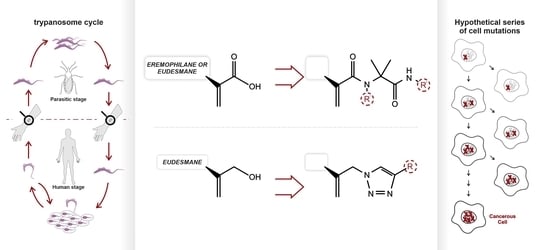
3.4. Spectroscopic and Physical Data
Compound [1]: White solid, m.p.: 155–156 °C. [α]D20: −143.4 (c 1.7, CHCl3). IR (KBr; υmax: cm−1): 2974, 1709, 1620, 1460, 1306, 1248, 1080. 1H-NMR (δ = ppm, 200 MHz): 0.98 (3H, s, Me-15); 1.08 (3H, s, Me-14); 1.51–1.68 (1H, m, H-6); 1.76–1.93 (2H, m, H-6, H-8); 1.93–2.12 (1H, m, H-8); 2.23–2.40 (4H, m, H-4, H-3, H-3a, H-9); 2.48–2.69 (2H, m, H-9, H-7); 5.69 (1H, s, H-13); 5.90 (1H, s, H-1); 6.36 (1H, s, H-13). 13C-NMR (δ = ppm, 50 MHz): 15.4 (C-15); 19.1 (C-14); 28.9 (C-9); 29.2 (C-8); 32.3 (C-7); 35.8 (C-4); 39.4 (C-6); 40.3 (C-5); 42.0 (C-3); 125.3 (C-13); 125.7 (C-1); 143.8 (C11); 171.8 (C-12); 173.9 (C-10); 199.6 (C-2). HRMS-ES (m/z): [M + Na]+: Calcd. for C15H19O3Na: 293.1130; found: 293.1127.
Compound [2]: Yellowish solid, m.p.: 181–182 °C. [α]D20: −32.8 (c 5.1; CHCl3). IR (KBr; υmax: cm−1): 3431, 2929, 1691, 1452, 1244, 1041, 863. 1H-NMR (δ = ppm, 200 MHz): 0.91 (3H, s, Me-14); 1.03–1.17 (1H, m, H-9); 1.17–1.25 (1H, m, H-6); 1.11 (3H, s, Me-15); 1.25–1.39 (2H, m, H-3, H-5); 1.39–1.52 (4H, m, H-1, H-2, H-3, H-9); 1.55–1.71 (3H, m, H-2, H-8, H-8a); 1.76-1.89 (1H, m, H-3); 1.91–2.03 (1H, m, H-6); 2.40–2.66 (1H, m, H-7); 5.0 (1H, s, H-13); 6.29 (1H, s, H-13). 13C-NMR (δ = ppm, 50 MHz): 18.7 (C-14); 20.1 (C-8); 22.4 (C-15); 26.6 (C-6); 27.1 (C-2); 34.6 (C-10); 40.0 (C-7); 40.9 (C-9); 43.4 (C-3); 44.5 (C-1); 55.0 (C-5); 72.4 (C-4); 124.5 (C-13); 145.1 (C-11); 171.6 (C-12). HRMS-ES (m/z): [M + Na]+: Calcd. for C15H19O3Na: 275.1623; found: 275.1620.
Compound [3]: White solid, m.p.: 134–135 °C. [α]D20: −45.0 (c 2.2; CHCl3). IR (KBr; υmax: cm−1): 3265, 2924, 2845, 1605, 1444, 1385, 1171, 1072, 889. 1H-NMR (δ = ppm, 200 MHz): 0.90 (3H, s, Me-14); 1.088–1.15 (1H, m, H-9); 1.11 (3H, s, Me-15); 1.15–1.31 (3H, m, H-1, H-5, H-6); 1.35–1.47 (3H, m, H-1, H-3, H-9); 1.47–1.66 (4H, m, H-2, H-2a, H-8, H-8a); 1.73–1.86 (1H, m, H-3); 1.88–1.97 (1H, m, H-6); 1.99–2.21 (1H, m, H-7); 4.14 (2H, s, H-12); 4.91 (1H, s, H-13); 5.02 (1H, s, H-13). 13C-NMR (δ = ppm, 50 MHz): 18.7 (C-14); 20.1 (C-8); 22.6 (C-15); 26.5 (C-6); 27.1 (C-2); 34.6 (C-10); 41.0 (C-9); 41.8 (C-7); 43.3 (C-3); 44.6 (C-1); 54.8 (C-5); 65.2 (C-12); 72.4 (C-4); 107.9 (C-13); 153.9 (C-11). HRMS-ES (m/z): [M + Na]+: Calcd. for C15H26O2Na: 261.1830; found: 261.1831.
Compound [4]: White solid, m.p.: 50–52 °C. [α]D20: −73.2 (c 10.21; CHCl3). IR (KBr; υmax: cm−1): 3442, 2962, 2359, 1653, 1508, 1362, 1279, 1163, 706. 1H-NMR: (δ = ppm, 600 MHz): 0.94 (3H, d, J = 7 Hz, Me-15); 1.03 (3H, s, Me-14); 1.38–1.39 (6H, s, H-1′, H-2′); 1.41 (9H, s; H-7′; H-8′; H-9′); 1.47–1.50 (1H, m, H-6); 1.65–1.68 (1H, m, H-6); 1.68–1.72 (2H, m, H-8); 1.99–2.07 (2H; m; H-4, H-7); 2.16–2.22 (1H, m, H-9); 2.24–2.30 (2H, m, H-3); 2.46–2.53 (1H, m, H-9); 4.94 (1H, d, J = 2 Hz, H-13); 4.99 (1H, s, H-13); 5.70 (1H, s, H-5′); 5.80 (1H, s, H-1′); 7.31–7.33 (3H, m, H-11′, H-13′, H-15′); 7.23–7.25 (2H, m, H12′, H-14′). 13C-NMR (δ = ppm, 150 MHz):15.5 (C-15); 19.0 (C-14); 25.5, 25.6 (C-1′,C-2′); 28.6 (C-7′,8′,9′); 28.7 (C-8); 28.8 (C-9); 34.4 (C-7); 36.3 (C-4); 38.8 (C-6); 40.1 (C-5); 42.2 (C-3); 51.1 (C-6′); 63.1 (C-3′); 115.5 (C-13); 125.5 (C-1); 128.1 (C-13′); 128.6 (C-11′,C15′); 131.2 (C-12′,C14′); 139.8 (C-10′); 149.5 (C-11); 171.2 (C-12); 173.6 (C-4′); 173.7 (C-10); 199.2 (C-2). HRMS-ES (m/z) [M + Na]+: Calcd. for C29H40N2O3Na: 487.2937; found 487.2927.
Compound [5]: Yellow solid, m.p.: 65–67 °C. [α]D20: −13.7 (c 8.35; CHCl3). IR (KBr; υmax: cm−1): 3421, 2926, 2360, 1653, 1541, 1458, 1171. 1H-NMR: (δ = ppm, 200 MHz): 0.93 (3H, d, J = 7 Hz, Me-15); 1.05 (3H, s, Me-14); 1.36 (9H, s; H-7′; H-8′; H-9′); 1.42–1.43 (6H, s, H-1′, H-2′); 1.57–1.63 (1H, m, H-6); 1.80–1.92 (3H, m, H-6, H-8, H-8a); 1.93–2.04 (1H, m, H-4); 2.19–2.28 (3H; m; H-3, H-3a, H-9); 2.32–2.42 (1H, m, H-7); 2.51–2.61 (1H, m, H-9); 4.70 (2H, s, H-10′); 5.03 (1H, s, H-13); 5.20 (1H, s, H-13); 5.5 (1H, s, H-5′); 5.8 (1H, s, H-1′); 7.25–7.27 (1H, m, H-14′), 7.33–7.37 (4H, m, H-12′, H-13′, H-15′,H-16′). 13C-NMR (δ = ppm, 50 MHz): 15.5 (C-15); 19.1 (C-14); 24.4, 24.5 (C-1′,C-2′); 28.4 (C-8); 28.6 (C-7′,8′,9′); 28.8 (C-9); 35.1 (C-7); 36.1 (C-4); 39.0 (C-6); 40.1 (C-5); 42.2 (C-3); 50.3 (C-10′); 51.0 (C-6′); 62.9 (C-3′); 112.3 (C-13); 125.7 (C-1); 126.1 (C-12′,C-16′); 127.2 (C-14′); 128.8 (C-13′,C15′); 139.5 (C-11′); 149.5 (C-11); 173.0 (C-10); 173.4 (C-12); 173.9 (C-4′); 199.0 (C-2). HRMS-ES (m/z) [M + Na]+: Calcd. for C30H42N2O3Na: 501.3089; found: 501.3093.
Compound [6]: Yellow solid, m.p.: 70–71 °C. [α]D20: −90.5 (c 9.96; CHCl3). IR (KBr; υmax: cm−1): 3365, 2931, 2359, 1653, 1518, 1491, 1365, 1282, 1167, 702. 1H-NMR: (δ = ppm, 600 MHz): 0.93 (3H, d, J = 7 Hz, Me-15); 1.03 (3H, s, Me-14); 1.39–1.45 (1H, m, H-6); 1.42, 1.49 (6H, s, H-1′, H-2′); 1.64–1.68 (1H, m, H-8); 1.70–1.74 (2H, m; H-6,H-8); 2.04–2.09 (1H, m, H-4); 2.10–2.15 (1H, m, H-7); 2.17–2.22 (1H, m, H-9); 2.24–2.30 (2H, m, H-3, H-3a); 2.45–2.52 (1H, m, H-9); 4.60 (2H, d, J = 6 Hz, H-6′); 4.95 (1H, s, H-13); 4.98 (1H, s, H-13); 5.8 (1H, s, H-1′); 6.1 (1H, t, J = 5 Hz, H-5′); 7.25–7.28 (2H, m, H-15′, H-17′); 7.29–7.31 (1H, m, H-16′); 7.31–7.37 (5H, m, H-9′, H-10′, H-11′, H-14′, H-18′); 7.38–7.40 (2H, m, H-8′, H-12′). 13C-NMR (δ = ppm, 150 MHz): 15.4 (C-15); 19.1 (C-14); 25.4, 25.7 (C-1′,C-2′); 28.7 (C-9); 28.8 (C-8); 34.3 (C-7); 35.9 (C-4); 38.6 (C-6); 40.2 (C-5); 42.2 (C-3); 44.0 (C-6′); 62.7 (C-3′); 116.1 (C-13); 125.6 (C-1); 127.4 (C-16′); 127.8 (C-8′,C-12′); 128.2, 128.6, 128.7 (C-9′,C-10′,C-11′,C-12′,C-14′,C-18′); 131.3 (C-15′,C-17′); 138.5 (C-7′); 139.4 (C-13′); 149.0 (C-11); 171.5 (C-12); 173.7 (C-10); 174.5 (C-4′); 199.2 (C-2). HRMS-ES (m/z) [M + Na]+: Calcd. for C32H38N2O3Na: 521.2778; found: 521.2780.
Compound [7]: Yellow solid, m.p.: 70–72 °C. [α]D20: −74.7 (c 8.53; CHCl3). IR (KBr; υmax: cm−1): 3361, 2935, 2359, 1653, 1522, 1362, 1456, 1171, 698. 1H-NMR: (δ = ppm, 600 MHz): 0.93 (3H, d, J = 7 Hz, Me-15); 1.07 (3H, s, Me-14); 1.50–1.54 (1H, m, H-6); 1.50, 1.54 (6H, s, H-1′, H-2′); 1.83–1.90 (3H, m, H-6, H-8, H-8a); 1.97–2.02 (1H, m, H-4); 2.22–2.27 (3H, m, H-3, H-3a, H-9); 2.38–2.45 (1H, m, H-7); 2.52–2.59 (1H, m, H-9); 4.51 (2H; t; J = 5 Hz, H-6′); 4.78 (2H, d, J = 3 Hz, H-13′); 5.05 (1H, d, J = 2 Hz, H-13); 5.3 (1H, s, H-13); 5.8 (1H, s, H-1′); 6.0 (1H, t, J = 5 Hz, H-5′); 7.27–7.31 (2H, m, H-10′, H-17′); 7.34–7.38 (6H, m, H8′, H-9′, H-11′, H-12′, H-16′, H-18′); 7.41–7.44 (2H, m, H-15′, H-19′). 13C-NMR (δ = ppm, 150 MHz): 15.5 (C-15); 19.1 (C-14); 24.3, 24.6 (C-1′,C-2′); 28.4 (C-8); 28.8 (C-9); 34.9 (C-4); 35.8 (C-7); 38.7 (C-6); 40.2 (C-5); 42.1 (C-3); 43.9 (C-6′); 50.3 (C-13′); 62.5 (C-3′); 112.6 (C-13); 125.7 (C-1); 126.1 (C-15′,C-19′); 127.2, 127.4 (C-10′, C17′); 127.8, 128.7, 128.8 (C-8′, C-9′, C-11′, C-12′, C16′, C-18′); 138.5 (C-7′); 139.3 (C-14′); 149.0 (C-11); 173.2 (C-10); 173.6 (C-12); 174.7 (C-4′); 199.1 (C-2). HRMS-ES (m/z) [M + Na]+: Calcd. for C33H40N2O3Na: 535.2934; found: 535.2937.
Compound [8]: Amorphous solid, m.p.: 73–74 °C. [α]D20: −85.9 (c 9.66; CHCl3). IR (KBr; υmax: cm−1): 3385, 2927, 2360, 1653, 1541, 1456, 1363, 1169, 669. 1H-NMR: (δ = ppm, 600 MHz): 0.92 (3H, d, J = 7 Hz, Me-15); 1.00 (3H, s, Me-14); 1.12–1.23 (3H, m, H-7′/H-11′, H-9′); 1.34–1.43 (2H, m, H-8′/H-10′, H-6); 1.37, 1.40 (6H, s, H-1′, H-2′); 1.58–1.73 (7H, m, H-6, H-8, H-8a, H-8′/H-10′, H-9′); 1.92–2.10 (4H, m, H-4, H-7, H-7′/H-11′); 2.14–2.23 (1H, m, H-9); 2.23–2.36 (2H, m, H-3, H-3a); 2.37–2.56 (1H, m, H-9); 3.78 (1H; m, H-6′); 4.91 (1H, s, H-13); 4.97 (1H, s, H-13); 5.66 (1H, d, J = 8 Hz, H-5′); 5.78 (1H, s, H-1); 7.19–7.26 (2H, m, H-13′, H-17′); 7.27–7.34 (3H, m, H-14′, H-15′, H-16′). 13C-NMR: (δ = ppm, 150 MHz): 15.4 (C-15); 19.0 (C-14); 24.2, 24.4 (C-1′,C-2′); 24.9 (C-8, C-10′); 25.6 (C-9′); 28.8 (C-8, C-9); 33.0 (C-7′, C-11′); 34.2 (C-7); 36.0 (C-4); 38.7 (C-6); 40.1 (C-5); 42.2 (C-3); 48.5 (C-6′); 62.7 (C-3′); 115.6 (C-13); 125.5 (C-1); 128.1 (C-15′); 128.5 (C-14′,C-16′); 131.2 (C13′,C17′); 139.9 (C-12′); 149.3 (C-11); 171.4 (C-12); 173.6 (C-4′,C-10); 199.1 (C-2). HRMS-ES (m/z) [M + Na]+: Calcd. for C31H42N2O3Na: 513.3093; found: 513.3099.
Compound [9]: Amorphous solid, m.p.: 75–77 °C. [α]D20: −62.6 (c 9.96; CHCl3). IR (KBr; υmax: cm−1): 3423, 2931, 2852, 1655, 1522, 1387, 1179, 729. 1H-NMR: (δ = ppm, 600 MHz): 0.92 (3H, d, J = 7 Hz, Me-15); 1.04 (3H, s, Me-14); 1.16–1.27 (3H, m, H-7′/H-11′, H-9′); 1.29–1.37 (2H, m, H-8′/H-10′); 1.43, 1.45 (6H, s, H-1′, H-2′); 1.48–1.56 (1H, m, H-6); 1.555–1.634 (1H, m, H-9′); 1.692–1.777 (2H, m, H-8′/H-10′); 1.803–1.917 (3H, m; H-6, H-8, H-8a); 1.92–1.99 (3H, m, H-4, H-7′/H-11′); 2.18–2.26 (3H, m, H-3, H-3a, H-9); 2.30–2.44 (1H, m, H-7); 2.47–2.64 (1H, m, H-9); 3.74 (1H, m, H-6′); 4.70 (2H, s, H-12′); 5.0 (1H, s, H-13); 5.2 (1H, s, H-13); 5.5 (1H, d, J = 8 Hz, H-5′); 5.8 (1H, s, H-1); 7.27–7.32 (1H, m, H-16′); 7.34–7.39 (2H, t, J = 7 Hz, H-15′, H-17′); 7.41–7.48 (2H, m, H-14′, H-18′). 13C-NMR: (δ = ppm, 150 MHz): 15.4 (C-15); 19.1 (C-14); 24.2, 24.4 (C-1′, C-2′); 24.9 (C-8, C-10′); 25.6 (C-9′); 28.4 (C-8); 28.8 (C-9); 33.0 (C-7′, C-11′); 34.7 (C-7); 35.8 (C-4); 38.6 (C-6); 40.1 (C-5); 42.1 (C-3); 48.5 (C-6′); 50.0 (C-12′); 62.4 (C-3′); 112.3 (C-13); 125.7 (C-1); 126.1 (C-14′, C-18′); 127.2 (C-16′); 128.7 (C-15′, C-17′); 139.5 (C-7′); 149.2 (C-11); 173.2 (C-10); 173.4 (C-12); 173.8 (C-4′); 199.1 (C-2). HRMS-ES (m/z) [M + Na]+: Calcd. for C32H44N2O3Na: 527.3250; found: 527.3246.
Compound [10]: Amorphous solid, m.p.: 44–46 °C. [α]D20: −23.6 (c 10.81; CHCl3). IR (KBr; υmax: cm−1): 3429, 2926, 1655, 1493, 1363, 1228, 1171, 706. 1H-NMR: (δ = ppm, 600 MHz): 0.82 (3H, s, Me-14); 0.97–1.03 (1H, m, H-6); 1.03–1.09 (2H, m, H-1, H-9); 1.10 (3H, s, Me-15); 1.19 (1H, dd, J = 2 y 12 Hz, H-5); 1.25–1.33 (2H, m, H-2); 1.35, 1.43 (6H, s, H-1′, H-2′); 1.37–1.39 (3H, m, H-1, H3, H-9); 1.41 (9H, s, H-7′,H-8′, H-9′); 1.50–1.56 (2H, m, H-8,); 1.79–1.81 (1H, m, H-3); 1.85–1.89 (1H, m, H-6); 1.94–2.00 (1H, m, H-7); 4.92, 4.96 (2H, s, H-13); 5.8 (1H, s, H-5′); 7.26–7.29 (3H, m, H-12′, H-13′, H-14′); 7.34–7.36 (2H, m, H-11′, H-15′). 13C-NMR: (δ = ppm, 150 MHz): 18.6 (C-14); 20.0 (C-8); 22.8 (C-15); 25.1, 25.8 (C-1′, C-2′); 25.6 (C-6); 26.1 (C-2); 28.7 (C-7′, C-8′, C-9′); 34.5 (C-10); 40.9 (C-9); 41.9 (C-7); 42.3 (C-3); 44.3 (C-1); 51.1 (C-6′); 54.9 (C-5); 63.2 (C-3′); 72.0 (C-4); 114.4 (C-13); 128.1 (C-13′); 128.5 (C11′,C-15′); 131.4 (C-12′,C-14′); 139.6 (C-10′); 150.9 (C-11); 172.0 (C-12); 173.8 (C-4′). HRMS-ES (m/z) [M + Na]+: Calcd. for C29H44N2O3Na: 491.3251; found: 491.3250.
Compound [11]: White amorphous solid, m.p.: 70–71 °C. [α]D20: −13.2 (c 9.11; CHCl3). IR (KBr; υmax: cm−1): 3446, 2922, 1624, 1454, 1362, 1232, 1171, 908. 1H-NMR: (δ = ppm, 600 MHz): 0.80 (3H, s, Me-14); 0.98 (1H, m, H-9); 1.00 (3H, s, Me-15); 1.06–1.09 (1H, m, H-6); 1.09–1.13 (1H, m, H-1); 1.18 (1H, dd, J = 2 y 12 Hz, H-5); 1.25 (9H, s, H-7′, H-8′, H-9′); 1.26–1.29 (2H, m, H-9, H-3); 1.29, 1.32 (6H, s, H-1′, H-2′); 1.33–1.36 (1H, m, H-1); 1.37–1.40 (1H, m, H-2); 1.41-1.47 (2H, m, H-8); 1.53–1.59 (1H, m, H-2); 1.67–1.72 (1H, dd, J = 12 Hz, H-3);1.92–1.97 (1H, m, H-6); 2.20 (1H, m, H-7); 4.6 (2H, s, H-10′); 4.92, 5.08 (2H, s, H-13); 5.5 (1H, s, H-5′); 7.17 (1H, m, H-14′); 7.25–7.27 (4H, m, H-12′, H-13′, H-15′, H-16′). 13C-NMR: (δ = ppm, 150 Hz): 18.6 (C-14); 20.0 (C-8); 22.9 (C-15); 24.4, 24.6 (C-1′,C-2′); 25.5 (C-6); 26.1 (C-2); 28.6 (C-7′, C-8′, C-9′); 34.5 (C-10); 41.0 (C-9); 42.9 (C-7); 42.6 (C-3); 44.2 (C-1); 51.0 (C-6′); 54.8 (C-5); 63.0 (C-3′); 71.8 (C-4); 111.1 (C-13); 126.4 (C-12′,C-16′); 127.2 (C-14′); 128.7 (C-13′,C15′); 139.5 (C11′); 150.8 (C-11); 174.0 (C-4′); 174.1 (C-12). HRMS-ES (m/z) [M + Na]+: Calcd. for C30H46N2O3Na: 505.3401; found: 505.3404.
Compound [12]: Amorphous solid, m.p.: 61–62 °C. [α]D20: −27.5 (c 13.09; CHCl3). IR (KBr; υmax: cm−1): 3450, 2926, 1655, 1491, 1369, 1169, 700. 1H-NMR: (δ = ppm, 600 MHz): 0.80 (3H, s, Me-14); 0.97–1.01 (1H, m, H-6); 1.05 (3H, s, Me-15); 1.08–1.06 (1H, m, H-9); 1.10 (1H, dd, J = 4 y 13 Hz, H-1); 1.15 (1H, dd, J = 2 y 12 Hz, H-5); 1.24–1.28 (1H, m, H-2); 1.34–1.38 (3H, m, H-1, H-3, H-9); 1.44, 1.45 (6H, s, H-1′, H-2′); 1.48–1.56 (2H, m, H-8,); 1.74–1.77 (1H, m, H-3); 1.81–1.84 (1H, m, H-6); 2.01–2.05 (1H, m, H-7); 4.51, 4.58 (2H, dd, J = 6 Hz, H-6′); 4.94 (2H, s, H-13); 6.2 (1H, t, J = 6 Hz, H-5′); 7.26–7.31 (1H, m, H-10′); 7.28–7.31 (2H, m, H15′, H-17′); 7.34–7.37 (5H, m, H-9′, H-11′, H-14′, H16′, H-18′); 7.38–7.40 (2H, m, H-8′, H12′). 13C-NMR (δ = ppm, 150 MHz): 18.5 (C-14); 20.0 (C-8); 22.7 (C-15); 25.2, 25.6 (C-1′, C-2′); 25.9 (C-6); 26.1 (C-2); 34.4 (C-10); 40.9 (C-9); 41.8 (C-7); 42.7 (C-3); 43.9 (C-6′); 44.3 (C-1); 54.8 (C-5); 62.8 (C-3′); 72.0 (C-4); 114.8 (C-13); 127.3 (C-10′); 127.8 (C-8′,C-12′); 128.2 (C-16′); 128.5, 128.6 (C-9′, C-11′, C-14′, C-18′); 131.4 (C-15′, C-17′); 139.5 (C-13′); 150.4 (C-11); 172.2 (C-12); 174.6 (C-4′). HRMS-ES (m/z) [M + Na]+: Calcd for C33H42N2O3Na: 525.3091; found: 525.3093.
Compound [13]: Amorphous solid. m.p.: 75–76 °C. [α]D20: −15.5 (c 10.53; CHCl3). IR (KBr; υmax: cm−1): 3361, 2926, 1647, 1456, 1385, 1362, 908; 698. 1H-NMR: (δ = ppm, 600 MHz): 0.88 (3H, s, Me-14); 1.08–1.10 (1H, m, H-9); 1.10 (3H, s, Me-15); 1.16–1.21 (2H, m, H-1; H-6); 1.22–1.25 (1H, m, H-5); 1.33–1.37 (1H, m, H-3); 1.37–1.41 (1H, m, H-9); 1.43–1.46 (1H, m, H-1); 1.48, 1.52 (6H, s, H-1′, H-2′); 1.50–1.51 (1H, m, H-2); 1.53–1.59 (2H, m, H-8); 1.66–1.71 (1H, m, H-2); 1.76–1.80 (1H, m, H-3); 2.00–2.04 (1H, m, H-6); 2.35–2.40 (1H, m, H-7); 4.5 (2H, ddd, J = 6 Hz, H-6′); 4.81 (2H, s, H-13′); 5.03, 5.20 (2H, s, H-13); 6.1 (1H, t, J = 6 Hz, H-5′); 7.26–7.28, 7.29–7.30 (2H, m, H-10′, H-17′); 7.36–7.39 (6H, m, H-8′, H-9′, H-11′, H-12′, H-16′, H-18′); 7.41 (2H, d, J = 7 Hz, H-15′, H-19′). 13C-NMR (δ = ppm, 150 MHz): 18.6 (C-14); 20.0 (C-8); 22.7 (C-15); 24.4, 24.5 (C-1′,C-2′); 25.6 (C-6); 26.2 (C-2); 34.6 (C-10); 40.9 (C-9); 42.8 (C-7); 42.7 (C-3); 43.9 (C-6′); 44.3 (C-1); 50.5 (C-13′); 54.8 (C-5); 62.7 (C-3′); 72.0 (C-4); 111.1 (C-13); 126.4 (C-15′,C-19′); 127.2, 127.3 (C-10′,C-17′); 127.9 (C-8′yC-12′); 128.6, 128.7 (C-9′,C-11′,C-16′,C-18′); 138.7 (C-7′); 139.3 (C-14′); 150.3 (C-11); 174.2 (C-12); 174.9 (C-4′). HRMS-ES (m/z) [M + Na]+: Calcd. for C33H44N2O3Na: 539.3246; found: 539.3250.
Compound [14]: Amorphous solid, m.p.: 78–79 °C. [α]D20: −17.8 (c 9.3; CHCl3). IR (KBr; υmax: cm−1): 3448, 2931, 2361, 1655, 1543, 1460, 1176. 1H-NMR: (δ = ppm, 600 MHz): 0.79 (3H, s, Me-14); 0.96–1.02 (1H, m, H-6); 1.03–1.10 (2H, m, H-9, H-1); 1.07 (3H, s, Me-15); 1.16–1.27 (5H, m, H-5, H-7′, H-9′, H-9′, H-11′,); 1.30–1.38 (5H, m, H-1, H-3, H-9, H-8′, H-10′); 1.34, 1.42 (6H, s, H-1′, H-2′); 1.40–1.447 (2H, m, H-2); 1.54–1.58 (2H, m, H-8); 1.67–1.74 (2H, m, H-8′,H-10′); 1.76–1.82 (2H, m, H-6,H-3); 1.93–2.20 (3H, m, H-7; H-7′-H-11′); 3.70–3.85 (1H, m, H-6′); 4.91 (1H, s, H-13); 4.96 (1H, s, H-13); 5.7475 (1H, d, J = 8 Hz, H-5′); 7.23–7.28 (2H, m, H-14′, H-16′); 7.30–7.36 (3H, m, H-13′, H-15′, H-17′). 13C-NMR (δ = ppm, 150 MHz): 18.5 (C-14); 19.9 (C-8); 22.7 (C-15); 24.8 (C-8′/C10′); 25.05, 25.73 (C-1′,C-2′); 25.6 (C-6)*; 25.7 (C-9′); 26.2 (C-2); 32.9, 33.0 (C-7′,C11′); 34.4 (C-10); 40.8 (C-9); 42.6 (C-7); 42.6 (C-3); 44.3 (C-1); 48.4 (C-6′); 54.8 (C-5); 62.7 (C-3′); 71.9 (C-4); 114.5 (C-13); 128.1 (C-13′,C-17′); 128.4 (C-15′); 131.5 (C-14′y C-16′); 139.6 (C-12′); 150.7 (C-11); 172.0 (C-12); 173.7 (C-4′). HRMS-ES (m/z) [M + Na]+: Calcd. for C31H46N2O3Na: 517.3405; found: 517.3406.
Compound [15]: Amorphous solid, m.p.: 57–59 °C. [α]D20: −13.7 (c 8.35; CHCl3). IR (KBr; υmax: cm−1): 3421, 2926, 2360, 1653, 1541, 1458, 1171. 1H-NMR: (δ = ppm, 600 MHz): 0.87 (3H, s, Me-14); 1.10–1.11 (1H, m, H-9); 1.10 (3H, s, Me-15); 1.14–1.20 (4H, m, H-6, H-7′, H-9′, H-11′); 1.21–1.24 (1H, m, H-1); 1.27–1.29 (1H, m, H-5); 1.37–1.40 (3H, m, H-3, H-9, H-8′/H-10′); 1.33–1.37 (1H, m, H-8′/H-10′); 1.43–1.45 (1H, m, H-1); 1.44, 1.44 (6H,, H-2′); 1.46–1.50 (1H, m, H-2); 1.53–1.57 (2H, m, H-8); 1.59–1.62 (1H, m, H-9′); 1.66–1.73 (3H, m, H-2, H-8′/H-10′); 1.78–1.81 (1H, m, H-3); 1.93–1.99 (2H, m, H-7′, H-11′), 1.99–2.03 (1H, m, H-6); 2.29–2.42 (1H, m, H-7); 3.74 (1H, m, H-6′); 4.74 (2H, d, J = 5 Hz, H-12); 5.02 (1H, s, H-13); 5.19 (1H, s, H-13); 5.63 (1H, d, J = 8 Hz, H-5′); 7.29–7.27 (1H, m, H-16′); 7.37 (2H, t, J = 7 Hz, H-15′, H-17′); 7.42 (2H, d, J = 7 Hz, H-14′, H-18′). 13C-NMR (δ = ppm, 150 MHz): 18.6 (C-14); 20.0 (C-8); 22.8 (C-15); 24.3, 24.5 (C-1′,C-2′); 24.83, 24.9 (C-8′,C-10′); 25.5 (C-6); 25.7 (C-9′); 26.2 (C-2); 32.9, 30.0 (C-7′,C11′); 34.6 (C-10); 41.0 (C-9); 42.6 (C-7); 42.7 (C-3); 44.3 (C-1); 48.4 (C-6′); 50.2 (C-12′); 54.8 (C-5); 62.6 (C-3′); 71.9 (C-4); 111.2 (C-13); 126.4 (C-14′,C-18′); 127.1 (C-16′); 128.6 (C-15′y C-17′); 139.5 (C-13′); 150.5 (C-11); 174.0 (C-4′); 174.1 (C-12′). HRMS-ES (m/z) [M + Na]+: Calcd. for C32H48N2O3Na: 531.3565; found: 531.3563.
Compound [16]: Colorless oil. [α]D20: −10.2 (c 6.17; CHCl3). IR (KBr; υmax: cm−1): 3294, 2926, 2854, 1713, 1468, 1261, 1093, 802. 1H-NMR (δ = ppm, 600 MHz): 0.88 (3H, s, Me-14); 0.88 (3H, t, J = 8.0 Hz Me-12′); 1.04–1.12 (1H, m, H-9a); 1.10 (3H, s, Me-15); 1.14–1.21 (2H, m, H-1a, H-2a); 1.21–1.25 (1H, m, H-5); 1.25–1.35 (14H, m, H-5′, H-6′, H-7′, H-8′, H-9′, H-10′, H-11′); 1.35-1.37 (1H, m, H-3a); 1.37–1.40 (1H, m, H-9b); 1.40–1.44 (2H, m, H-1b, H-6a); 1.50–1.60 (3H, m, H-6b, H-8a, H-8b); 1.63–1.70 (2H, m, H-4′); 1.78–1.83 (1H, m, H-3b); 1.86–1.94 (2H, m, H-2b, H-7); 2.7 (2H, t, J = 8.8 Hz, H-3′); 4.83, 5.08 (2H, s, H-13a, H-13b); 4.95 (2H, c, J = 9.0 Hz, H-12); 7.26 (1H, s, H-1′). 13C-NMR (δ = ppm, 50 MHz): 14.1 (C-12′); 18.2 (C-6′); 18.7 (C-15); 20.1 (C-8); 22.6 (C-14); 25.2 (C-3′); 26.1 (C-2); 27.0 (C-6); 28.4 (C-4′); 27.9 (C-5′); 34.6 (C-10); 40.9 (C-9); 42.4 (C-7); 43.5 (C-3); 44.4 (C-1); 54.2 (C-12); 54.8 (C-5); 64.5 (C-8′); 72.1 (C-4); 84.4 (C-7′); 112.8 (C-13); 120.8 (C-1′); 148.1 (C-2′); 148.7 (C-11). HRMS (m/z) [M]+: Calcd. for C27H47N3O: 429.3719; found 429.3716.
Compound [17]: Colorless oil. [α]D20: −30.8 (c 1.59; CHCl3). IR (KBr; υmax: cm−1): 2962, 1741, 1477, 1261, 1089, 798. 1H-NMR: (δ = ppm, 600 MHz): 0.87 (3H, s, Me-14); 1.02–1.08 (1H, m, H-9); 1.09 (3H, s, Me-15); 1.41.24 (3H, m, H-1, H-2, H-5); 1.32–1.37 (1H, m, H-3); 1.37–1.46 (3H, m, H-1, H-6, H-9); 1.52–1.61 (5H, m, H-6, H-8, H-8a, H-5′, H-5′a); 1.74–1.83 (3H, m, H-3, H-4′, H-4′a); 1.83–1.91 (2H, m, H-2, H-7); 1.94 (1H, s, H-8′); 2.2 (2H, t, J = 11 Hz, H-6′); 2.7 (2H, t, J = 9 Hz, H-3′); 4.8 (1H, s, H-13); 4.94 (2H, c, J = 15 Hz, H-12); 5.10 (1H, s, H-13); 7.26 (1H, s, H-1′). 13C-NMR (δ = ppm, 150 MHz): 18.2 (C-6′); 18.7 (C-15); 20.1 (C-8); 22.6 (C-14); 25.2 (C-3′); 26.1 (C-2); 27.0 (C-6); 28.4 (C-4′); 27.9 (C-5′); 34.6 (C-10); 40.9 (C-9); 42.4 (C-7); 43.5 (C-3); 44.4 (C-1); 54.2 (C-12); 54.8 (C-5); 64.5 (C-8′); 72.1 (C-4); 84.4 (C-7′); 112.8 (C-13); 120.8 (C-1′); 148.1 (C-2′); 148.7 (C-11). HRMS (m/z) [M]+: Calcd. for C22H32N3O: 355.2624; found 355.2646.
Compound [18]: Colorless oil. [α]D20: −20.5 (c 4.60; CHCl3). IR (KBr; υmax: cm−1): 3477, 3330, 2927, 1648, 1467, 1172, 763. 1H-NMR: (δ = ppm, 600 MHz): 0.86 (3H, s, Me-14); 1.04–1.09 (1H, m, H-9); 1.07 (3H, s, Me-15); 1.12–1.173 (3H, m, H-1, H-2, H-5); 1.29–1.35 (1H, m, H-3); 1.36–1.42 (3H, m, H-1, H-6, H-9); 1.49–1.55 (3H, m, H-6, H-8, H-8a); 1.75–1.81 (1H, m, H-3); 1.82–1.88 (2H, m, H-2, H-7); 4.09 (2H, s, H-3′); 4.8 (1H, s, H-13); 4.92 (2H, c, J = 14 Hz, H-12); 5.04 (1H, s, H-13); 7.15 (1H, s, H-1′); 7.22 (1H, m, H-7′); 7.22–7.25 (2H, m, H-5′, H-9′); 7.29 (2H, m, H-6′, H-8′). 13C-NMR (δ = ppm, 150 MHz): 18.8 (C-14); 20.2 (C-8); 22.8 (C-15); 26.3 (C-2); 27.1 (C-6); 32.5 (C-3′); 34.7 (C-10); 41.0 (C-9); 42.6 (C-7); 43.7 (C-3); 44.6 (C-1); 54.4 (C-12); 55.0 (C-5); 72.2 (C-4); 113.0 (C-13); 121.8 (C-1′); 126.2 (C-7′); 128.7, 128.8 (C-5′ y 9′,C-6′ y 8′); 139.3 (C-4′); 148.1 (C-2′); 148.6 (C-11). HRMS (m/z) [M]+: Calcd. for C24H332N3O: 379.2604; found: 379.2619.
Compound [19]: Amorphous white solid, m.p.: 100–101 °C. [α]D20: −6.0 (c 5.41; CHCl3). IR (KBr; υmax: cm−1): 3475, 3338, 2927, 2357, 1647, 1466, 1173, 908. 1H-NMR: (δ = ppm, 200 MHz) 0.88 (3H, s, Me-14); 1.06–1.13 (1H, m, H-9); 1.10 (3H, s, Me-15); 1.15–1.24 (3H, m, H-1, H-2, H-5); 1.25–1.32 (1H, m, H-3); 1.32–1.43 (3H, m, H-1, H-6, H-9); 1.53–1.64 (3H, m, H-6, H-8, H-8a); 1.74–1.84 (1H, m, H-3); 1.88–2.03 (2H, m, H-2, H-7); 4.92 (1H, s, H-13); 5.03 (2H, s, H-12); 5.13 (1H, s, H-13); 7.29–7.37 (1H, m, H-6′); 7.37–7.48 (2H, m, H-5′, H-7′); 7.81–7.88 (2H, m, H-4′, H-8′); 7.74 (1H, s, H-1′). 13C-NMR (δ = ppm, 50 MHz): 18.7 (C-14); 20.1 (C-8); 22.5 (C-15); 26.1 (C-2); 27.0 (C-6); 34.6 (C-10); 40.9 (C-9); 42.5 (C-7); 43.6 (C-3); 44.4 (C-1); 54.4 (C-12); 54.9 (C-5); 72.1 (C-4); 113.1 (C-13); 119.6 (C-1′); 125.7 (C-4′, C-8′); 128.1 (C-6′); 128.8 (C-5′, C-7′); 130.7 (C-3′); 148.0 (C-2′); 148.6 (C-11). HRMS-ES (m/z) [M + Na]+: Calcd. for C23H31N3ONa: 388.2369; found: 388.2365.