Growth Inhibition and Apoptotic Effect of Pine Extract and Abietic Acid on MCF-7 Breast Cancer Cells via Alteration of Multiple Gene Expressions Using In Vitro Approach
Abstract
:1. Introduction
2. Results
2.1. Abietic Acid Induces Growth Inhibition in MCF-7 Sensitive Cell Line
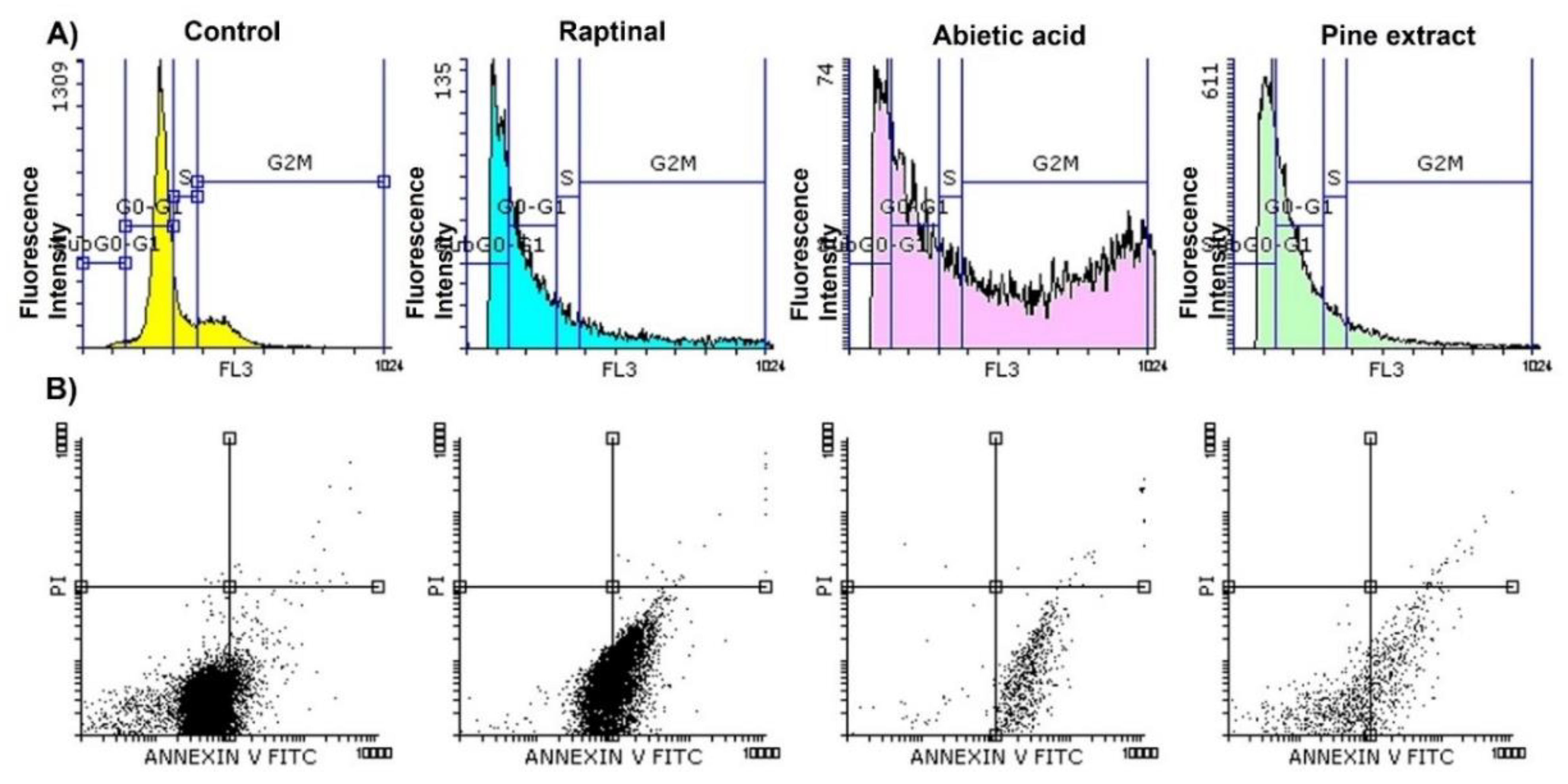
2.2. Abietic Acid Induces MCF-7 Cells Apoptosis with Cell Cycle Arrests at Both G0-G1 and G2/M Phases and SubG0-G1 Sub-Population
2.3. Abietic Acid Modulates Key- Genes Regulating Multiple Controlling Pathways
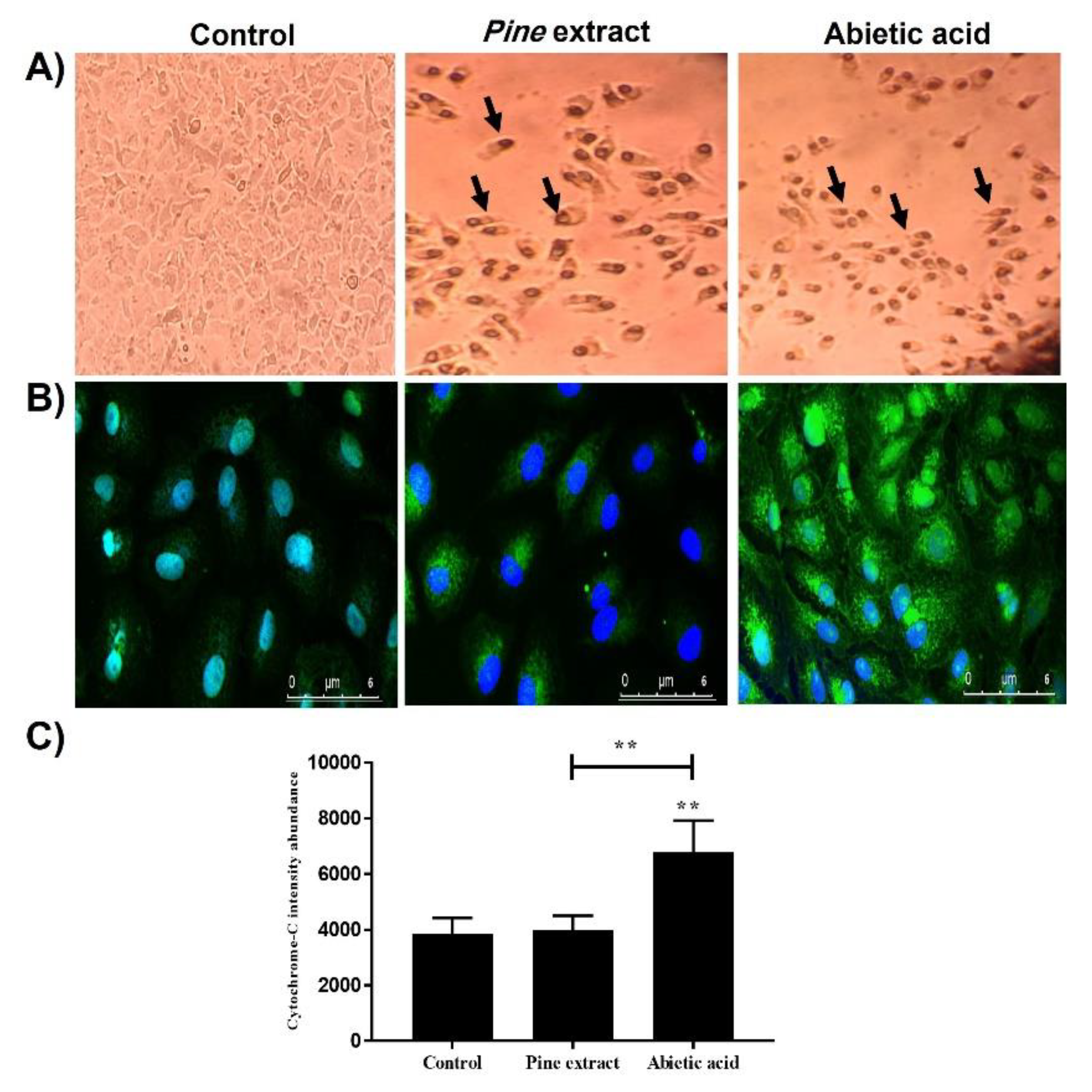
2.4. Abietic Acid Induces Increased Protein Level of Cytochrome-C Confirmed by Immunocytochemistry (ICC) Analysis
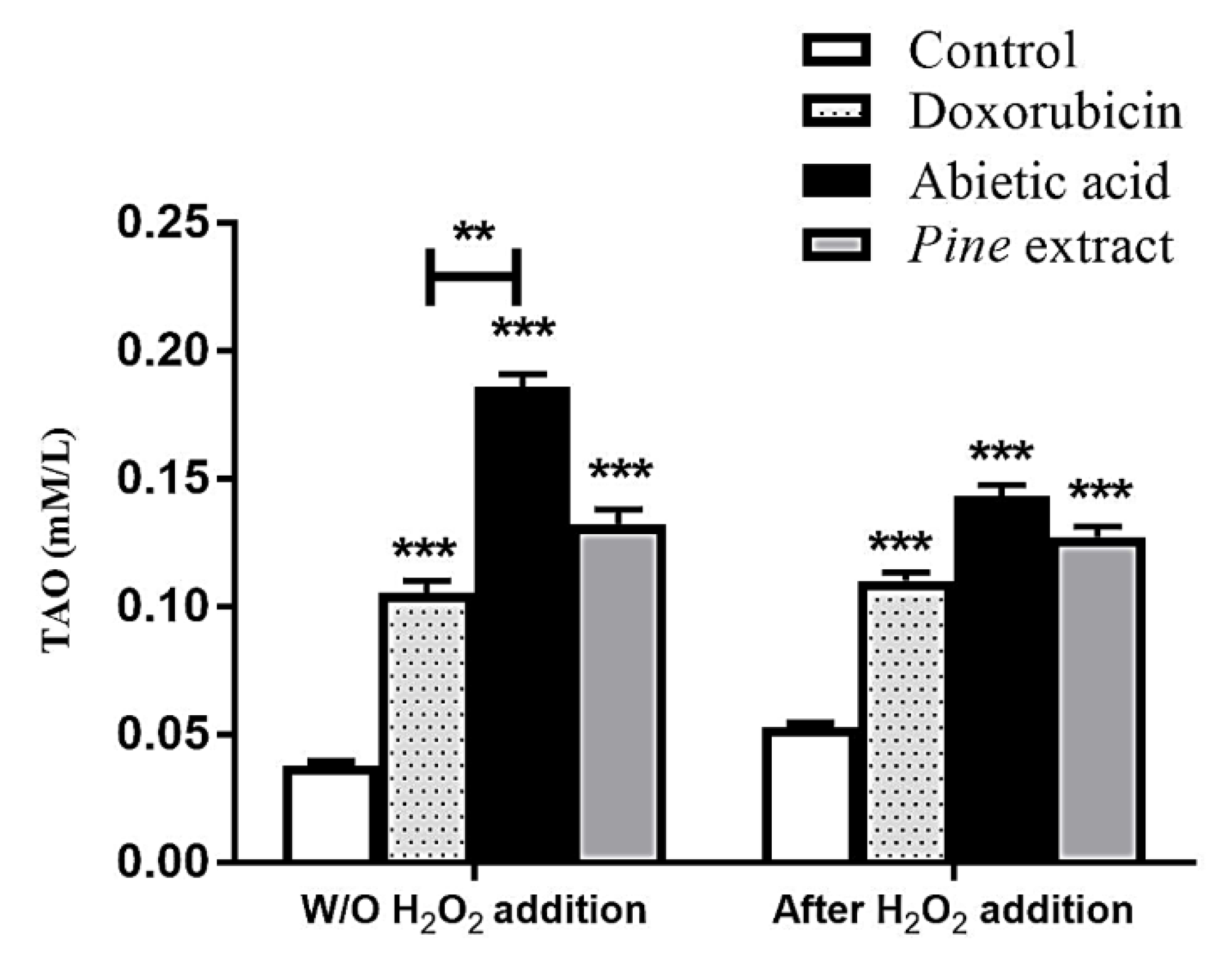
2.5. Total Antioxidant Assay
3. Discussion
4. Materials and Methods
4.1. Plant Material Preparation
4.1.1. Plant Material and Resin Collection
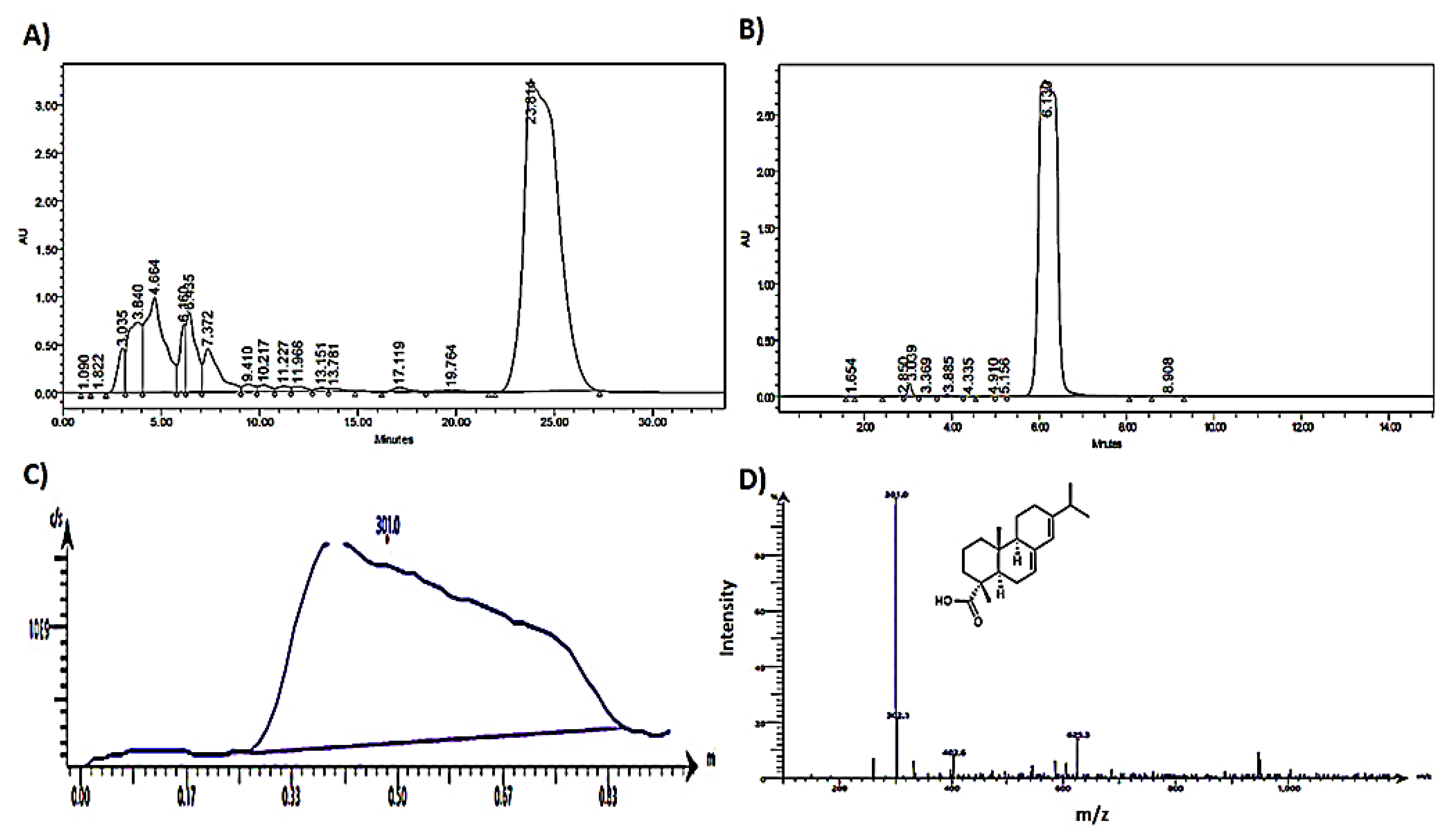
4.1.2. Extraction and Purification of Abietic Acid
4.1.3. HPLC Analysis of Abietic Acid and Percent Purity
4.1.4. MS Analysis of Abietic Acid
4.1.5. Preparation of P. paliustris Total Extract, Abietic Acid and Standard Control Solutions
4.2. In Vitro Study
4.2.1. Cell Culture
4.2.2. Viability Assay
4.2.3. Flow Cytometry and Apoptosis Assay
4.2.4. Real-Time Quantitative PCR (qPCR)
4.2.5. Immunocytochemistry
4.2.6. Total Antioxidant Capacity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ebrahim, H.Y.; Akl, M.R.; Elsayed, H.E.; Hill, R.A.; El Sayed, K.A. Usnic acid benzylidene analogues as potent mechanistic target of rapamycin inhibitors for the control of breast malignancies. J. Nat. Prod. 2017, 80, 932–952. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.; Pinto, D.C. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benarba, B. Medicinal plants used by traditional healers from South-West Algeria: An ethnobotanical study. J. Intercult. Ethnopharmacol. 2016, 5, 320–330. [Google Scholar] [CrossRef]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Akkol, E.K. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Intercult. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef]
- Javadi, B.; Iranshahy, M.; Emami, S.A. Anticancer plants in Islamic traditional medicine. In Complementary Therapies for the Body, Mind and Soul, 1st ed.; Saad, M., Ed.; InTech Open: London, UK, 2015; Chapter 5; pp. 111–144. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). Biochem. Mol. Biol. Plants 2000, 24, 1250–1319. [Google Scholar]
- Bohlmann, J. Pine terpenoid defences in the mountain pine beetle epidemic and in other conifer pest interactions: Specialized enemies are eating holes into a diverse, dynamic and durable defence system. Tree Physiol. 2012, 32, 943–945. [Google Scholar] [CrossRef]
- Savluchinske-Feio, S.; Curto, M.J.M.; Gigante, B.; Roseiro, J.C. Antimicrobial activity of resin acid derivatives. Appl. Microbiol. Biotechnol. 2006, 72, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Evidente, A. Phytotoxic terpenes produced by phytopathogenic fungi and allelopathic plants. Nat. Prod. Commun. 2014, 9, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhaoyu, L.; Xiangming, F.; Chunyi, L.; Jiayu, P.; Lu, S.; Jitao, C.; Liangcai, C.; Jifang, L. Abietic acid attenuates allergic airway inflammation in a mouse allergic asthma model. Int. Immunopharmacol. 2016, 38, 261–266. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J.; Yuan, Y. Abietic acid attenuates IL-1β-induced inflammation in human osteoarthritis chondrocytes. Int. Immunopharmacol. 2018, 64, 110–115. [Google Scholar] [CrossRef]
- Talevi, A.; Cravero, M.S.; Castro, E.A.; Bruno-Blanch, L.E. Discovery of anticonvulsant activity of abietic acid through application of linear discriminant analysis. Bioorganic Med. Chem. Lett. 2007, 17, 1684–1690. [Google Scholar] [CrossRef]
- Hwang, K.-H.; Ahn, J.-Y.; Kim, S.; Park, J.-H.; Ha, T.-Y. Abietic acid has an anti-obesity effect in mice fed a high-fat diet. J. Med. Food 2011, 14, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Correa-Royero, J.; Agudelo, L.; Mesa, A.; Betancur-Galvis, L. Synthesis and biological evaluation of abietic acid derivatives. Eur. J. Med. Chem. 2009, 44, 2468–2472. [Google Scholar] [CrossRef]
- Zapata, B.; Rojas, M.; Betancur-Galvis, L.; Mesa-Arango, A.C.; Pérez-Guaita, D.; González, M.A. Cytotoxic, immunomodulatory, antimycotic, and antiviral activities of semisynthetic 14-hydroxyabietane derivatives and triptoquinone C-4 epimers. MedChemComm 2013, 4, 1239–1246. [Google Scholar] [CrossRef]
- Agudelo-Gómez, L.S.; Betancur-Galvis, L.A.; González, M.A. Anti HHV-1 and HHV-2 activity in vitro of abietic and dehydroabietic acid derivatives. Pharmacologyonline 2012, 1, 36–42. [Google Scholar]
- Yoshida, N.; Takada, T.; Yamamura, Y.; Adachi, I.; Suzuki, H.; Kawakami, J. Inhibitory effects of terpenoids on multidrug resistance-associated protein 2-and breast cancer resistance protein-mediated transport. Drug Metab. Dispos. 2008, 36, 1206–1211. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, W.; Liu, Q.; Dai, J. Abietic acid suppresses non-small-cell lung cancer cell growth via blocking IKKβ/NF-κB signaling. Onco Targets Ther. 2019, 12, 4825–4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palchaudhuri, R.; Lambrecht, M.J.; Botham, R.C.; Partlow, K.C.; Van Ham, T.J.; Putt, K.S.; Nguyen, L.T.; Kim, S.-H.; Peterson, R.T.; Fan, T.M. A small molecule that induces intrinsic pathway apoptosis with unparalleled speed. Cell Rep. 2015, 13, 2027–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, I.; Jenner, A.; Bruchelt, G.; Niethammer, D.; Halliwell, B. Effect of concentration on the cytotoxic mechanism of doxorubicin—apoptosis and oxidative DNA damage. Biochem. Biophys. Res. Commun. 1997, 230, 254–257. [Google Scholar] [CrossRef]
- Pascale, F.; Bedouet, L.; Baylatry, M.; Namur, J.; Laurent, A. Comparative chemosensitivity of VX2 and HCC cell lines to drugs used in TACE. Anticancer Res. 2015, 35, 6497–6503. [Google Scholar]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [PubMed]
- Rashidi, M.; Seghatoleslam, A.; Namavari, M.; Amiri, A.; Fahmidehkar, M.A.; Ramezani, A.; Eftekhar, E.; Hosseini, A.; Erfani, N.; Fakher, S. Selective Cytotoxicity and apoptosis-induction of Cyrtopodion scabrum extract against digestive cancer cell lines. Int. J. Cancer Manag. 2017, 10, 7. [Google Scholar] [CrossRef]
- Rusdi, M.; Alam, G.; Manggau, M.A. Selective Cytotoxicity evaluation in Anticancer drug screening of Boehmeria virgata (Forst) Guill leaves to several human cell lines: HeLa, WiDr, T47D and Vero. Dhaka Univ. J. Pharm. Sci. 2013, 12, 87–90. [Google Scholar]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin. Med. 2011, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Tram, N.T.T.; Anh, D.H.; Thuc, H.H.; Tuan, N.T. Investigation of chemical constituents and cytotoxic activity of the lichen Usnea undulata. Vietnam J. Chem. 2020, 58, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [Green Version]
- Shah, F.; Leung, L.; Barton, H.A.; Will, Y.; Rodrigues, A.D.; Greene, N.; Aleo, M.D. Setting clinical exposure levels of concern for drug-induced liver injury (DILI) using mechanistic in vitro assays. Toxicol. Sci. 2015, 147, 500–514. [Google Scholar] [CrossRef] [Green Version]
- Juarez, M.; Schcolnik-Cabrera, A.; Dueñas-Gonzalez, A. The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018, 8, 317–331. [Google Scholar]
- Quayle, L.A.; Pereira, M.G.; Scheper, G.; Wiltshire, T.; Peake, R.E.; Hussain, I.; Rea, C.A.; Bates, T.E. Anti-angiogenic drugs: Direct anti-cancer agents with mitochondrial mechanisms of action. Oncotarget 2017, 8, 88670–88688. [Google Scholar] [CrossRef] [Green Version]
- Haffez, H.; Taha, H.; Farrag, N.S.; Amin, A.M.; Hassan, Z.A. Biological Screening and Radiolabeling of Raptinal as a Potential Anticancer Novel Drug in Hepatocellular Carcinoma Model. Eur. J. Pharm. Sci. 2021, 158, 105653. [Google Scholar] [CrossRef]
- Meli, M.; Shafie, N.H.; Loh, S.P.; Rahmat, A. Anti-proliferative and apoptosis-inducing effects of Morinda citrifolia L. shoot on breast, liver, and colorectal cancer cell lines. Mal. J. Med. Health Sci. 2019, 15, 129–135. [Google Scholar]
- Kuete, V.; Mafodong, F.D.; Celik, I.; Fobofou, S.; Ndontsa, B.; Karaosmanoğlu, O.; Weissjohann, L.; Tane, P.; Koparal, A.; Sivas, H. In vitro cytotoxicity of compounds isolated from Desbordesia glaucescens against human carcinoma cell lines. S. Afr. J. Bot. 2017, 111, 37–43. [Google Scholar] [CrossRef]
- Yeh, R.-D.; Chen, J.-C.; Lai, T.-Y.; Yang, J.-S.; Yu, C.-S.; Chiang, J.-H.; Lu, C.-C.; Yang, S.-T.; Yu, C.-C.; Chang, S.-J. Gallic acid induces G0/G1 phase arrest and apoptosis in human leukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer Res. 2011, 31, 2821–2832. [Google Scholar] [PubMed]
- Kubatka, P.; Kello, M.; Kajo, K.; Samec, M.; Liskova, A.; Jasek, K.; Koklesova, L.; Kuruc, T.; Adamkov, M.; Smejkal, K. Rhus coriaria L. (Sumac) demonstrates oncostatic activity in the therapeutic and preventive model of breast carcinoma. Int. J. Mol. Sci. 2021, 22, 183. [Google Scholar] [CrossRef]
- Huang, X.-C.; Jin, L.; Wang, M.; Liang, D.; Chen, Z.-F.; Zhang, Y.; Pan, Y.-M.; Wang, H.-S. Design, synthesis and in vitro evaluation of novel dehydroabietic acid derivatives containing a dipeptide moiety as potential anticancer agents. Eur. J. Med. Chem. 2015, 89, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-C.; Wang, M.; Pan, Y.-M.; Tian, X.-Y.; Wang, H.-S.; Zhang, Y. Synthesis and antitumor activities of novel α-aminophosphonates dehydroabietic acid derivatives. Bioorganic Med. Chem. Lett. 2013, 23, 5283–5289. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [Green Version]
- Azimian, H.; Dayyani, M.; Toossi, M.T.B.; Mahmoudi, M. Bax/Bcl-2 expression ratio in prediction of response to breast cancer radiotherapy. Iran. J. Basic Med. Sci. 2018, 21, 325–332. [Google Scholar] [PubMed]
- Khodapasand, E.; Jafarzadeh, N.; Farrokhi, F.; Kamalidehghan, B.; Houshmand, M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran. Biomed. J. 2015, 19, 69–75. [Google Scholar]
- Bensky, D.; Clavey, S.; Stõger, E. Chinese Herbal Medicine: Materia Medica, 3rd ed.; Eastland Press: Seattle, WA, USA, 2004; pp. 3–6. [Google Scholar]
- Wood, M. The Earthwise Herbal: A Complete Guide to Old World Medicinal Plants; North Atlantic Books: Berkeley, CA, USA, 2008; Volume 1. [Google Scholar]
- Moore, M. Medicinal plants of the Mountain West; Museum of New Mexico Press: Santa Fe, NM, USA, 2003. [Google Scholar]
- Jenab, E.; Mussone, P.; Nam, G.; Bressler, D. Production of renewable hydrocarbons from thermal conversion of abietic acid and tall oil fatty acids. Energy Fuels 2014, 28, 6988–6994. [Google Scholar] [CrossRef]
- Roh, S.-S.; Park, M.-K.; Kim, Y.-u. Abietic acid from Resina Pini of Pinus species as a testosterone 5α-reductase inhibitor. J. Health Sci. 2010, 56, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Mo, Q.; Cui, Y.; Tang, J. Inhibition of nasopharyngeal cells mediated via G2/M cell cycle arrest, blocking PI3K/AKT/mTOR signalling pathway and suppression of cell migration and invasion. J. King Saud Univ. Sci. 2020, 32, 1325–1331. [Google Scholar] [CrossRef]
- Huang, L.; Huang, R.; Pang, F.; Li, A.; Huang, G.; Zhou, X.; Li, Q.; Li, F.; Ma, X. Synthesis and biological evaluation of dehydroabietic acid-pyrimidine hybrids as antitumor agents. RSC Adv. 2020, 10, 18008–18015. [Google Scholar] [CrossRef]
- Levenson, A.S.; Jordan, V.C. MCF-7: The first hormone-responsive breast cancer cell line. Cancer Res. 1997, 57, 3071–3078. [Google Scholar]
- Park, J.Y.; Lee, Y.K.; Lee, D.-S.; Yoo, J.-E.; Shin, M.-S.; Yamabe, N.; Kim, S.-N.; Lee, S.; Kim, K.H.; Lee, H.-J. Abietic acid isolated from pine resin (Resina Pini) enhances angiogenesis in HUVECs and accelerates cutaneous wound healing in mice. J. Ethnopharmacol. 2017, 203, 279–287. [Google Scholar] [CrossRef] [PubMed]
- El-Hallouty, S.M.; Soliman, A.A.; Nassrallah, A.; Salamatullah, A.; Alkaltham, M.S.; Kamal, K.Y.; Hanafy, E.A.; Gaballa, H.S.; Aboul-Soud, M.A. Crude Methanol Extract of Rosin Gum Exhibits Specific Cytotoxicity against Human Breast Cancer Cells via Apoptosis Induction. Anticancer Agents Med. Chem. 2020, 20, 1028–1036. [Google Scholar] [CrossRef]
- Carmona, F.; Pereira, A.M.S. Herbal medicines: Old and new concepts, truths and misunderstandings. Rev Bras Farmacogn. 2013, 23, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1+1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How can synergism of traditional medicines benefit from network pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Thumanu, K.; Tanthanuch, W. Synergistic anticancer effect of the extracts from Polyalthia evecta caused apoptosis in human hepatoma (HepG-2) cells. Asian Pac. J. Trop. Biomed. 2012, 2, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocasap, P.; Nonpunya, A.; Weerapreeyakul, N. Pinus kesiya Royle ex Gordon induces apoptotic cell death in hepatocellular carcinoma HepG-2 cell via intrinsic pathway by PARP and Topoisomerase I suppression. Biomed. Pharmacother. 2021, 139, 111628. [Google Scholar] [CrossRef]
- Faustino, C.; Neto, Í.; Fonte, P.; Macedo, A. Cytotoxicity and chemotherapeutic potential of natural rosin abietane diterpenoids and their synthetic derivatives. Curr. Pharm. Des. 2018, 24, 4362–4375. [Google Scholar] [CrossRef] [PubMed]
- Zinkel, S.; Gross, A.; Yang, E. BCL2 family in DNA damage and cell cycle control. Cell Death Dis. 2006, 13, 1351–1359. [Google Scholar] [CrossRef]
- Kulsoom, B.; Shamsi, T.S.; Afsar, N.A.; Memon, Z.; Ahmed, N.; Hasnain, S.N. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: Are we ready for Bcl-2-directed therapy? Cancer Manag. Res. 2018, 10, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Hemann, M.; Lowe, S. The p53-BCL-2 connection. Cell Death Dis. 2006, 13, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Rubinsztein, D. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Dis. 2007, 14, 1247–1250. [Google Scholar] [CrossRef] [Green Version]
- Parveen, A.; Subedi, L.; Kim, H.W.; Khan, Z.; Zahra, Z.; Farooqi, M.Q.; Kim, S.Y. Phytochemicals targeting VEGF and VEGF-related multifactors as anticancer therapy. J. Clin. Med. 2019, 8, 350–388. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct. Target. Ther. 2018, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhou, Y.; Peng, J.; Xie, B.; Shou, Q.; Wang, J. Silencing c-Myc Enhances the Antitumor Activity of Bufalin by Suppressing the HIF-1α/SDF-1/CXCR4 Pathway in Pancreatic Cancer Cells. Front. Pharmacol. 2020, 11, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.W.; Zhang, M.M.; Zhou, H.; Lam, K.Y.C.; Chan, P.L.; Law, C.K.M.; Yue, P.Y.K.; Liu, L. Saikosaponin-d enhances the anticancer potency of TNF-via overcoming its undesirable response of activating NF-Kappa B signalling in cancer cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 745295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicenas, J.; Zalyte, E.; Bairoch, A.; Gaudet, P. Kinases and cancer. Cancers 2018, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Ways, D.K.; Kukoly, C.A.; de Vente, J.; Hooker, J.L.; Bryant, W.O.; Posekany, K.J.; Fletcher, D.J.; Cook, P.P.; Parker, P.J. MCF-7 breast cancer cells transfected with protein kinase C-alpha exhibit altered expression of other protein kinase C isoforms and display a more aggressive neoplastic phenotype. J. Clin. Investig. 1995, 95, 1906–1915. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Orhan, Y.C.; Zha, X.; Esencan, E.; Chatterton, R.T.; Bulun, S.E. AMP-activated protein kinase and energy balance in breast cancer. Am. J. Transl. Res. 2017, 9, 197–213. [Google Scholar]
- Niu, Y.; Xu, J.; Sun, T. Cyclin-dependent kinases 4/6 inhibitors in breast cancer: Current status, resistance, and combination strategies. J. Cancer 2019, 10, 5504. [Google Scholar] [CrossRef]
- Fujisawa, K.; Terai, S.; Takami, T.; Yamamoto, N.; Yamasaki, T.; Matsumoto, T.; Yamaguchi, K.; Owada, Y.; Nishina, H.; Noma, T. Modulation of anti-cancer drug sensitivity through the regulation of mitochondrial activity by adenylate kinase 4. J. Exp. Clin. Cancer Res. 2016, 35, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Martinou, J.-C.; Green, D.R. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2001, 2, 63–67. [Google Scholar] [CrossRef]
- Cain, K.; Bratton, S.B.; Cohen, G.M. The Apaf-1 apoptosome: A large caspase-activating complex. Biochimie 2002, 84, 203–214. [Google Scholar] [CrossRef]
- Waterhouse, N.; Trapani, J. A new quantitative assay for cytochrome c release in apoptotic cells. Cell Death Dis. 2003, 10, 853–855. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Yang, J.; Jones, D. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta Bioenerg. 1998, 1366, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Henzel, W.J.; Liu, X.; Lutschg, A.; Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c–dependent activation of caspase-3. Cell 1997, 90, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S.-g. Correlation between oxidative stress, nutrition, and cancer initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qu, T.; Wang, P.; Li, X.; Qiang, J.; Xia, Z.; Duan, H.; Huang, J.; Zhu, L. Unravelling the relationship between macroautophagy and mitochondrial ROS in cancer therapy. Apoptosis 2016, 21, 517–531. [Google Scholar] [CrossRef]
- Lee, M.H.; Cha, H.J.; Choi, E.O.; Han, M.H.; Kim, S.O.; Kim, G.Y.; Hong, S.H.; Park, C.; Moon, S.K.; Jeong, S.J. Antioxidant and cytoprotective effects of morin against hydrogen peroxide-induced oxidative stress are associated with the induction of Nrf-2-mediated HO-1 expression in V79-4 Chinese hamster lung fibroblasts. Int. J. Mol. Med. 2017, 39, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive oxygen species and cancer: A complex interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahim, H.Y.; Osman, S.A.; Haffez, H.R.; Hassan, Z.A. In-vitro screening of some plant extracts for their potential anticancer activity. Afr. J. Tradit. Complement. Altern. Med. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Abdelaal, M.R.; Soror, S.H.; Elnagar, M.R.; Haffez, H. Revealing the Potential Application of EC-Synthetic Retinoid Analogues in Anticancer Therapy. Molecules 2021, 26, 506. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Li, Y.; Chung, H.Y.; Ye, W. 2β-(Isobutyryloxy) florilenalin, a sesquiterpene lactone isolated from the medicinal plant Centipeda minima, induces apoptosis in human nasopharyngeal carcinoma CNE cells. Molecules 2009, 14, 2135–2146. [Google Scholar] [CrossRef] [Green Version]
- Prieto, A.; Díaz, D.; Barcenilla, H.; García-Suárez, J.; Reyes, E.; Monserrat, J.; San Antonio, E.; Melero, D.; de la Hera, A.; Orfao, A. Apoptotic rate: A new indicator for the quantification of the incidence of apoptosis in cell cultures. Cytom. J. Int. Soc. Anal. Cytol. 2002, 48, 185–193. [Google Scholar] [CrossRef]
- Singh, A.; Bhatia, P.; Trehan, A.; Bansal, D.; Singh, A.; Bhatia, A. Low spontaneous apoptosis index at diagnosis predicts a high-risk phenotype in paediatric acute lymphoblastic leukaemia. Indian J. Med. Res. 2018, 147, 248. [Google Scholar] [PubMed]
- Naguib, R.N.; Sherbet, G.V. Artificial Neural Networks in Cancer Diagnosis, Prognosis, and Patient Management; CRC press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Sherbet, G.V. Genetic Recombination in Cancer, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Mahalingaiah, P.K.S.; Singh, K.P. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS ONE 2014, 9, e87371. [Google Scholar] [CrossRef]

| Studied Plants & Reference Standard Drug | * IC50 ± SEM and Corresponding SI | ||||||
|---|---|---|---|---|---|---|---|
| Wi-38 | HepG-2 | MCF-7 | HCT-116 | ||||
| ** IC50 | SI | ** IC50 | SI | ** IC50 | SI | ||
| Raptinal | 5.2 ± 0.9 | 0.74 ± 0.1 | 7 | 4.1 ± 0.76 | 1.3 | 96.2 ± 1.5 | 0.05 |
| Doxorubicin | 0.1± 0.1 | 0.8 ± 1.1 | 0.11 | 1.3 ± 1.0 | 0.07 | 0.97 ± 0.72 | 0.1 |
| Pine extract | 0.42 ± 0.1 | 0.2 ± 0.76 | 2.1 | 0.11 ± 0.76 | 3.8 | 0.25 ± 0.1 | 1.7 |
| Abietic acid | N/A | N/A | --- | 0.06 ± 0.5 | >1 | N/A | --- |
| Cell Cycle Parameters on MF-7 Cells | Control | Raptinal | Abietic Acid | Pine Extract |
|---|---|---|---|---|
| % Gated Sub G0-G1 phase | 1.52 ± 0.3 | 36.18 *** ± 6.2 | 20.96 *** ± 1.6 | 37.30 *** ± 7.5 |
| % Gated G0-G1 phase | 66.59 ± 4.8 | 33.62 ** ± 3.2 | 24.98 ** ± 5.2 | 42.07 * ± 9.1 |
| % Gated S phase | 13.16 ± 3.7 | 11.66 ± 1.8 | 4.96 * ± 1.04 | 9.29 * ± 1.5 |
| % Gated G2M phase | 18.73 ± 0.2 | 18.63 ± 3.8 | 49.17 ** ± 2.3 | 11.34 ± 2.3 |
| G2M/G0-G1 ratio | 0.28 ± 0.01 | 0.55 ± 0.1 | 2.0 ± 0.1 | 0.27 ± 0.1 |
| Apoptotic Parameters on MF-7 Cells | Control | Raptinal | Abietic Acid | Pine Extract |
|---|---|---|---|---|
| % Viable cells (C− −) | 93.65 ± 10.7 | 48.55 ** ± 4.8 | 8.48 *** ± 2.9 | 28.55 *** ± 10.9 |
| % Early apoptotic cells (C+ −) | 6.30 ± 2.4 | 51.35 *** ± 11.5 | 91.41 *** ± 12.9 | 71.39 *** ± 7.6 |
| % Late apoptotic cells (C− +) | 0.01 | 0.00 | 0.01 | 0.00 |
| % Necrotic cells (C+ +) | 0.04 | 0.1 | 0.1 | 0.06 |
| Apoptotic index (AI) | 0.06 ± 0.03 | 0.51 ± 0.4 | 1.0 ± 0.35 | 0.71 ± 0.11 |
| Genes | Pine Total Extract-RQ, Fold-Change (Mean ± SEM) | Abietic Acid-RQ, Fold-Change (Mean ± SEM) | ||||
|---|---|---|---|---|---|---|
| 4 h | 8 h | 24 h | 4 h | 8 h | 24 h | |
| Apoptotic genes | ||||||
| Fas | 145.0 ± 10.2 | 308.0 ± 5.7 | 146.0 ± 6.4 | 7.0 ± 0.9 | 32.0 ± 2.7 | 33.0 ± 8.7 |
| FasL | 26.0 ± 2.3 | 319.0 ± 8.7 | 88.0 ± 12.5 | 10.0 ± 1.4 | 27 ± 5.4 | 5.0 ± 1.8 |
| BINP3 | 0.15 | 57.0 ± 6.7 | 5.0 ± 2.6 | 7.0 ± 2.3 | 16.0 ± 4.7 | 0.22 |
| Casp3 | 1.8 ± 0.2 | 28.0 ± 5.2 | 19.0 ± 6.9 | 0.009 | 0.02 | 1.1 ± 0.1 |
| Casp8 | 2794 ± 15.7 | 1285.0 ± 51.9 | 37.0 ± 4.2 | 42.0 ± 5.2 | 7.0 ± 4.9 | 3.0 ± 0.7 |
| Cyt-C | 3712 ± 20.8 | 5036.0 ± 44.2 | 1009.0 ± 10.4 | 4.0 ± 1.4 | 772.0 ± 13.8 | 2688.0 ± 17.5 |
| Bax | 0.01 | 0.07 | 2.0 ± 0.5 | 2.0 ± 0.7 | 5.0 ± 1.2 | 0.05 |
| Bcl-2 | 10 ± 1.7 | 8.0 ± 2.8 | 3.0 ± 0.4 | 5.0 ± 1.8 | 3.0 ± 1.1 | 0.36 |
| Bax/Bcl-2 ratio | 0.01 | 0.01 | 0.7 | 0.01 | 0.7 | 13.9 ± 1.4 |
| ATG5 | 0.45 | 1.1 ± 0.1 | 6.0 ± 1.7 | 0.14 | 0.88 | 1.5 ± 0.4 |
| P53 | 0.45 | 0.13 | 0.53 | 0.06 | 0.81 | 2.0 ± 0.6 |
| Proliferation genes | ||||||
| VEGF | 0.45 | 0.52 | 0.03 | 3.0 ± 2.7 | 4.0 ± 1.3 | 0.03 |
| TGF-β1 | 0.25 | 0.09 | 0.01 | 0.77 | 0.57 | 0.02 |
| IGF1R | 0.32 | 0.99 | 0.80 | 2.0 ± 0.7 | 1.3 ± 0.2 | 0.04 |
| ATG12 | 0.20 | 0.28 | 0.22 | 0.12 | 0.36 | 0.03 |
| Oncogenic genes | ||||||
| C-myc | 0.16 | 0.15 | 0.03 | 0.18 | 0.29 | 0.05 |
| TNF-α | 2.0 ± 0.5 | 4.0 ± 0.9 | 4.0 ± 0.7 | 0.02 | 0.04 | 0.01 |
| NF-κB | 0.03 | 0.44 | 0.17 | 1.4 ± 0.2 | 2.6 ± 0.7 | 1.0 ± 0.1 |
| Kinase’s genes | ||||||
| PKC-α | 21.0 ± 8.7 | 2.0 ± 0.7 | 0.88 | 3.0 ± 0.9 | 2.0 ± 0.4 | 0.30 |
| PRKAA1 | 0.49 | 42.0 ± 16.7 | 50.0 ± 7.8 | 0.77 | 0.5 | 7.0 ± 2.4 |
| CDK-4 | 0.1 | 1.6 ± 0.3 | 0.47 | 9.0 ± 2.6 | 6.0 ± 1.5 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haffez, H.; Osman, S.; Ebrahim, H.Y.; Hassan, Z.A. Growth Inhibition and Apoptotic Effect of Pine Extract and Abietic Acid on MCF-7 Breast Cancer Cells via Alteration of Multiple Gene Expressions Using In Vitro Approach. Molecules 2022, 27, 293. https://doi.org/10.3390/molecules27010293
Haffez H, Osman S, Ebrahim HY, Hassan ZA. Growth Inhibition and Apoptotic Effect of Pine Extract and Abietic Acid on MCF-7 Breast Cancer Cells via Alteration of Multiple Gene Expressions Using In Vitro Approach. Molecules. 2022; 27(1):293. https://doi.org/10.3390/molecules27010293
Chicago/Turabian StyleHaffez, Hesham, Shimaa Osman, Hassan Y. Ebrahim, and Zeinab A. Hassan. 2022. "Growth Inhibition and Apoptotic Effect of Pine Extract and Abietic Acid on MCF-7 Breast Cancer Cells via Alteration of Multiple Gene Expressions Using In Vitro Approach" Molecules 27, no. 1: 293. https://doi.org/10.3390/molecules27010293






