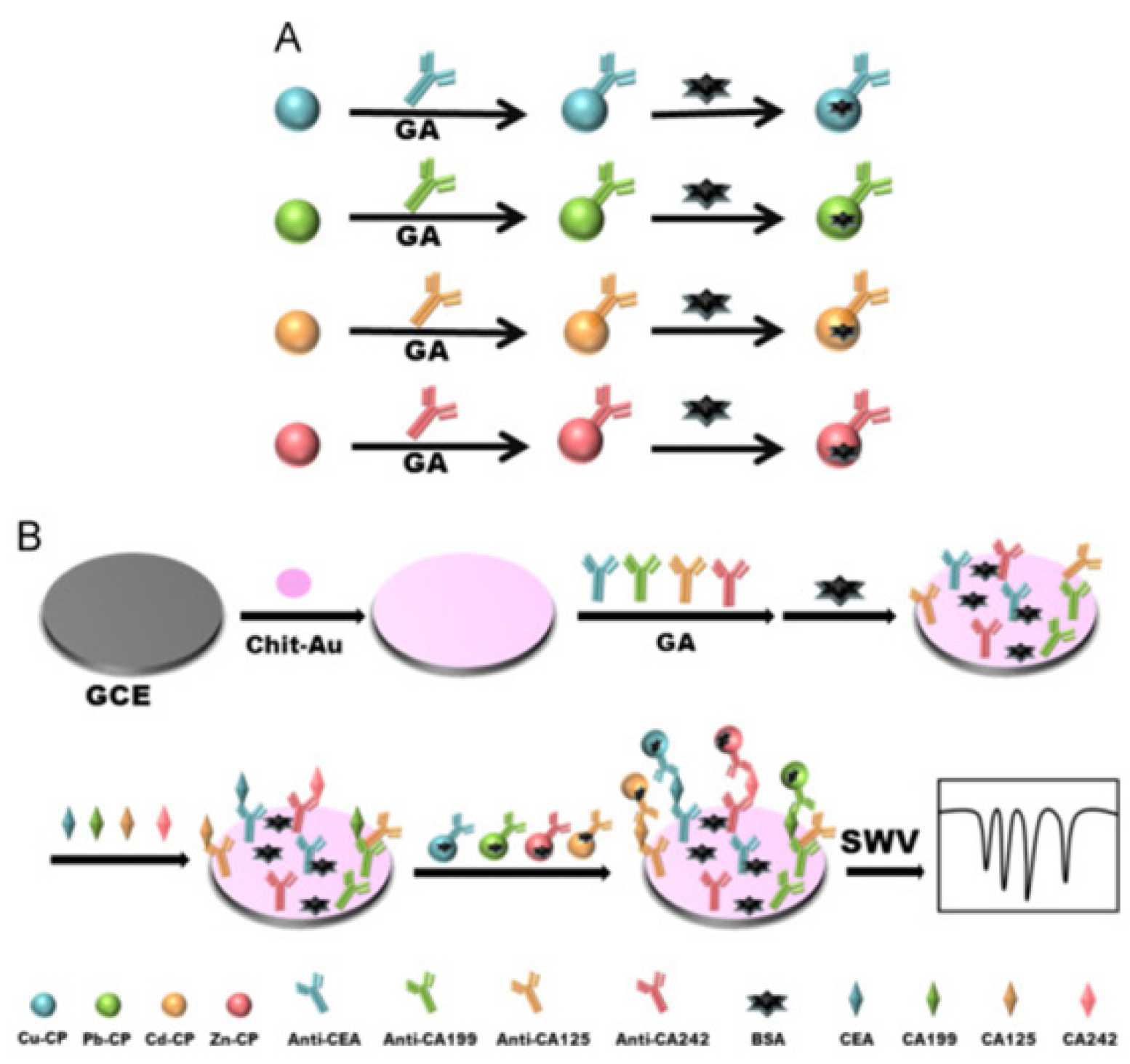
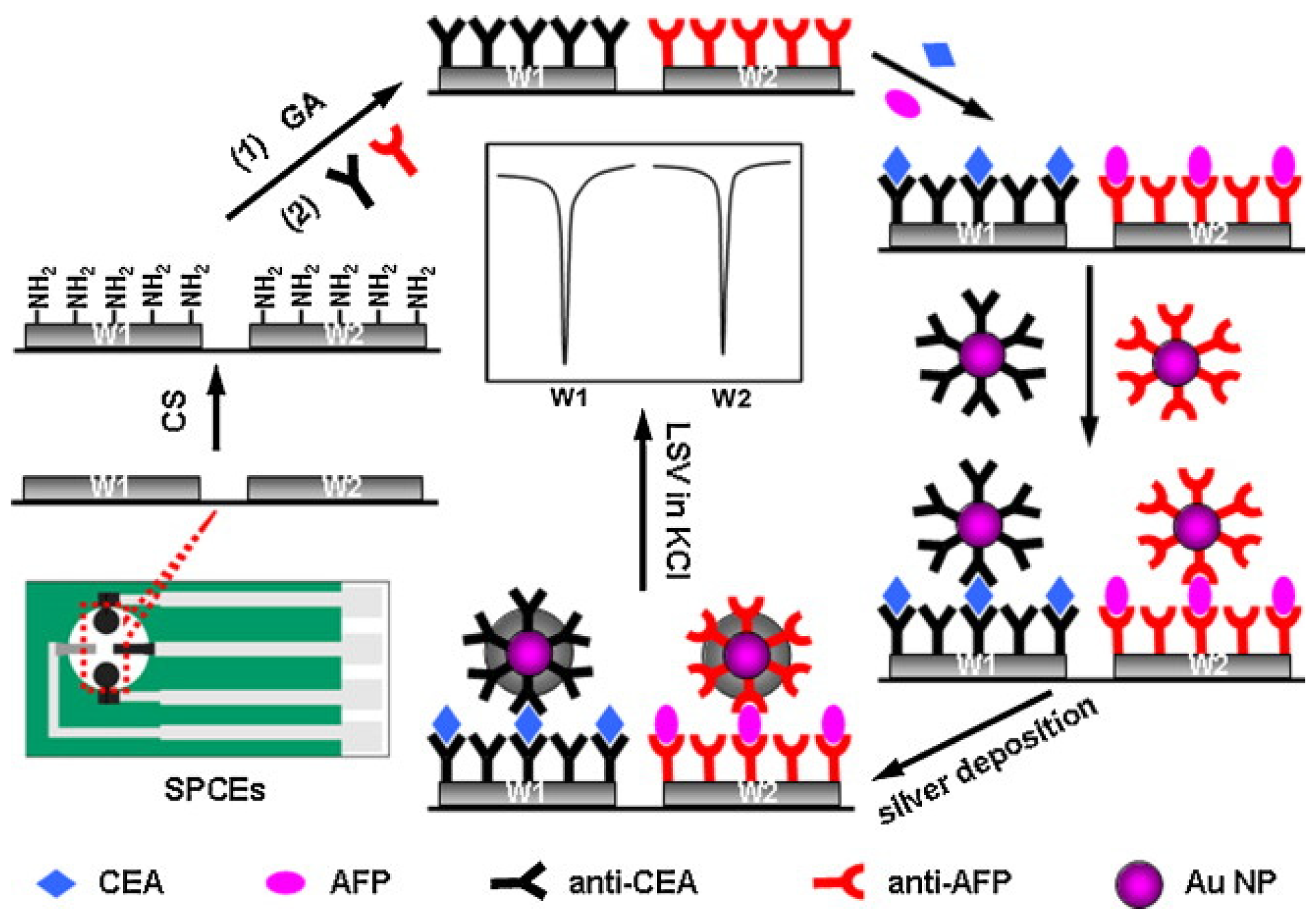
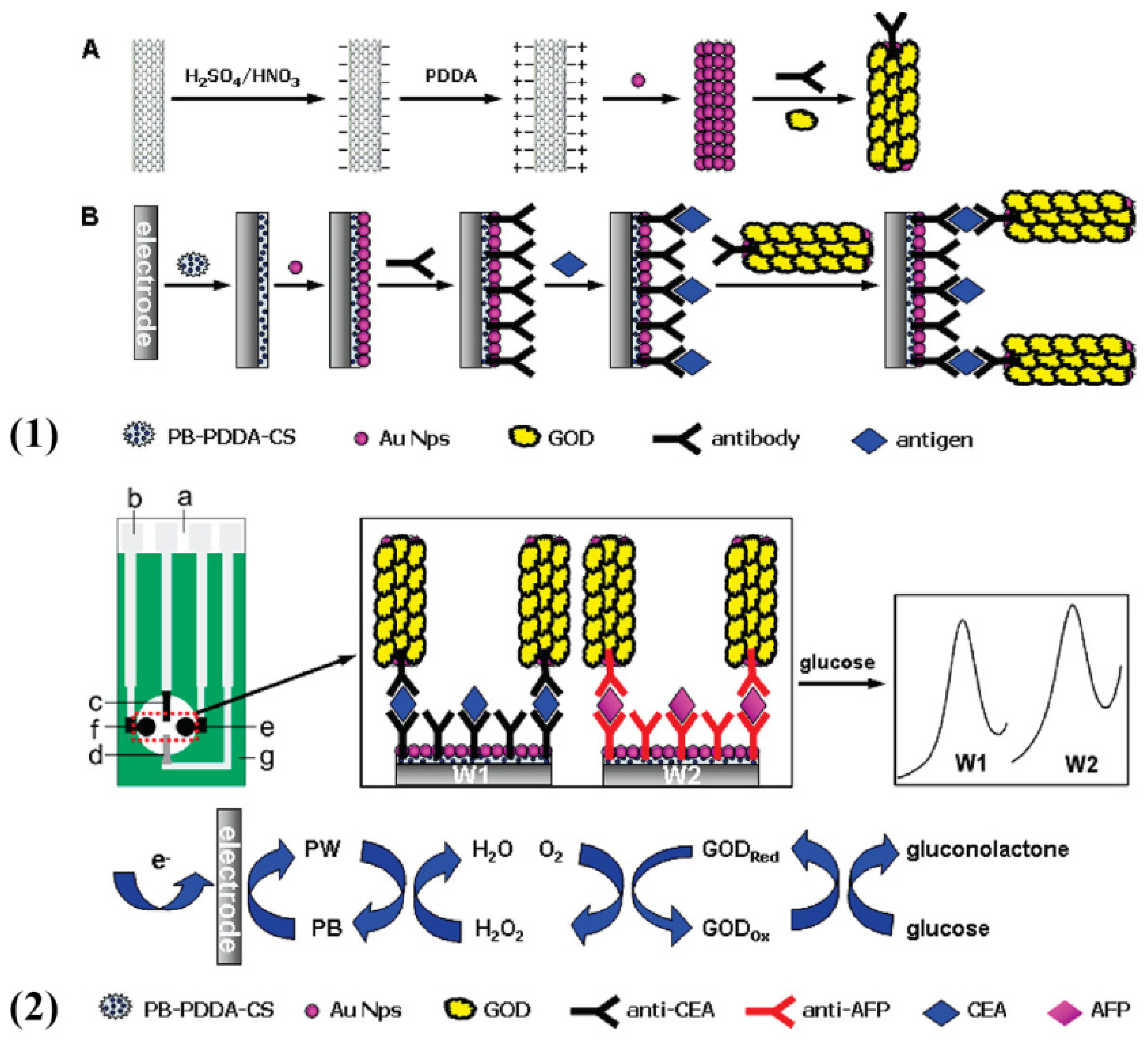
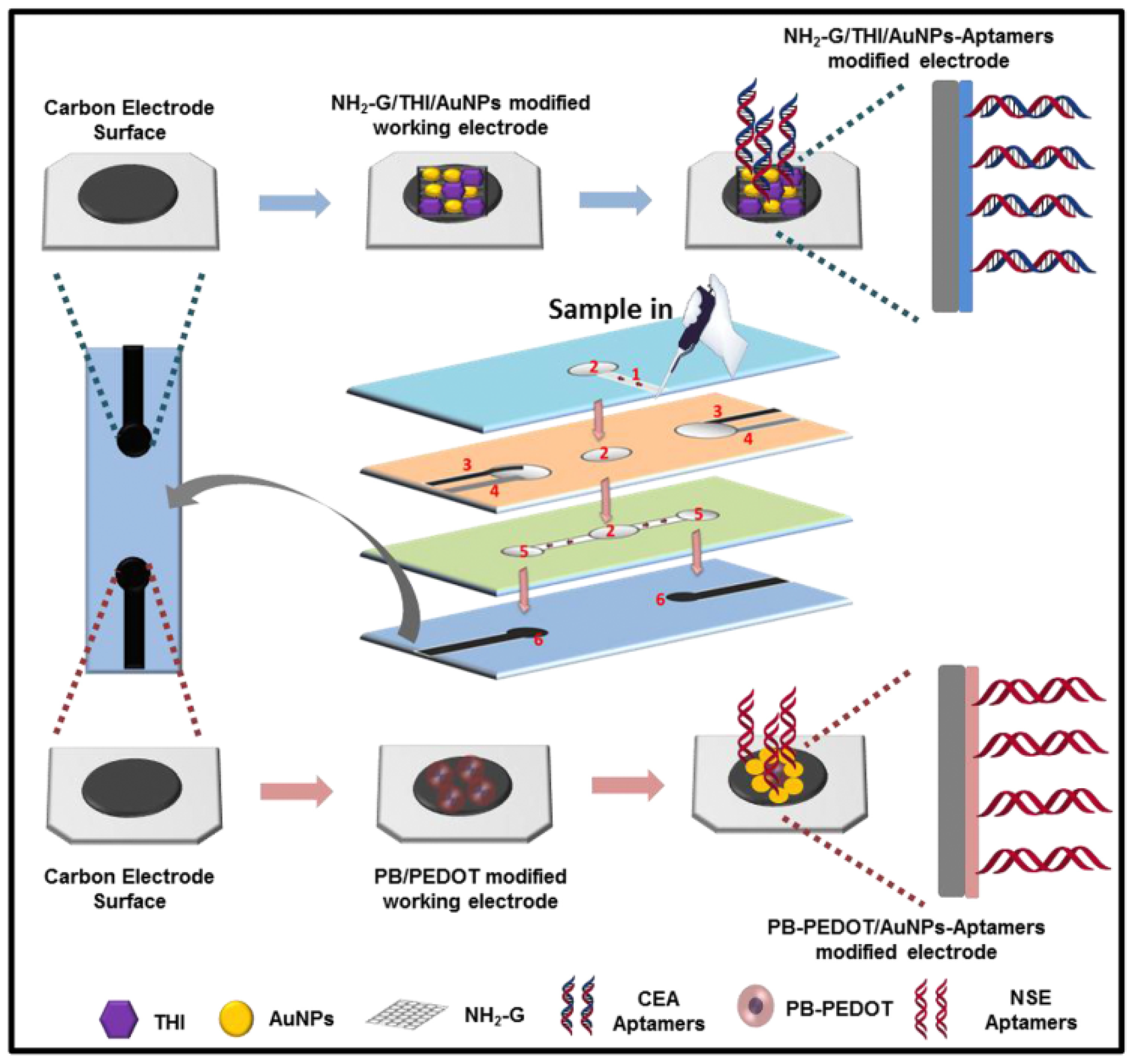
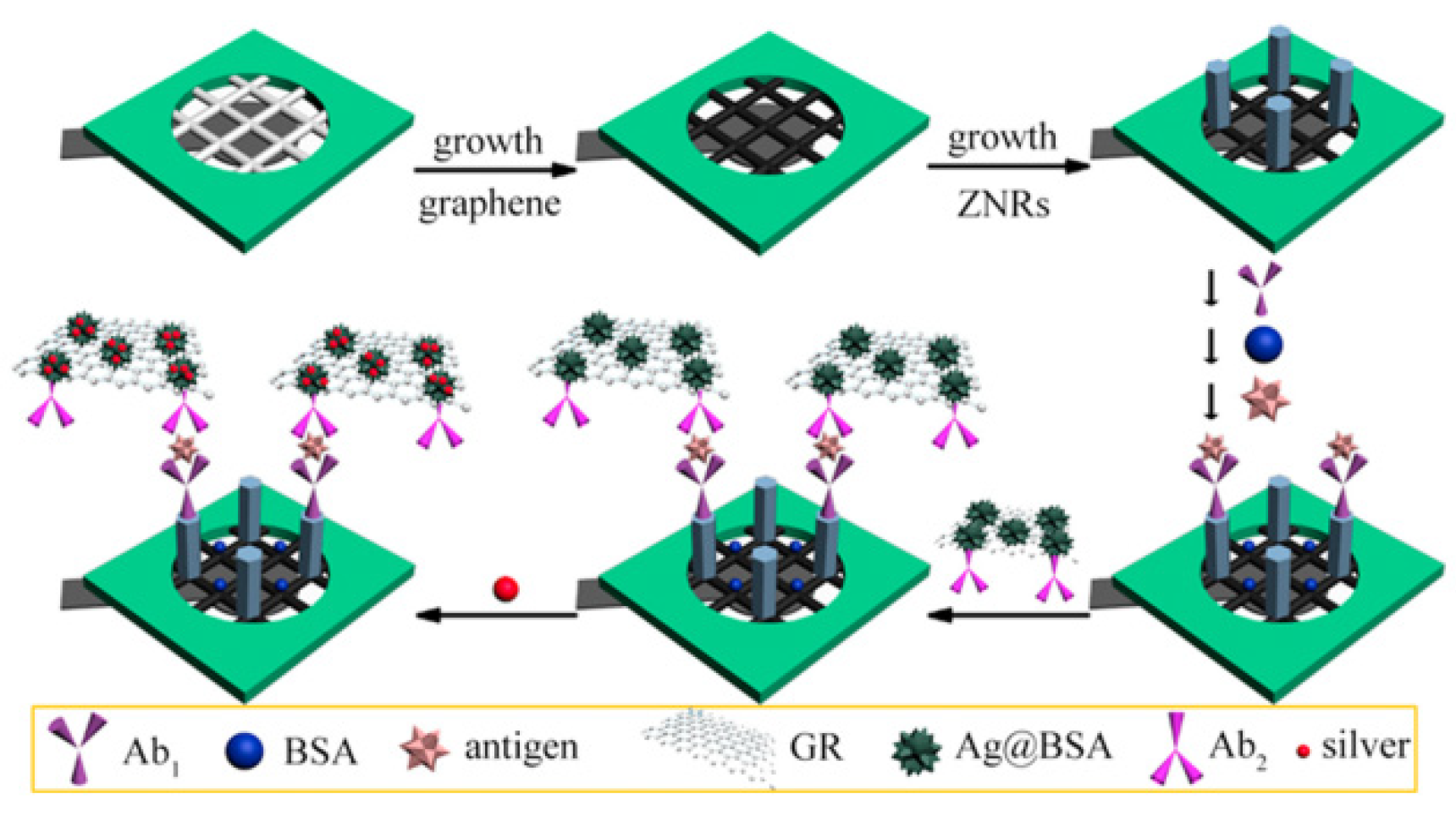
| Species | Advantages | Limitations | Electrochemical Signal Substance | Detection Object | Ref. |
|---|---|---|---|---|---|
| Organic molecules | Easy accessibility, Simple loading, Excellent redox spikes | Unstable physisorption to cause leakage, Fussy covalent bonding to lead time-consuming, Environmental contamination | Methylene blue, Thionine | CEA, AFP | [53] |
| Thionine, Ferrocene | CEA, AFP | [54] | |||
| Anthraquinone 2-carboxylic acid, Thionine, Tris(2,2(-bipyridine-4,4(-dicarboxylic acid) cobalt(III), Ferrocene | CEA, CA 19-9, CA 12-5, CA 24-2 | [55] | |||
| Metal ions | Easy accessibility, Sharp electrochemical signal peaks | Consideration of heavy metal ions contamination | Copper(II) ions, Lead(II) ions, Titanium(IV) ions | CEA, NSE, CYFRA 21-1 | [43] |
| Copper(II) ions, Lead(II) ions, Cadmium(II) ions, zinc(II) ions | CEA, CA 19-9, CA 12-5, CA24-2 | [62] | |||
| Copper(II) ions, Lead(II) ions, Cadmium(II) ions, Silver ions | CEA, AFP, PSA, IL-8 | [63] | |||
| Metal nanoparticles | Small size, High surface-to-bulk ratio, High catalytic activity | Easily oxidation for a long period of storage | Silver nanoparticles | CEA, CA 12-5, CA 15-3 | [70] |
| Silver nanoparticles | CEA, AFP | [71] | |||
| Prussian blue | High catalytic activity, Excellent electrochemical signal peaks | Requirement of pre-preparation | PB-PDDA-CS | CEA, AFP | [81] |
| PB-PEDOT | CEA, NSE | [82] | |||
| Other substances | Excellent catalytic system Needlessness of signal molecules labeling | Common usage of noble metal-based nanozyme | Pt NPs-functionalized mesoporous silica | Diethylstilbestrol Estradiol | [88] |
| BSA-stabilized silver nanoparticles | CEA, PSA, HCG | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Chu, C.; Ma, Z. Electrochemical Signal Substance for Multiplexed Immunosensing Interface Construction: A Mini Review. Molecules 2022, 27, 267. https://doi.org/10.3390/molecules27010267
Feng J, Chu C, Ma Z. Electrochemical Signal Substance for Multiplexed Immunosensing Interface Construction: A Mini Review. Molecules. 2022; 27(1):267. https://doi.org/10.3390/molecules27010267
Chicago/Turabian StyleFeng, Jiejie, Changshun Chu, and Zhanfang Ma. 2022. "Electrochemical Signal Substance for Multiplexed Immunosensing Interface Construction: A Mini Review" Molecules 27, no. 1: 267. https://doi.org/10.3390/molecules27010267





