Distinct Role of Mono-2-ethylhexyl Phthalate in Neuronal Transmission in Rat CA3 Hippocampal Neurons: Involvement of Ion Channels
Abstract
:1. Introduction
2. Results
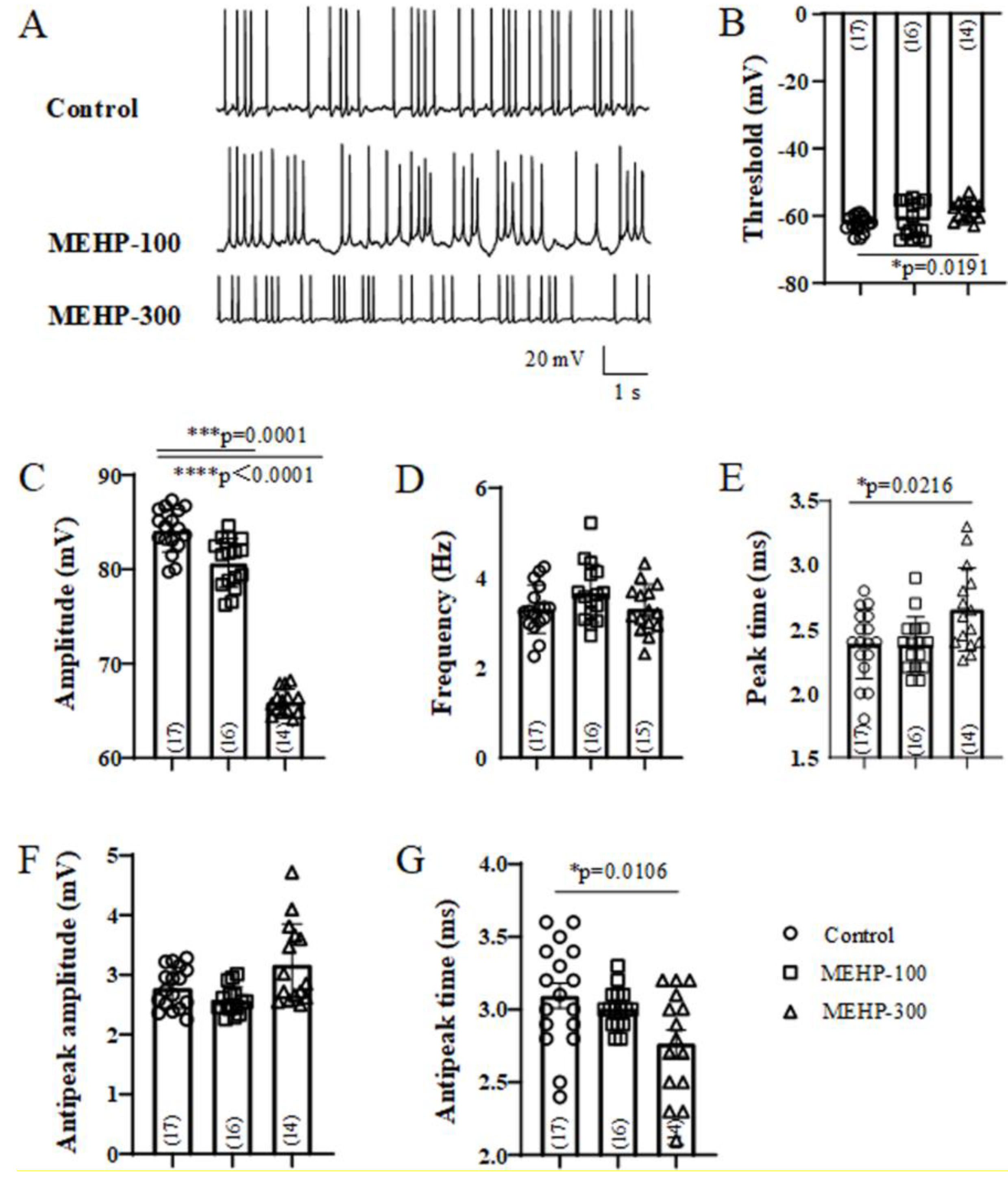
2.1. MEHP Suppressed the Excitability of Rat Hippocampal CA3 Neurons
2.2. MEHP Decreased the Na+ Current Recorded from Rat CA3 Hippocampal Neurons
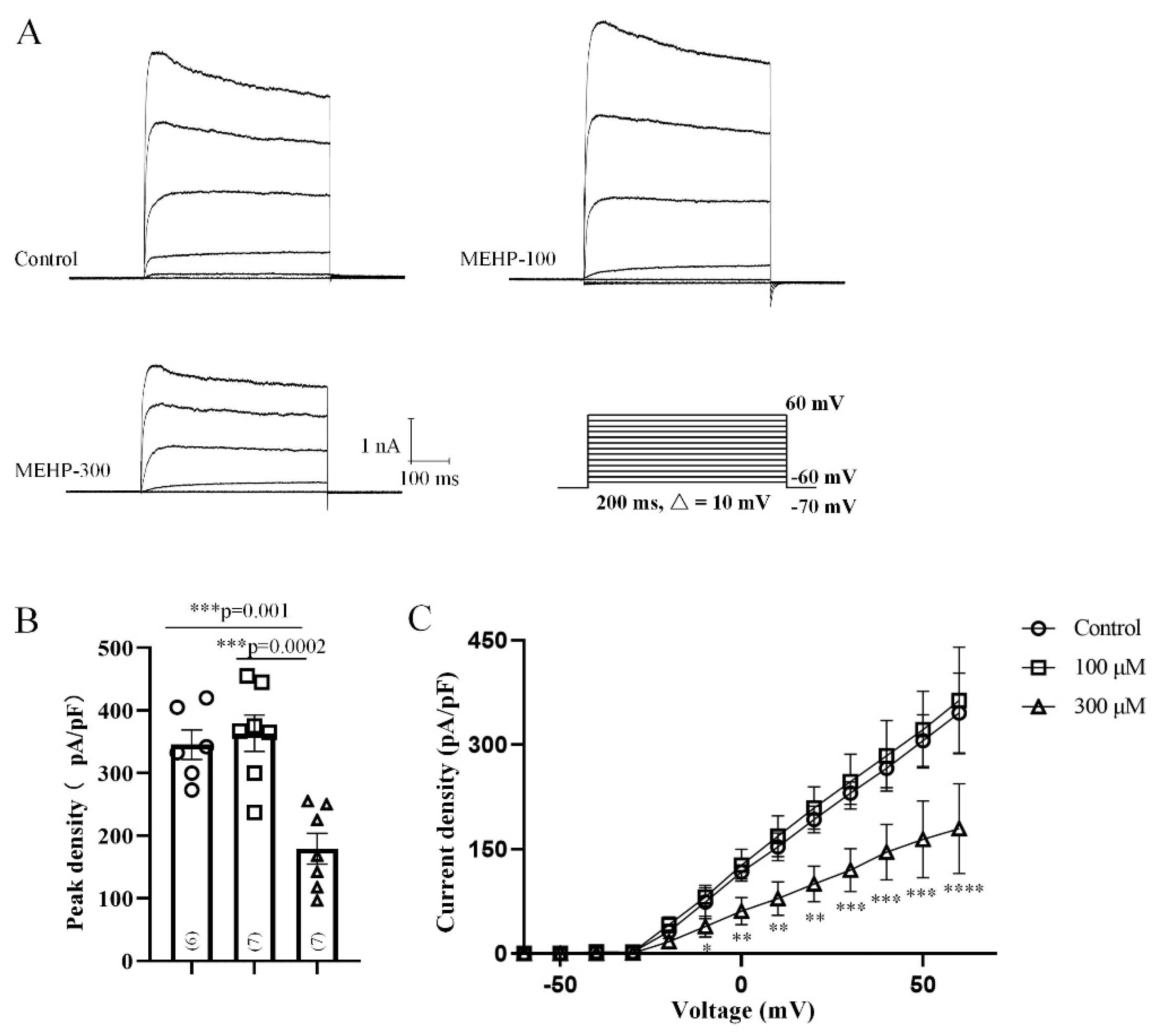
2.3. MEHP Decreased the Total Kv Current Recorded from Rat CA3 Hippocampal Neurons
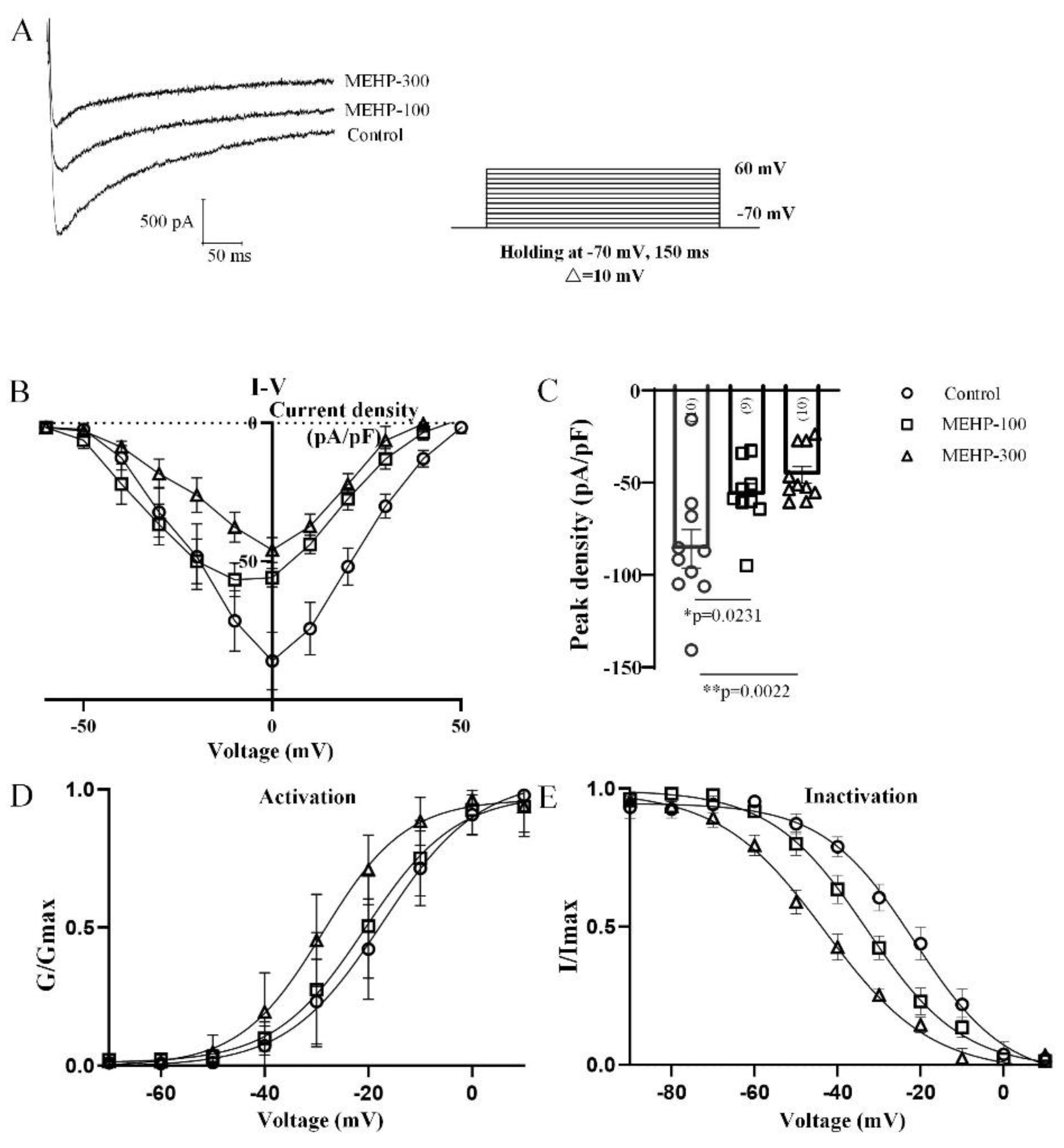
2.4. MEHP Decreased the Total Calcium Current Recorded from Rat CA3 Hippocampal Neurons
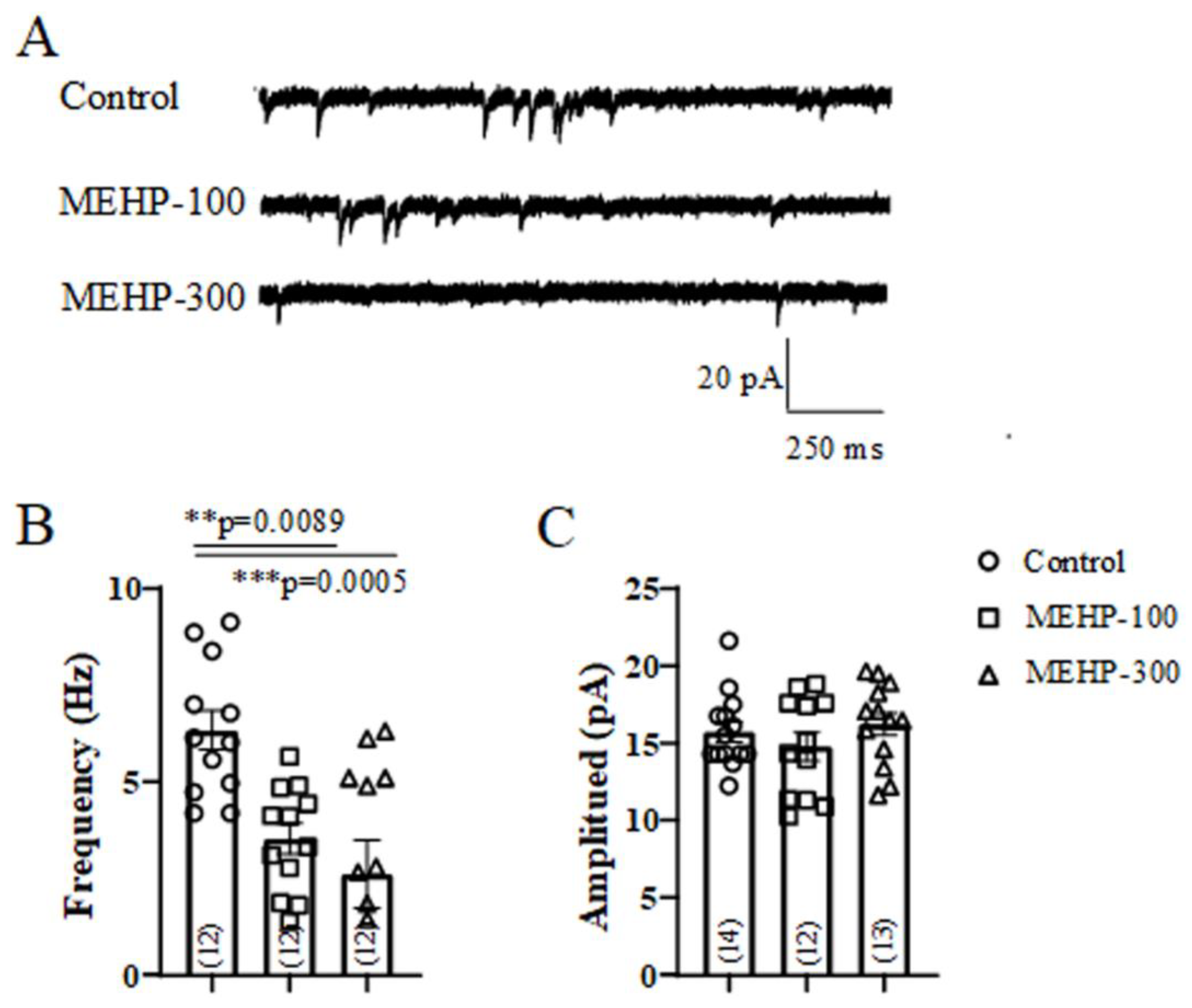
2.5. MEHP Reduced the Frequency of mEPSC Recorded from Rrat CA3 Hippocampal Neurons
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Treatment
4.3. Brain Tissue Preparation
4.4. Whole-Cell Electrophysiological Recordings
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Koch, H.M.; Preuss, R.; Angerer, J. Di(2-ethylhexyl) phthalate (DEHP): Human metabolism and internal exposure-an update and latest results1. Int. J. Androl. 2006, 29, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, L.; Latini, G.; Felice, C.; Razzi, S.; Paris, I.; Ruggieri, F.; Mazzeo, P.; Petraglia, F. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum. Reprod. 2003, 18, 1512–1515. [Google Scholar] [CrossRef]
- Latini, G.; De Felice, C.; Verrotti, A. Plasticizers, infant nutrition and reproductive health. Reprod. Toxicol. 2004, 19, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Hauser, R.; Calafat, A.M.; Weuve, J.; Schettler, T.; Ringer, S.; Huttner, K.; Hu, H. Use of Di(2-ethylhexyl) Phthalate–Containing Medical Products and Urinary Levels of Mono(2-ethylhexyl) Phthalate in Neonatal Intensive Care Unit Infants. Environ. Health Perspect. 2005, 113, 1222–1225. [Google Scholar] [CrossRef] [Green Version]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 13–43. [Google Scholar] [CrossRef] [Green Version]
- Albro, P.W.; Lavenhar, S.R. Metabolism of di(2-ethylhexyl) phthalate. Drug Metab. 1989, 21, 13–14. [Google Scholar] [CrossRef]
- Huber, W.W.; Grasl-Kraupp, B.; Schulte-Hermann, R. Hepatocarcinogenic Potential of Di(2-Ethylhexyl) phthalate in Rodents and its Implications on Human Risk. Crit. Rev. Toxicol. 1996, 26, 365–481. [Google Scholar] [CrossRef]
- Li, X.-N.; Li, H.-X.; Yang, T.-N.; Li, X.-W.; Huang, Y.-Q.; Zhu, S.-Y.; Li, J.-L. Di-(2-ethylhexyl) phthalate induced developmental abnormalities of the ovary in quail (Coturnix japonica) via disruption of the hypothalamic-pituitary-ovarian axis. Sci. Total Environ. 2020, 741, 140293. [Google Scholar] [CrossRef]
- Zou, Q.-Y.; Hong, S.-L.; Kang, H.-Y.; Ke, X.; Wang, X.-Q.; Li, J.; Shen, Y. Effect of di-(2-ethylhexyl) phthalate (DEHP) on allergic rhinitis. Sci. Rep. 2020, 10, 14625. [Google Scholar] [CrossRef]
- Seth, P.K. Hepatic effects of phthalate esters. Env. Health Perspect 1982, 45, 27–34. [Google Scholar] [CrossRef]
- Stermer, A.R.; Murphy, C.J.; Ghaffari, R.; Di Bona, K.R.; Voss, J.J.; Richburg, J.H. Mono-(2-ethylhexyl) phthalate-induced Sertoli cell injury stimulates the production of pro-inflammatory cytokines in Fischer 344 rats. Reprod. Toxicol. 2017, 69, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Berrios, C.A.; Vélez, C.; Zayas, B. Mitochondrial permeability and toxicity of diethylhexyl and monoethylhexyl phthalates on TK6 human lymphoblasts cells. Toxicol. In Vitro 2011, 25, 2010–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, D.; Cai, S.; Wu, H.; Gu, H. Di (2-ethylhexyl) phthalate modulates cholinergic mini-presynaptic transmission of projection neurons in Drosophila antennal lobe. Food Chem. Toxicol. 2012, 50, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.; Luo, Y.; Gan, Z.; Liu, J.; Yang, J. Neural mechanisms underlying the deficit of learning and memory by exposure to Di(2-ethylhexyl) phthalate in rats. Ecotoxicol. Environ. Saf. 2019, 174, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Holahan, M.R. Reduced hippocampal dendritic spine density and BDNF expression following acute postnatal exposure to di(2-ethylhexyl) phthalate in male Long Evans rats. PLoS ONE 2014, 9, e109522. [Google Scholar] [CrossRef] [Green Version]
- Dhanya, C.R.; Indu, A.R.; Deepadevi, K.V.; Kurup, P.A. Inhibition of membrane Na (+)-K+ Atpase of the brain, liver and RBC in rats administered di (2-ethyl hexyl) phthalate (DEHP) a plasticizer used in polyvinyl chloride (PVC) blood storage bags. Indian J. Exp. Biol. 2003, 41, 814–820. [Google Scholar]
- Magliozzi, R.; Howell, O.W.; Reeves, C.; Roncaroli, F.; Nicholas, R.; Serafini, B.; Aloisi, F.; Reynolds, R. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 2010, 68, 477–493. [Google Scholar] [CrossRef]
- Le Duigou, C.; Simonnet, J.; Teleñczuk, M.T.; Fricker, D.; Miles, R.M. Recurrent synapses and circuits in the CA3 region of the hippocampus: An associative network. Front. Cell. Neurosci. 2014, 7, 262. [Google Scholar] [CrossRef]
- Spencer, W.; Kandel, E.R. Hippocampal neuron responses to selective activation of recurrent collaterals of hippocampofugal axons. Exp. Neurol. 1961, 4, 149–161. [Google Scholar] [CrossRef]
- Manns, J.R.; Eichenbaum, H. Time and treason to the trisynaptic teachings: Theoretical comment on Kesner et al. (2005). Behav. Neurosci. 2005, 119, 1140–1143. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Gan, Z. Effects of Mono-2-ethylhexyl Phthalate on the Neural Transmission of PNs in Drosophila Antennal Lobe. Neurotox. Res. 2021, 39, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, P.O.; Bondesson, U.G.; Sedin, E.G.; Gustafsson, J.P. Exposure of newborn infants to plasticizers. Plasma levels of di-(2- ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate during exchange transfusion. Transfusion 1985, 25, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Grimwood, P.D.; Morris, R.G.M. Synaptic Plasticity and Memory: An Evaluation of the Hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef] [Green Version]
- Suwabe, T.; Mistretta, C.M.; Krull, C.; Bradley, R.M. Pre- and postnatal differences in membrane, action potential, and ion channel properties of rostral nucleus of the solitary tract neurons. J. Neurophysiol. 2011, 106, 2709–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandel, G. Sodium channel regulation in the nervous system: How the action potential keeps in shape. Curr. Opin. Neurobiol. 1993, 3, 278–282. [Google Scholar] [CrossRef]
- Kim, J.; Wei, D.-S.; Hoffman, D.A. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J. Physiol. 2005, 569, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.C.; Galarraga, E.; Bargas, J.; Cristancho, M.; Aceves, J. Charybdotoxin and apamin sensitivity of the calcium-dependent repolarization and the afterhyperpolarization in neostriatal neurons. J. Neurophysiol. 1992, 68, 287–294. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, J.; Wang, H.; Gan, Z.; Ran, D. Cellular Mechanism Underlying rTMS Treatment for the Neural Plasticity of Nervous System in Drosophila Brain. Int. J. Mol. Sci. 2019, 20, 4625. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Liu, S.; Gao, P.; Cao, P.; Xu, H. Effect of diisobutyl phthalate on learning and memory behavior and apoptosis of hippocampus cells in mice. Wei Sheng Yan Jiu J. Hyg. Res. 2013, 42, 57–61. [Google Scholar]
- Zeliger, H.I. Exposure to lipophilic chemicals as a cause of neurological impairments, neurodevelopmental disorders and neurodegenerative diseases. Interdiscip. Toxicol. 2013, 6, 103–110. [Google Scholar] [CrossRef]
- Smith, C.; MacDonald, A.; Holahan, M. Acute postnatal exposure to di(2-ethylhexyl) phthalate adversely impacts hippocampal development in the male rat. Neurosci. 2011, 193, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Purves, D. Neuroscience, 4th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2008. [Google Scholar]
- Uzman, A.; Lodish, H.; Berk, A.; Matsudaira, P.; Kaiser, C.; Krieger, M.; Scott, M.; Zipursky, L.; Darnell, J. Molecular Cell Biology, 4th ed.; Mac Millan: Basingstoke, UK, 2000. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol 1952, 116, 449–472. [Google Scholar] [CrossRef] [PubMed]
- Venkateshwaran, M.; Cosme, A.; Han, L.; Banba, M.; Satyshur, K.; Schleiff, E.; Parniske, M.; Imaizumi-Anraku, H.; Ané, J.-M. The Recent Evolution of a Symbiotic Ion Channel in the Legume Family Altered Ion Conductance and Improved Functionality in Calcium Signaling. Plant Cell 2012, 24, 2528–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. BioMed Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittassek, M.; Angerer, J. Phthalates: Metabolism and exposure. Int. J. Androl. 2008, 31, 131–138. [Google Scholar] [CrossRef]
- Leeder, J.S.; Kearns, G.L. Pharmacogenetics in pediatrics. Implications for practice. Pediatr. Clin. N. Am. 1997, 44, 55–77. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Zhang, H.; Liu, Y.; Cao, C.; Dong, R.; Yuan, Y.; Wang, M.; Lu, Y.; Wu, M.; et al. Association between urinary concentration of phthalate metabolites and impaired renal function in Shanghai adults. Environ. Pollut. 2019, 245, 149–162. [Google Scholar] [CrossRef]
- Chen, T.; Yang, W.; Li, Y.; Chen, X.; Xu, S. Mono-(2-ethylhexyl) phthalate impairs neurodevelopment: Inhibition of proliferation and promotion of differentiation in PC12 cells. Toxicol. Lett. 2011, 201, 34–41. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Wang, H.; Yang, J.; Jiang, W.; Xin, H.; Luo, Y.; Loya-López, S.; Gu, H.; Ran, D. Distinct Role of Mono-2-ethylhexyl Phthalate in Neuronal Transmission in Rat CA3 Hippocampal Neurons: Involvement of Ion Channels. Molecules 2022, 27, 3082. https://doi.org/10.3390/molecules27103082
Lu Y, Wang H, Yang J, Jiang W, Xin H, Luo Y, Loya-López S, Gu H, Ran D. Distinct Role of Mono-2-ethylhexyl Phthalate in Neuronal Transmission in Rat CA3 Hippocampal Neurons: Involvement of Ion Channels. Molecules. 2022; 27(10):3082. https://doi.org/10.3390/molecules27103082
Chicago/Turabian StyleLu, Yi, Hong Wang, Junqing Yang, Wengao Jiang, Hong Xin, Ying Luo, Santiago Loya-López, Huaiyu Gu, and Dongzhi Ran. 2022. "Distinct Role of Mono-2-ethylhexyl Phthalate in Neuronal Transmission in Rat CA3 Hippocampal Neurons: Involvement of Ion Channels" Molecules 27, no. 10: 3082. https://doi.org/10.3390/molecules27103082




