The Active Phytohormone in Microalgae: The Characteristics, Efficient Detection, and Their Adversity Resistance Applications
Abstract
:1. Introduction
2. Characteristics of Plant Hormones
2.1. Auxin (IAA)
2.2. Cytokinin (CK)
2.3. Abscisic Acid (ABA)
2.4. Ethylene (ETH)
2.5. Others
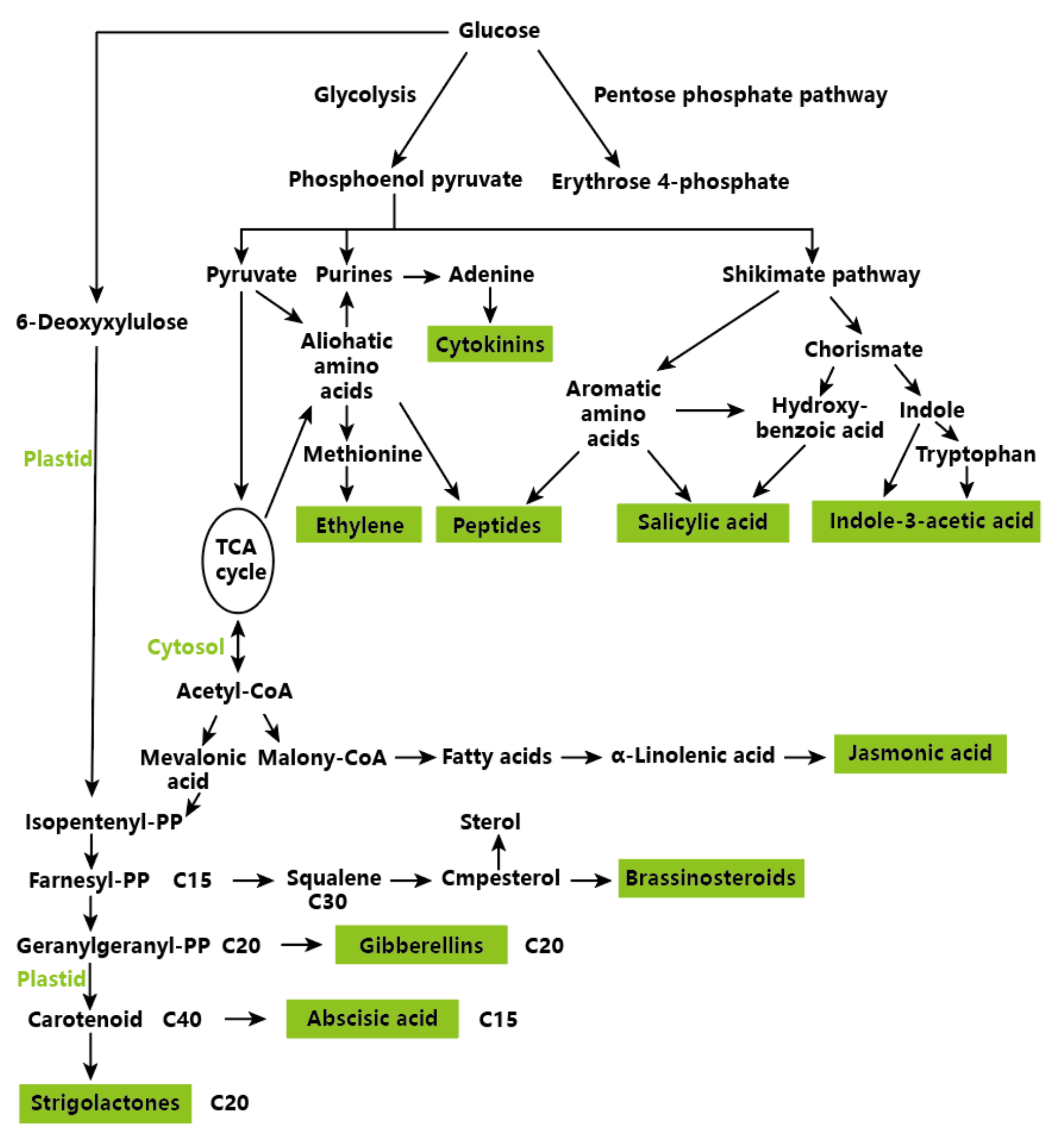
3. Biosynthesis of Plant Hormones
4. Determination and Analysis of Plant Hormones
4.1. Physical Chemistry Method
4.1.1. High-Performance Liquid Chromatography (HPLC)
4.1.2. Gas Chromatography (GC)
4.2. Electrochemical Analysis
4.3. Others
4.3.1. Biological Assay
4.3.2. Immunoassay
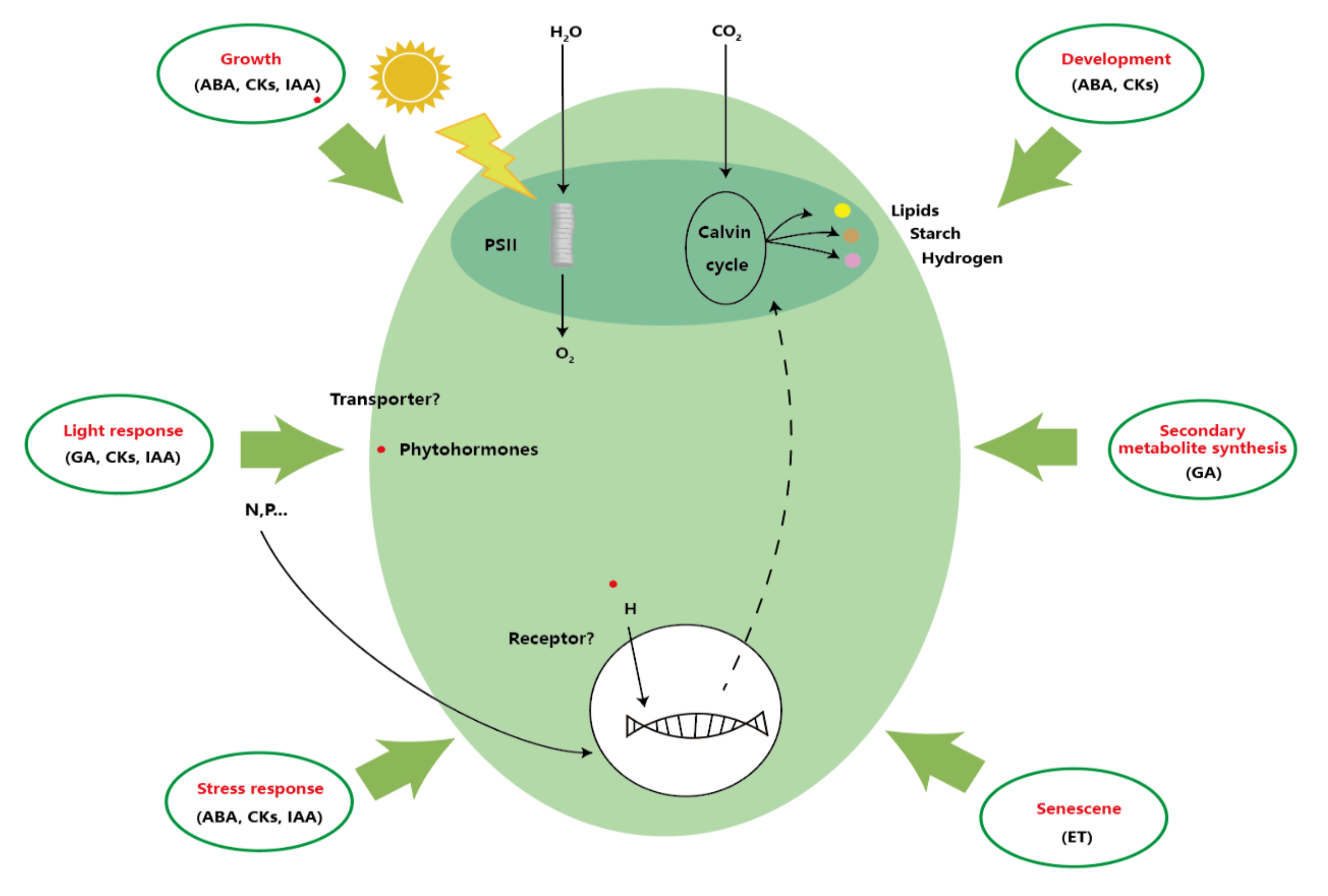
5. Application of Plant Hormones
5.1. Physiological Response
5.1.1. The Effect of Plant Hormones on Growth and Primary Metabolites
5.1.2. Effects of Plant Hormone on Oil Products
5.2. Stress Resistance Response of Plant Hormones
5.2.1. Nutrients
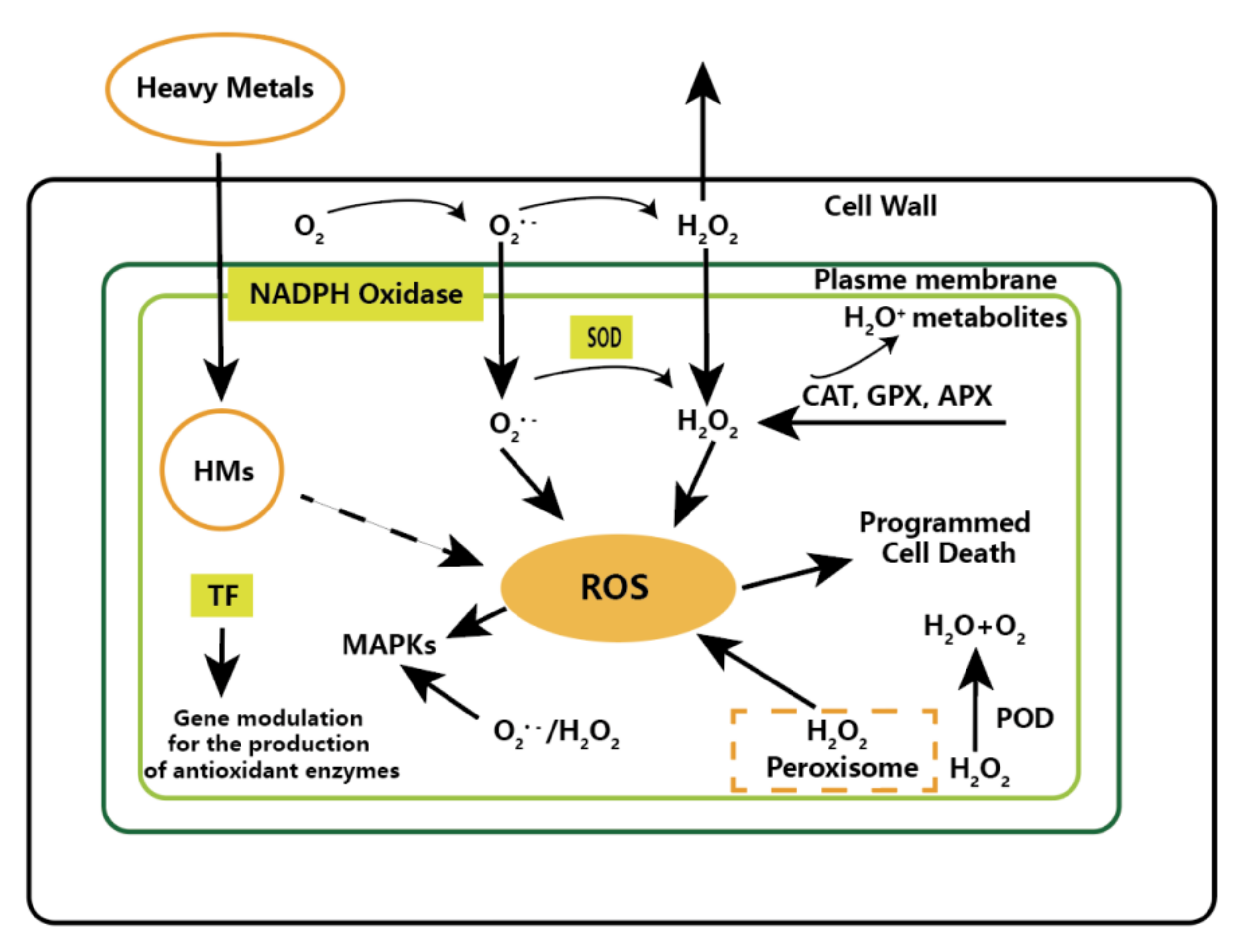
5.2.2. Heavy Metals
5.2.3. Light and Temperature
6. Future Perspectives and Conclusions
- (1)
- in-depth study of the molecular mechanism of plant hormones that regulate the metabolism of energy storage substances in microalgae;
- (2)
- the response and regulation mechanism of the metabolic process of microalgae, under stimulation of plant hormones and systematically studied by multi-omics combined analyses, and;
- (3)
- screening of cheaper and more effective plant hormones for metabolic regulation of microalgae under other stress conditions, such as high salinity and high light intensity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Other Ethics Statements
References
- Show, P.L.; Tang, M.S.Y.; Nagarajan, D.; Ling, T.C.; Ooi, C.W.; Chang, J.S. A holistic approach to managing microalgae for biofuel applications. Int. J. Mol. Sci. 2017, 18, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruk, G.; Gifuni, I.; Illiano, A.; Roxo, M.; Pinto, G.; Amoresano, A.; Marzocchella, A.; Piccoli, R.; Wink, M.; Olivieri, G.; et al. Simultaneous production of antioxidants and starch from the microalga Chlorella sorokiniana. Algal Res. 2018, 34, 164–174. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Imran, K.M.; Hyuk, S.J.; Deog, K.J. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell. Fact. 2018, 17, 1–21. [Google Scholar]
- Puja, O.; Renu, B.; Shagun, B.; Ravinderjit, K.; Shivam, J.; Anjali, K.; Parihar, R. The common molecular players in plant hormone crosstalk and signaling. Curr. Protein Pept. Sci. 2015, 16, 369–388. [Google Scholar]
- Voβ, U.; Bishopp, A.; Farcot, E.; Bennett, M.J. Modelling hormonal responseand development. Trends Plant Sci. 2014, 19, 311–319. [Google Scholar]
- Zhao, Y.T.; Wang, H.P.; Han, B.Y.; Yu, Y.Y. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: A review. Bioresour. Technol. 2019, 275, 549–556. [Google Scholar] [CrossRef]
- Park, W.K.; Yoo, G.; Moon, M.; Kim, C.W.; Choi, Y.-E.; Yang, J.W. Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Appl. Biochem. Biotechnol. 2013, 171, 1128–1142. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Babu, A.G.; Wu, X.G.; Kabra, A.K.; Kim, D. Cultivation of an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitation. Algal Res. 2017, 23, 178–185. [Google Scholar] [CrossRef]
- Yu, X.H.; Chen, L.; Zhang, W.W.; Angelis, M.D. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Squires, T.; Ambrose, S.; Abrams, S.; Andrew, R.S.; Adrian, R.; Cutler, A. Rapid extraction of abscisic acid and its metabolites for liquid chromatography-tandemmass spectrometry. J. Chromatogr. Biomed. Appl. 2003, 1010, 75–85. [Google Scholar]
- Rotino, G.L.; Perri, E.; Zottini, M.; Sommer, H.; Spena, A. Genetic engineering of parthenocarpic plants. Nat. Biotechnol. 1997, 15, 1398–1401. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Karnachuk, R.A.; Tishchenko, S.Y.; Golovatskaya, I.F. Endogenous Phytohormones and Regulation of Morphogenesis of Arabidopsis thalianaby Blue Light. Russ. J. Plant Physiol. 2001, 48, 226–230. [Google Scholar] [CrossRef]
- Pierik, R.; Tholen, D.; Poorter, H.; Visser, E.J.; Voesenek, L.A. The Janus face of ethylene: Growth inhibition and stimulation. Trends Plant Sci. 2006, 11, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.V.; Trewavas, A.J. Is Algal Development Controlled by Plant Growth Substances? Eur. J. Phycol. 1991, 27, 322–326. [Google Scholar] [CrossRef]
- Janmes, G.U. Green Algae and the Origins of Multicellularity in the Plant Kingdom. Cold Spring Harb. Perspect. Biol. 2014, 6, a016170. [Google Scholar]
- Leliaert, F.; Smith, R.D.; Moreau, H.; Herron, D.M.; Verbruggen, H.; Delwiche, F.C.; Clerck, D.O. Phylogeny and Molecular Evolution of the Green Algae. Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Kenrick, P.; Crane, P.R. The origin and early evolution of plants on land. Nature 1997, 389, 33–39. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Staden, J.V. Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Van Staden, J. Comparison of cytokinin-and auxin-like activity in some commercially used seaweed extracts. J. Appl. Phycol. 1996, 8, 503–508. [Google Scholar] [CrossRef]
- Romanenko, E.A.; Kosakovskaya, I.V.; Romanenko, P.A. Phytohormones of Microalgae: Biological Role and Involvement in the Regulation of Physiological Processes. Pt I. Auxins, Abscisic Acid, Ethylene. Int. J. Algae 2015, 17, 275–289. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Salama, E.S.; Kabra, A.N.; Ji, M.K.; Kim, J.R.; Min, B.; Jeon, B.H. Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour. Technol. 2014, 172, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Arumugam, M. Selective enrichment of Eicosapentaenoic acid (20:5n-3) in N. oceanica CASA CC201 by natural auxin supplementation. Bioresour. Technol. 2017, 242, 329–333. [Google Scholar]
- Srivastava, L.M. Plant growth and development: Hormones and environment. Ann. Bot. 2003, 92, pp1. [Google Scholar]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; Staden, J.V. Auxin and cytokinin relationships in 24 microalgal strains. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, P.; Morowvat, M.; Montazeri-Najafab, N.; Abolhassanz, Z.; Ghasemi, Y. Investigating the effects of phytohormones on growth and β-carotene production in a naturally isolates stain of Dunaliella salina. J. Appl. Pharm. Sci. 2016, 6, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Tomá, W.; Michael, R.; Eva, K.; Isabel, B.M. Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J. Plant Growth Regul. 2003, 116, 241–252. [Google Scholar]
- Anantharaman, V.; Aravind, L. The CHASE domain: A predicted ligand-binding module in plant cytokinin receptors and other eukaryotic and bacterial receptors. Trends Biochem. Sci. 2001, 26, 579–582. [Google Scholar] [CrossRef]
- Calzada, A.; Villa-Lojo, M.C.; Beceiro-González, E.; Alonso-Rodrı, E.; Prada-Rodr, D. Determination of arsenic species in environmental samples: Use of the alga Chlorella vulgaris for arsenic (III) retention. Trends Anal. Chem. 1998, 17, 167–175. [Google Scholar] [CrossRef]
- Yoshida, K.; Igarashi, E.; Wakatsuki, E.; Miyamoto, K.; Hirata, K. Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress-derived oxidative damage in Chlamydomonas reinhardtii. Plant Sci. 2004, 167, 1335–1341. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hirai, N.; Kurimura, Y.; Ohigashi, H.; Tsuji, Y. Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Regul. 1997, 22, 79–85. [Google Scholar] [CrossRef]
- Ju, C.; Poel, B.V.D.; Cooper, E.D.; Thierer, J.H.; Chang, C. Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat. Plants. 2015, 1, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Claudia, H.; Georg, G. Novel insights into the transfer routes of the essential copper cofactor to the ethylene plant hormone receptor family. Plant Signaling Behav. 2020, 15, 1716512. [Google Scholar]
- Vo, T.T.; Lee, C.; Han, S.I.; Kim, J.Y.; Kim, S.; Choi, Y.E. Effect of the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid on different growth stages of Haematococcus pluvialis. Bioresour. Technol. 2016, 220, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Minges, A.; Groth, G. Interfering Peptides Targeting Protein–Protein Interactions in the Ethylene Plant Hormone Signaling Pathway as Tools to Delay Plant Senescence. Plant Chemical Genomics. 2021, 2213, 71–85. [Google Scholar]
- Kim, S.H.; Lim, S.R.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J. Agric. Food Chem. 2016, 64, 4807–4816. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The Current Status of Research on Gibberellin Biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.H.; Zhu, J.; Jiang, J.G. High-value bioproducts from microalgae: Strategies and progress. Front. Microb. 2018, 59, 2423–2441. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Sun, J.; Sun, Y.Q.; Zheng, J.Y.; Wang, Z. Metabolomics analysis of phytohormone gibberellin improving lipid and DHA accumulation in Aurantiochytrium sp. Biochem. Eng. J. 2016, 112, 258–268. [Google Scholar] [CrossRef]
- Pan, X.J.; Chang, F.Y.; Kang, L.J.; Liu, Y.D. Effects of gibberellin A (3) on growth and microcystin production in Microcystis aeruginosa (cyanophyta). J. Plant Physiol. 2008, 165, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Lin, Z.; Wang, Y.; Chai, H.; Zhou, J. Facilitating effects of plant hormoneson biomass production and nutrients removal by Tetraselmis cordiformis for advanced sewage treatment and its mechanism. Sci. Total Environ. 2019, 693, 133650. [Google Scholar] [CrossRef]
- Talarek-Karwel, M.; Andrzej, B.; Alicja, P.; Iwona, R. The effect of 24-epibrassinolide on the green alga Acutodesmus obliquus (Chlorophyceae). Plant Physiol. Biochem. 2018, 124, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Strnad, M.; Staden, J.; Ördög, V. Endogenous brassinosteroids in microalgae exposed to salt and low temperature stress. Eur. J. Phycol. 2018, 53, 273–279. [Google Scholar] [CrossRef]
- Andrzej, B.; Alicja, P.N. Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol. Biochem. 2013, 71, 290–297. [Google Scholar]
- Mirshekari, M.; Einali, A.; Valizadeh, J. Metabolic changes and activity pattern of antioxidant enzymes induced by salicylic acid treatment in green microalga Dunaliella salina under nitrogen deficiency. J. Appl. Phycol. 2019, 31, 1709–1719. [Google Scholar] [CrossRef]
- Xu, J.H.; Fan, X.J.; Li, X.X.; Liu, G.F.; Zhang, Z.Y.; Zhu, Y.C.; Fu, Z.W.; Qian, H.F. Effect of salicylic acid on fatty acid accumulation in Phaeodactylum tricornutum during stationary growth phase. J. Appl. Phycol. 2017, 29, 2801–2810. [Google Scholar] [CrossRef]
- Lee, J.E.; Cho, Y.U.; Kim, K.H.; Lee, D.Y. Distinctive metabolomic responses of Chlamydomonas reinhardtii to the chemical elicitation by methyl jasmonate and salicylic acid. Process Biochem. Int. 2016, 51, 1147–1154. [Google Scholar] [CrossRef]
- Kiseleva, A.A.; Tarachovskaya, E.R.; Shishova, M.F. Biosynthesis of phytohormones in algae. Russ. J. Plant Physiol. 2012, 59, 595–610. [Google Scholar] [CrossRef]
- Middleton, A.M.; Úbeda-Tomás, S.; Griffiths, J.; Holman, T.; Hedden, P.; Thomas, S.G.; Phillips, A.L.; Holdsworth, M.J.; Bennett, M.J.; King, J.R.; et al. Mathematical modeling elucidates the role of transcriptional feedback in gibberellin signaling. Proc. Natl. Acad. Sci. USA 2012, 19, 7571–7576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bail, A.L.; Billoud, B.; Kowalczyk, N.; Kowalczyk, M.; Gicquel, M.; Panse, S.L.; Stewart, S.; Scornet, D.; Cock, J.M.; Ljung, K.; et al. Auxin Metabolism and Function in the Multicellular Brown Alga Ectocarpus siliculosus. Plant Physiol. 2010, 153, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Bennett, T.; Leyser, O. The Auxin Question: A Philosophical Overview. Auxin Its Role Plant Dev. 2014, 1, 3–19. [Google Scholar]
- Wu, X.X.; Chen, J.L.; Xu, S.; Zhu, Z.W.; Zha, D.S. Exogenous 24-epibrassinolide alleviates zinc-induced toxicity in eggplant (Solanum melongena L.) seedlings by regulating the glutathione-ascorbate-dependent detoxification pathway. J. Pomol. Hortic. Sci. 2016, 91, 412–420. [Google Scholar]
- Srivastava, S.; Srivastava, A.K.; Suprasanna, P.; D’Souza, S.F. Identification and profiling of arsenic stress-induced microRNAs in Brassica juncea. J. Exp. Bot. 2012, 64, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska-Niczyporuk, A.; Bajguz, A. The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul. 2014, 73, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Tarkowska, D.; Tureckova, V.; Luo, T.; Xin, Y.; Li, J.; Wang, Q.; Jiao, N.; Strnad, M.; Xu, J. Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J. 2014, 80, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Hoyerová, K.; Gaudinová, A.; Malbeck, J.; Dobrev, P.; Kocábek, T.; Šolcová, B.; Trávníčková, A.; Kamínek, M. Efficiency of different methods of extraction and purification of cytokinins. Phytochemistry 2006, 67, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.J.; Zhu, J.; Ding, M.Y.; Lv, G.H. Simultaneous determination of gibberellicacid, indole-3-acetic acid and abscisic acid in wheat extracts by solid-phase extractionand liquid chromatography-electrospray tandem mass spectrometry. Talanta 2008, 76, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Birkemeyer, C.; Kolasa, A.; Kopka, J. Comprehensive chemical derivatization for gas chromatography-mass spectrometry-based multi-targeted profiling of the major phytohormones. J. Chromatogr. A 2003, 993, 89–102. [Google Scholar] [CrossRef]
- Chen, G.N.; Zhao, Z.F.; Wang, X.L.; Duan, J.P.; Chen, H.Q. Electrochemical behavior of tryptophan and its derivatives at a glassy carbon electrode modified with hemin. Anal. Chim. Acta. 2002, 452, 245–254. [Google Scholar]
- Hernández, P.; Dabrio-Ramos, M.; Patón, M.; Ballesteros, Y.; Hernández, L. Determination of abscisic acid by cathodic stripping square wave voltammetry. Talanta 1997, 44, 1783–1792. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.Y.; Xiao, L.T.; Zeng, G.M.; Huang, G.H.; Shen, G.L.; Yu, R.Q. A Novel Piezoelectric Biosensor for the Detection of Phytohormone β-Indole Acetic Acid. Anal. Sci. 2002, 18, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.; Eriksson, T. A Revised Medium for Growth of Pea Mesophyll Protoplasts. Physiol. Plant. 1977, 39, 257–260. [Google Scholar] [CrossRef]
- Plettner, I.; Steinke, M.; Malin, G. Ethene (ethylene) production in the marine macroalga Ulva (Enteromorpha) intestinalis L. (Chlorophyta, Ulvophyceae): Effect of light-stress and co-production withdimethyl sulphide. Plant Cell Environ. 2005, 28, 1136–1145. [Google Scholar] [CrossRef]
- Arney, S.E.; Mitchell, D.L. The Effect of Abscisic Acid on Stem Elongation and Correlative Inhibition. New Phytol. 1969, 69, 1001–1015. [Google Scholar] [CrossRef]
- Grayling, A.; Hanke, D.E. Cytokinins in exudates from leaves and roots of red Perilla. Pergamon Ser. Environ. Sci. 1992, 31, 1863–1868. [Google Scholar] [CrossRef]
- Régis, M.; Bernard, L.; Issam, S.; Bruno, S.; Lucienne, S.; Emile, M. A biotin-avidin-based enzyme immunoassay to quantify three phytohormones: Auxin, abscisic acid and zeatin-riboside. J. Immunol. Methods 1986, 90, 151–158. [Google Scholar]
- Swaczynová, J.; Novák, O.; Hauserová, E.; Fuksová, K.; Šíša, M.; Kohout, L.; Strnad, M. New Techniques for the Estimation of Naturally Occurring Brassinosteroids. J. Plant Growth Regul. 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and Effects on Growth and Metabolites of Microalgae: A Review. Fermentation 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Stirk, W.A.; Tarkowska, B.; Maroti, N.; Tureckova, L.; Ordog, S. Effect of light on growth and endogenous hormones in Chlorella minutissima (Trebouxiophyceae). Plant Physiol. Biochem. 2014, 79, 66–76. [Google Scholar] [CrossRef]
- Dao, G.H.; Wu, G.X.; Wang, X.X.; Zhuang, L.L.; Zhang, T.Y.; Hu, H.Y. Enhanced growth and fatty acidaccumulation of microalgae Scenedesmus sp. LX1 by two types of auxin. Bioresour. Technol. 2018, 247, 561–567. [Google Scholar] [CrossRef]
- Czerpak, R.; Bajguz, A.; Białecka, B.; Wierzchołowska, L.E.; Wolanska, M.M. Effect of auxin precursors andchemical analogues on the growth and chemical composition in Chlorella pyrenoidosa Chick. Acta Soc. Bot. Pol. 1994, 63, 279–286. [Google Scholar] [CrossRef]
- Czerpak, R.; Piotrowska, A.; Szulecka, K. Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris. Acta Physiol. Plant. 2006, 28, 195–203. [Google Scholar] [CrossRef]
- Bajguz, A. Effect of brassinosteroids on nucleic acids and protein content in cultured cells of Chlorella vulgaris. Plant Physiol. Biochem. 2000, 38, 209–215. [Google Scholar] [CrossRef]
- Piotrowska, A.; Czerpak, R. Cellular response of light/dark-grown green alga Chlorella vulgaris Beijerinck (Chlorophyceae) to exogenous adenine and phenylurea-type cytokinins. Acta Physiol. Plant. 2009, 31, 573–585. [Google Scholar] [CrossRef]
- Czerpak, R.; Bajguz, A.; Gromek, M.; Kozowska, G.; Nowak, I. Activity of salicylic acid on the growth and biochemism of Chlorella vulgaris Beijerinck. Acta Physiol. Plant. 2002, 24, 45–52. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Interactive effect of brassinosteroids and cytokinins on growth, chlorophyll, monosaccharide and protein content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol. Biochem. 2014, 80, 176–183. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, W.; Song, Y. Stimulatory effect of auxins on the growth and lipid productivity of Chlorella pyrenoidosa and Scenedesmus quadricauda. Algal Res. 2016, 18, 273–280. [Google Scholar] [CrossRef]
- ElSayed, T.H.; Almutairi, A.W. Effect of Phytohormones Supplementation under Nitrogen Depletion on Biomass and Lipid Production of Nannochloropsis oceanica for Integrated Application in Nutrition and Biodiesel. Sustainability. 2021, 13, 592. [Google Scholar]
- Du, K.; Tao, H.; Wen, X.; Geng, Y.; Li, Y. Enhanced growth and lipid production of Chlorella pyrenoidosa by plant growth regulator GA3. Fresenius Environ. Bull. 2015, 24, 3414–3419. [Google Scholar]
- Kozlova, T.A.; Hardy, B.P.; Krishna, P.; Levin, D.B. Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Res. 2017, 27, 325–334. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Singh, P.; Ansari, F.A.; Rawat, I.; Bux, F. Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energy Convers. Manag. 2017, 140, 14–23. [Google Scholar] [CrossRef]
- Warren, K.C.; Terry, J.H.; David, M.R.; Trevor, A.T. Ethylene as an endogenous inhibitor of root regeneration in tomato leaf discs cultured in vitro. Physiol. Plant. 1980, 48, 519–525. [Google Scholar]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.J.; Lovitt, R.W.; Flynn, K.J. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2020, 46, 703–726. [Google Scholar] [CrossRef] [Green Version]
- Salama, E.S.; Hwang, J.H.; El-Dalatony, H.M.; Kurade, M.B.; Kabra, A.N.; Abou-Shanab, R.A.I.; Kim, K.H.; Yang, I.S.; Govindwar, S.P.; Sunjoon, K.; et al. Enhancement of microalgal growth and biocomponent-based transformations for improved biofuel recovery: A review. Bioresour. Technol. 2018, 258, 365–375. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef]
- Imran, P.; Kaumeel, C.; Rahulkumar, M.; Khanjan, T.; Kumar, P.S.; Arup, G.; Sandhya, M. Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 189, 341–348. [Google Scholar]
- Kaumeel, C.; Imran, P.; Khanjan, T.; Basil, G.; Rahulkumar, M.; Arup, G.; Sandhya, M. Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour. Technol. 2015, 180, 162–171. [Google Scholar]
- Hockin, N.L.; Mock, T.; Mulholland, F.; Malin, K.G. The Response of Diatom Central Carbon Metabolism to Nitrogen Starvation is Different from That of Green Algae and Higher Plants. Plant Physiol. 2012, 158, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresour. Technol. 2017, 24, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Sulochana, S.B.; Arumugam, M. Influence of abscisic acid on growth, biomass and lipid yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresour. Technol. 2016, 213, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Salar, F.A.; Kazem, G.G. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016. [Google Scholar]
- Nguyen, H.N.; Kisiala, A.B.; Emery, R.J.N. The roles of phytohormones in metal stress regulation in microalgae. J. Appl. Phycol. 2020, 32, 3817–3829. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.; Lau, B.F.; Chang, J.S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Bashri, G.; Prasad, S.M. Impact of Cd stress on cellular functioning and its amelioration by phytohormones: An overview on regulatory network. Plant Growth Regul. 2016, 80, 253–263. [Google Scholar] [CrossRef]
- Virgilio, H.; Mario, J.; Olivia, R. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka-Szelewa, E.; Bralska, M. Exogenously applied auxins and cytokinins ameliorate lead toxicity by inducing antioxidant defence system in green alga Acutodesmus obliquus. Plant Physiol. Biochem. 2018, 132, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Falkowska, M.; Pietryczuk, A.; Piotrowska, A.; Bajguz, A.; Grygoruk, A.; Czerpak, R. The effect of gibberellicacid (GA3) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (Chlorophyceae) Beijerinck exposed to cadmium and lead stress. Pol. J. Environ. Stud. 2011, 20, 53–59. [Google Scholar]
- Bajguz, A. Brassinosteroids and lead as stimulators of phytochelatins synthesis in Chlorella vulgaris. J. Plant Physiol. 2013, 159, 321–324. [Google Scholar] [CrossRef]
- Raman, V.; Ravi, S. Effect of salicylic acid and methyl jasmonate on antioxidant systems of Haematococcus pluvialis. Acta Physiol. Plant. 2011, 33, 1043–1049. [Google Scholar] [CrossRef]
- Ding, W.; Zhao, Y.T.; Xu, J.W.; Zhao, P.; Li, T.; Ma, H.X.; Reiter, R.J.; Yu, X.Y. Melatonin: A multifunctional molecule that triggers defence responses against high light and nitrogen starvation stress in Haematococcus pluvialis. J. Agric. Food Chem. 2018, 66, 7701–7711. [Google Scholar] [CrossRef]
- Wang, Y.; He, B.; Sun, Z.; Chen, Y.F. Chemically enhanced lipid production from microalgae under low sub-optimal temperature. Algal Res. 2016, 16, 20–27. [Google Scholar] [CrossRef]
| Structural Formula | Targets Promoted | Reference | |
|---|---|---|---|
| Auxins |  | Growth Lipid Chlorophyll-a Soluble proteins | [25] [26] [27] |
| Cytokinin | 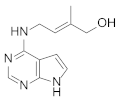 | Biomass Lipid Carbohydrate Proteins | [29] [30] |
| Abscisic acid | 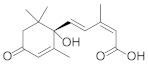 | Growth β-carotene Lipid Carotenoids | [35] |
| Ethylene | 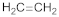 | A-tocopherol C-aminobutyric acid Proline Astaxanthin | [37] [38] [40] |
| Gibberellin A4 | 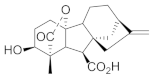 | Biomass Lipid Carbohydrate Proteins | [42] [43] [44] |
| Brassinolide | 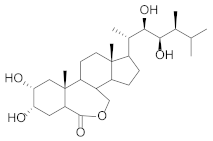 | Protein Nucleic acid Carbohydrate Photosynthetic pigments | [46] [47] |
| Jasmonoyl-isoleucine | 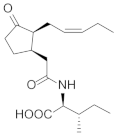 | Carbohydrates Proteins Lipids Carotenoids | [51,52,53] |
| Salicylic acid | 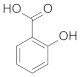 | Astaxanthin β-carotene Carbohydrates Proteins | [50] [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Qi, M.; Guo, J.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The Active Phytohormone in Microalgae: The Characteristics, Efficient Detection, and Their Adversity Resistance Applications. Molecules 2022, 27, 46. https://doi.org/10.3390/molecules27010046
Wang C, Qi M, Guo J, Zhou C, Yan X, Ruan R, Cheng P. The Active Phytohormone in Microalgae: The Characteristics, Efficient Detection, and Their Adversity Resistance Applications. Molecules. 2022; 27(1):46. https://doi.org/10.3390/molecules27010046
Chicago/Turabian StyleWang, Chun, Mei Qi, Jiameng Guo, Chengxu Zhou, Xiaojun Yan, Roger Ruan, and Pengfei Cheng. 2022. "The Active Phytohormone in Microalgae: The Characteristics, Efficient Detection, and Their Adversity Resistance Applications" Molecules 27, no. 1: 46. https://doi.org/10.3390/molecules27010046
APA StyleWang, C., Qi, M., Guo, J., Zhou, C., Yan, X., Ruan, R., & Cheng, P. (2022). The Active Phytohormone in Microalgae: The Characteristics, Efficient Detection, and Their Adversity Resistance Applications. Molecules, 27(1), 46. https://doi.org/10.3390/molecules27010046






